Abstract
Free full text

S200: IBRUTINIB VERSUS PLACEBO IN PATIENTS WITH ASYMPTOMATIC, TREATMENT-NAÏVE EARLY STAGE CHRONIC LYMPHOCYTIC LEUKEMIA (CLL): FINAL RESULTS OF THE PHASE 3, DOUBLE-BLIND, PLACEBO-CONTROLLED CLL12 TRIAL
Abstract Topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Background: We previously demonstrated that early intervention with ibrutinib lead to longer event-free survival in a subgroup of patients with early stage CLL. However, the results did not justify changing the current standard of care as survival analysis was pending.
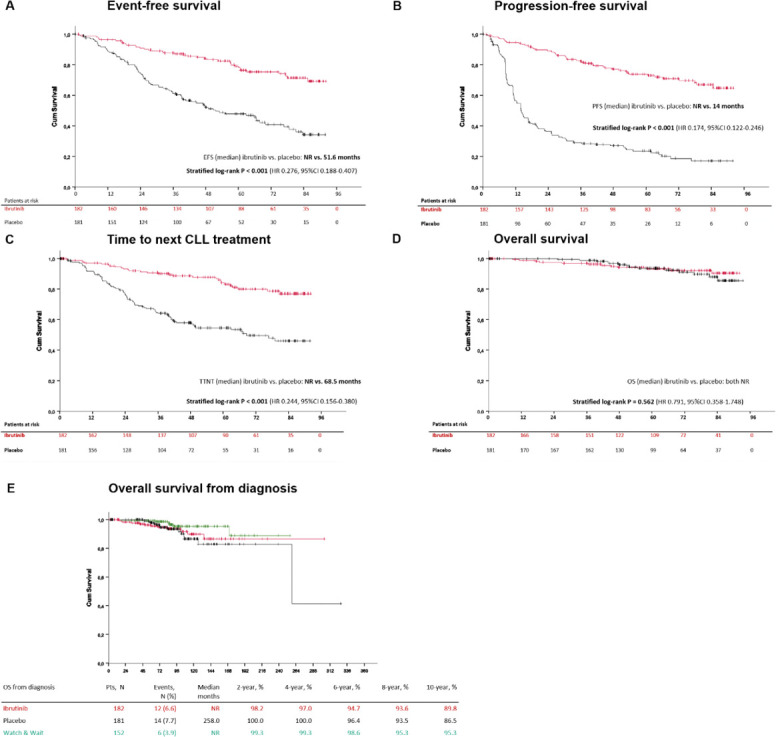
Aims: We present the final analysis of the phase 3, double-blind, placebo-controlled CLL12 trial evaluating ibrutinib in patients with early stage CLL at increased risk of progression defined by a comprehensive score (NCT02863718).
Methods: We randomly assigned patients with asymptomatic, treatment-naïve Binet stage A CLL at increased risk of progression in a 1:1 ratio to receive ibrutinib (n=182) or placebo (n=181) at a dose of 420 mg daily. Patients with low risk CLL were allocated to the watch and wait group (W&W; n=152). The final analysis evaluated event-free survival (EFS; defined as time to symptomatic progression, CLL treatment or death), progression-free survival (PFS), time to next treatment (TTNT), and overall survival (OS).
mg daily. Patients with low risk CLL were allocated to the watch and wait group (W&W; n=152). The final analysis evaluated event-free survival (EFS; defined as time to symptomatic progression, CLL treatment or death), progression-free survival (PFS), time to next treatment (TTNT), and overall survival (OS).
Results: At a median observation time of 69.3 months the overall response rate was 72.5% in the ibrutinib group. PFS, EFS and TTNT were not reached in the ibrutinib group as compared to 14 months (P<0.001; HR 0.174, 95%CI 0.122-0.246), 51.6 months (P<0.001; HR 0.276, 95%CI 0.188-0.407), and 68.5 months (P<0.001; HR 0.244, 95%CI 0.156-0.380) in the placebo group.
A total of 29 (15.9%), 79 (43.6%), and 25 (16.4%) subsequent treatment lines were administered in the ibrutinib, placebo and W&W group, respectively.
The median overall survival was not reached in neither treatment group (P=0.562, HR 0.791, 95%CI 0.358-1.748) with 12 (6.6%) deaths in the ibrutinib and 14 (7.7%) deaths in the placebo group. The estimated survival rate at 5 years for patients treated with ibrutinib and placebo was 93.3% and 93.6%, respectively.
A total of 6 (3.9%) deaths occurred in the W&W group with median survival from CLL diagnosis not reached, as compared to 258 months for the placebo and not reached for the ibrutinib group.
Causes of death were progressive disease (1x ibrutinib), Richter Transformation (3x placebo), treatment-related adverse event (1x ibrutinib, subdural hematoma), infection (2x ibrutinib, 1x placebo, 1x W&W), concomitant disease (4x ibrutinib, 5x placebo, 4x W&W), and other (4x ibrutinib, 5x placebo, 1x W&W).
Adverse events were documented in 99.4% of ibrutinib and placebo treated patients, with 71.8% and 66.1% CTC-grade 3-5 events, respectively.
More bleeding events (36.5% vs. 14.9%), cardiac arrhythmias (22.4% vs. 9.5%), other cardiac events (17.6% vs. 15.5%), diarrhoea (40.6% vs. 28.6%), and hypertensive disorders (19.4% vs. 8.3%) occurred in ibrutinib as compared to placebo-treated patients.
Summary/Conclusion: Early ibrutinib-treatment of patients with asymptomatic Binet stage A CLL at high risk of progression failed to demonstrate an overall survival benefit when compared to placebo. Based on these results, watch and wait should remain the standard of care of patients with early stage CLL with inactive disease - regardless of genetic, laboratory or clinical risk factors.
Keywords: ibrutinib, Phase III, B-CLL, Targeted therapy
Articles from HemaSphere are provided here courtesy of Wiley
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02863718