Abstract
Free full text

Altered Cytokine Production and Impaired Antimycobacterial Immunity in Protein-Malnourished Guinea Pigs
Abstract
Protein malnutrition leads to multiple detrimental alterations of host immune responses to mycobacterial infection. In this study, we demonstrated that splenocytes from low-protein (LP) guinea pigs vaccinated 6 weeks previously with attenuated Mycobacterium tuberculosis H37Ra failed to control the accumulation of virulent M. tuberculosis H37Rv in cocultured autologous peritoneal macrophages, despite the fact that they were able to control the accumulation of virulent tubercle bacilli in cocultured syngeneic peritoneal macrophages from normally nourished guinea pigs as successfully as did those from high-protein (HP) counterparts. Vaccine-induced growth control of virulent M. tuberculosis H37Rv in these cocultures appeared to be mediated by CD4 lymphocytes but not CD8 cells. Tuberculin (purified protein derivative [PPD])-induced lymphoproliferation was markedly impaired in vaccinated LP guinea pigs, and the depletion of CD4 lymphocytes significantly decreased lymphocyte proliferation whereas CD8 cell depletion did not. Protein malnutrition also impaired the abilities of cells from vaccinated LP guinea pigs to produce cytokines, including interferon, tumor necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β), in response to PPD, despite the demonstration of higher serum levels of TNF-α and TGF-β after an intravenous injection of PPD into LP guinea pigs. In contrast, peritoneal macrophages from protein-malnourished guinea pigs produced a higher level of TGF-β 4 days after infection in vitro with M. tuberculosis H37Rv than did those from protein adequate controls. These results suggest that dietary protein malnutrition impairs vaccine-induced resistance to M. tuberculosis, in part, by altering the cytokine profile to favor macrophage deactivation.
Tuberculosis causes approximately 1.5 billion latent infections, 8 million new clinical cases, and 3 million deaths annually, making it the most prevalent infectious disease in the world (9). The emergence of multidrug-resistant tuberculosis and the pandemic of AIDS have further exacerbated the situation (1, 11). The host protective immune mechanisms against infection with Mycobacterium tuberculosis depend critically on the interactions and cooperation between monocytes-macrophages, T lymphocytes, and their cytokines (22), which are sensitive to nutritional insult (5–8, 18).
The incidence of tuberculosis is unusually high among malnourished people, including the elderly, the homeless, alcoholics, drug abusers, and human immunodeficiency virus-infected individuals (43). The reactivation of latent or previously subclinical tuberculous infections may be related, in part, to deteriorating nutritional status (42). Protein malnutrition has been identified as an important risk factor for the predisposition to intracellular infections leading to death (47). There is a strong association between protein malnutrition and impairment of a range of immune functions, principally those mediated by T lymphocytes, which are known to be essential for resistance to tuberculosis (18, 23, 28). Studies of experimental animals indicate that protein deprivation significantly impairs macrophage activation and granuloma development (38) and alters T-cell functions and cytokine production (5–8, 18). Previous reports from this laboratory have demonstrated that chronic, moderate protein deficiency severely impairs T-cell functions and protective efficacy of M. bovis BCG vaccination (32–34). Recently, it was reported that protein calorie malnutrition markedly enhanced bacterial growth and dissemination in mice infected with the attenuated BCG vaccine strain (5) and diminished the expression of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) in the lungs of mice infected by the intravenous route with a very high dose of the virulent M. tuberculosis Erdman strain, resulting in rapidly fatal tuberculosis infection and a markedly elevated bacillary load in the lungs of protein-deficient mice (6). The precise mechanisms by which protein malnutrition impairs host antimycobacterial immunity remain largely unknown.
In this study, we investigated the effects of protein malnutrition on the interaction between immune lymphocytes and M. tuberculosis H37Rv-infected macrophages, the roles of CD4 and CD8 T-cell subpopulations, and the production of cytokines in M. tuberculosis H37Ra-vaccinated guinea pigs.
MATERIALS AND METHODS
Experimental animals.
