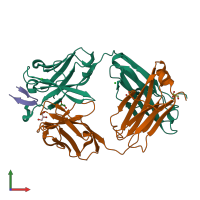
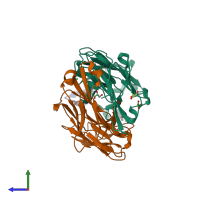
Function and Biology Details
Reactions catalysed:
Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1)
Hydrolysis of four peptide bonds in the viral precursor polyprotein, commonly with Asp or Glu in the P6 position, Cys or Thr in P1 and Ser or Ala in P1'.
NTP + H(2)O = NDP + phosphate
ATP + H(2)O = ADP + phosphate
Biochemical function:
- not assigned
Biological process:
- not assigned
Cellular component:
- not assigned
Structure analysis Details
Assembly composition:
hetero trimer (preferred)
Assembly name:
PDBe Complex ID:
PDB-CPX-541327 (preferred)
Entry contents:
3 distinct polypeptide molecules
Macromolecules (3 distinct):