Both pathogen-free inbred strain 2 guinea pigs (NCI-Frederick Cancer Research Facility, Frederick, Md.) and outbred Hartley strain guinea pigs (Charles River Breeding Laboratories, Inc., Wilmington, Mass.), weighing 200 to 300 g, were used in this study. The animals were individually housed in an air-filtered environment, within polycarbonate cages with stainless steel grid floors and feeders. They were given food and tap water ad libitum. Each animal was randomly assigned to an experimental diet.
Experimental diets.
Purified experimental diets were obtained from a commercial supplier (Dyets, Inc., Bethlehem, Pa.) and formulated to our specifications by inversely varying the proportions of cornstarch and ovalbumin to obtain the desired protein content. The exact diet composition was published previously (30). The low-protein (LP) diet contained 10% ovalbumin, and the high-protein (HP) diet contained 30% ovalbumin. The diets were isocaloric and identical in every nutrient except protein. The third diet used was a commercial guinea pig chow (Ralston Purina, St. Louis, Mo.).
M. tuberculosis H37Ra vaccination.
Two weeks after the experimental diets were started, guinea pigs were infected with viable, attenuated M. tuberculosis H37Ra (ATCC 25177; American Type Culture Collection, Rockville, Md.). Each animal received 0.1 ml of sterile physiological saline containing about 2 × 103 viable bacilli subcutaneously in the left inguinal area. The viability of the inoculum was determined by plating appropriate dilutions on Middlebrook 7H10 agar (Difco Laboratories, Detroit, Mich.).
Necropsy procedure.
Three weeks after M. tuberculosis H37Ra vaccination, all guinea pigs were treated daily with streptomycin (40 mg/kg of body weight) and rifampin (20 mg/kg) for 3 weeks to sterilize the animals, as determined by plating lung and spleen tissue dilutions on M7H10 agar and observing no bacillary growth. Some guinea pigs were injected via the ear vein with purified protein derivative (PPD; 1 mg/kg) 2 h before euthanasia. Euthanasia was carried out by the intramuscular injection of 2 ml of sodium pentobarbital (Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). A blood sample was obtained immediately via cardiac puncture. Sera were separated by centrifugation and stored at −70°C until use. The abdominal cavity of each guinea pig was opened aseptically, and the spleen was removed for lymphocyte isolation by gentle homogenization with a glass homogenizer. The homogenates were treated with ACK lysing buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA [pH 7.2 to 7.4]) for 3 to 5 min at room temperature to destroy erythrocytes, the cells were washed three times in RPMI 1640 medium, viability was determined by trypan blue exclusion, and the number of cells was adjusted to the desired concentration in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 μM l-glutamine (working medium).
Isolation of peritoneal macrophages.
Guinea pigs were injected intraperitoneally with 5 ml of 3% thioglycolate and euthanized 4 to 5 days later by intramuscular injection of 2 ml of sodium pentobarbital. The peritoneal cavity was washed with 20 ml of phosphate-buffered saline (PBS) containing 20 U of heparin per ml and 2% FBS. Peritoneal cells were collected, washed three times with and suspended in RPMI 1640 working medium, and incubated in plastic petri dishes at 37°C for 1 to 2 h. The dishes were then placed at 4°C for 20 min, and the adherent cells were scraped off, collected, washed twice in working medium, counted by the trypan blue exclusion method, and adjusted to the desired concentration in working medium without streptomycin.
Depletion of CD4 T cells, CD8 T cells, or both.
For depletion of T-lymphocyte subpopulations, the following monoclonal antibodies against the specified guinea pig T-cell markers were used: CT7, anti-CD4 (35, 44); CT6, anti-CD8 (35, 44); and CT5, anti-pan-T-cell marker (4, 44). All were purchased from Serotec Inc., Partners 1, Raleigh, N.C. Different T-cell subpopulations were depleted by treatment of 5 × 106 to 1 × 107 cells with a 1:500 dilution of anti-CD4, anti-CD8, or anti-pan-T-cell marker monoclonal antibody and a 1:8 dilution of normal guinea pig serum in RPMI 1640 working medium. Lymphocyte suspensions were incubated with monoclonal antibodies for 45 min at 4°C, washed twice with 3 to 5 ml of cold PBS, and resuspended in working medium containing diluted guinea pig serum. After incubation for 30 min at 37°C, cells were washed twice and incubated with diluted serum for another 30 min at 37°C. After incubation, cells were washed three times, assessed for viability by trypan blue exclusion, and adjusted to 2 × 106 cells/ml in working medium without streptomycin. Pan-T cells, CD4 T cells, and CD8 T cells in the suspensions accounted, on average, for 34.1, 26.9, and 13.6%, respectively, before depletion and 5.4, 6.2, and 3.4%, respectively, after depletion, as determined by flow cytofluorometric analysis (26). Therefore, these depletion procedures, on average, reduced T cells by 84%, CD4 cells by 77%, and CD8 cells by 75%.
Coculture of infected macrophages and lymphocytes.
Adherent peritoneal macrophages were cultured with live, dispersed virulent M. tuberculosis H37Rv organisms (ATCC 27294) at a ratio of approximately 100 macrophages per mycobacterium at 37°C for 2 to 4 h. Then differently treated lymphocytes were added, and the cells were cultured for another 7 days. The cultures were lysed with distilled water and briefly sonicated for 3 to 5 s to disperse the mycobacteria, using an Ultrasonics sonicator (Heat System-Ultrasonics, Inc., Plainview, N.Y.) with an output control setting of 3.0. The lysates were diluted in physiological saline, vigorously vortexed, and plated onto M7H10 agar plates. After incubation at 37°C for 3 weeks, the colonies were counted and expressed as CFU of mycobacteria per culture.
Preparation of lymphocyte and peritoneal macrophage culture supernatants.
Peritoneal macrophages at 106 cells/ml in RPMI 1640 containing 15 μg of PPD (Statens Seruminstitut, Copenhagen, Denmark) per ml were cultured in 24-well culture plates at 1 ml per well. After incubation at 37°C in 5% CO2 for 24 or 48 h, cell-free supernatants were collected after centrifugation of the cultures at 1,500 rpm for 10 min and stored at −70°C until use. For M. tuberculosis H37Rv-induced production of TNF-α and transforming growth factor β1 (TGF-β1), 0.25 ml of peritoneal macrophages at 4 × 106 cells/ml was well mixed with 0.25 ml of live, dispersed M. tuberculosis H37Rv organisms at a multiplicity of infection of 1:1 and cultured at 37°C for 4 h, and then 0.5 ml of splenocytes at 2 × 105 cells/ml was added. After incubation for another 2 or 4 days, cell-free supernatants were collected after centrifugation of the cultures at 1,500 rpm for 10 min and stored at −70°C until use.
To assess the effect of diet on IFN production, primed splenocytes at 2 × 106 cells per ml in RPMI 1640 containing 15 μg of PPD per ml were cultured in 24-well culture plates in a total volume of 1 ml per well. After incubation at 37°C in 5% CO2 for 48 h, cell-free supernatants were collected after centrifugation of the cultures at 1,500 rpm for 10 min and stored at −70°C until use.
TGF-β bioassay.
The Mv-1-Lu mink lung cell line (ATCC CCL-64) was grown in Eagle’s minimal essential medium containing 10% FBS and used to measure TGF-β bioactivity as described previously (37). Briefly, triplicate cultures were set up by seeding 2 × 104 Mv-1-Lu cells per well in 100 μl of medium in 96-well plates and adding 100 μl of diluted samples, followed by incubation for 24 h at 37°C in a 5% CO2 humidified incubator. All samples were treated with 0.12 N HCl for 15 min at room temperature and then neutralized with 0.1 M HEPES–0.144 N NaOH before use. The cell cultures were labeled with 1 μCi of [3H]thymidine (6.7 Ci/mmol; ICN Radiochemicals, Irvine, Calif.) per well for the final 8 h and harvested onto fiberglass filter disks, and the uptake of [3H]thymidine was measured in a liquid scintillation counter (LS8000; Beckman Instruments, Fullerton, Calif.). A standard curve was generated with recombinant human TGF-β1 (R&D Systems, Minneapolis, Minn.) over a concentration range of 0.008 to 4 ng/ml. This assay was sensitive to TGF-β activity of 40 pg/ml.
TNF-α bioassay.
Macrophage culture supernatants were assessed for TNF-α activity by measuring their cytotoxicity on L929 cells as described by Espevik et al. (12). Briefly, 0.1-ml aliquots of L929 cell suspension (4 × 105 cells/ml) were pipetted into 96-well culture plates and incubated overnight. The following day, 0.1 ml of dilutions of culture supernatants or various concentrations of recombinant human TNF-α (rhTNF-α; R&D Systems) and actinomycin D at a final concentration of 2 μg/ml were added to each well. After incubation for 18 h, 10 μl of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] at a concentration of 5 mg/ml in PBS was added, and the cells were further cultured for 4 h. After the supernatants were decanted, 0.1 ml of isopropanol with 0.04 N HCl was added to each well, and the plates were incubated for 3 h. The plates were read by an enzyme-linked immunosorbent assay (ELISA) reader (Dynatech MR 5000; Dynatech Laboratories, Chantilly, Va.), using a test wavelength of 570 nm and a reference wavelength of 630 nm. A standard curve was generated with rhTNF-α. To verify that the assay was detecting TNF-α-induced cytotoxicity, some of the supernatant samples were incubated with rabbit anti-rhTNF-α neutralizing antibody (R&D Systems) and some with normal rabbit immunoglobulin G at 37°C for 2 h prior to the addition of the supernatants to the target cell cultures. This assay was sensitive to TNF-α activity of 25 pg/ml.
IFN bioassay.
Total IFN bioactivity in the culture supernatants was measured by inhibition of the cytopathic effect of vesicular stomatitis virus (VSV) on mouse L929 cells (19). Briefly, serial twofold dilutions of 50 μl of IFN-containing supernatants were made in Iscove’s modified Dulbecco medium (GIBCO BRL, Grand Island, N.Y.) without serum in a 96-well plate, and 0.1 ml of L929 cells (5 × 104 cells) was added per well. Following overnight incubation, cells were challenged with 50 μl of VSV at a multiplicity of infection of 1. After incubation for 24 h, the supernatant from each well was removed, and the cells were washed and fixed with 0.1 ml of 5% formalin at room temperature for 10 min. Then the cells were washed and stained with 0.1 ml of crystal violet solution for 10 min. Finally, the plate was rinsed and dried. After addition of 0.1 ml of 100% methanol to each well, the plates were read at 595 nm on a microtiter ELISA plate reader. A standard curve was generated with recombinant human IFN-γ (R&D Systems). This assay was sensitive to IFN-γ activity of 20 U/ml but detects all three species of IFN.
Lymphocyte blastogenesis.
Splenocytes were distributed into 96-well flat-bottom tissue culture plates (Corning Glass Works, Corning, N.Y.) at 2 × 105 cells per well in RPMI 1640 working medium. Triplicate cultures were stimulated with PPD at a final concentration of 15 μg/ml. For some experiments, 10 μg of anti-human interleukin-2 (IL-2) antibody (Genzyme Corporation, Cambridge, Mass.) per ml was added to the cultures. The cultures were incubated for 3 days at 37°C in a 5% CO2 atmosphere, labeled with 1.0 μCi of tritiated thymidine per well for another 18 h, and harvested onto glass wool fiber filters, using a cell harvester. [3H]thymidine incorporation into cellular DNA was measured in a liquid scintillation counter. The results were expressed as mean net counts per minute (counts per minute in stimulated wells minus counts per minute in control wells).
Statistical analysis.
The analysis of variance was used to test for the effect of dietary treatment on the dependent variables measured. When appropriate, Student’s t test was used to analyze the significance of differences between group means. A 95% confidence level was set for all tests.
RESULTS
Influence of dietary protein malnutrition on the growth containment of M. tuberculosis H37Rv induced in peritoneal macrophages by M. tuberculosis H37Ra-primed T lymphocytes.
The initial experiments showed that cocultures of peritoneal macrophages with syngeneic splenocytes from inbred strain 2 guinea pigs vaccinated 6 weeks previously with viable, attenuated M. tuberculosis H37Ra inhibited the growth of virulent tubercle bacilli in a dose-dependent fashion (Fig. (Fig.1).1). In cocultures with syngeneic peritoneal macrophages derived from normally nourished donor guinea pigs, splenocytes from LP guinea pigs vaccinated 6 weeks previously with M. tuberculosis H37Ra were able to control the accumulation of virulent tubercle bacilli in cultured macrophages as successfully as those from HP guinea pigs (Fig. (Fig.2).2). Only the CD4 T-cell population appeared to be necessary in both diet groups to mediate antituberculosis resistance, as evidenced by the abrogation of this vaccine-induced bacillary control by the depletion of CD4 T lymphocytes, or CD4 plus CD8 T cells, but not by the depletion of CD8 cells alone (Fig. (Fig.2).2).
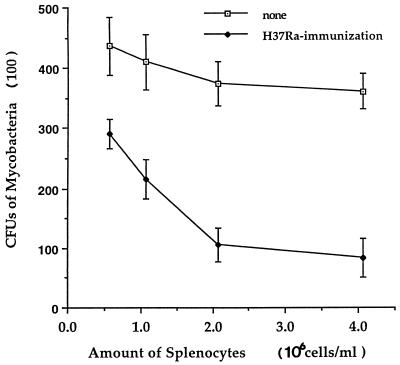
Growth containment of virulent M. tuberculosis H37Rv organisms induced in peritoneal macrophages by M. tuberculosis H37Ra-primed lymphocytes. Peritoneal macrophages were infected with live, dispersed virulent M. tuberculosis H37Rv at 37°C for 2 to 4 h and then cocultured for 7 days with different numbers of syngeneic splenocytes derived from M. tuberculosis H37Ra-vaccinated and nonvaccinated inbred strain 2 guinea pigs. The cultures were harvested and streaked onto M7H10 agar plates. After 3 weeks of incubation, the CFU were counted. The means ± standard errors of the means for four animals in each group are shown.
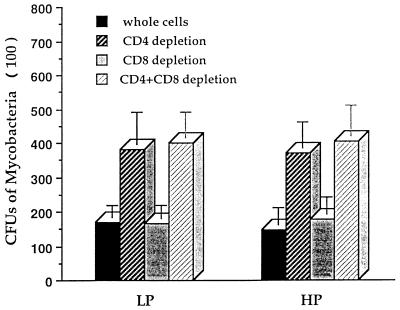
Influence of dietary protein malnutrition on the interactions between lymphocytes and macrophages. Peritoneal macrophages from normally nourished donors were infected with live, dispersed virulent M. tuberculosis H37Rv at 37°C for 2 to 4 h and then cocultured for 7 days with whole syngeneic splenocytes or splenocytes depleted of CD4 T cells, CD8 T cells, or both. The splenocytes were derived from inbred strain 2 guinea pigs vaccinated with M. tuberculosis H37Ra and maintained on the LP or HP diet. The cultures were harvested and streaked onto M7H10 agar plates. After 3 weeks of incubation, the CFU were counted. The means ± standard errors of the means for four animals in each group are shown.
Despite the fact that splenocytes from vaccinated LP guinea pigs were able to control the growth of virulent tubercle bacilli as successfully as cells from HP guinea pigs did in coculture with syngeneic peritoneal macrophages derived from normally nourished guinea pigs (Fig. (Fig.2),2), they failed to do so in coculture with autologous (i.e., protein-deprived) peritoneal macrophages, as indicated by a significant increase in the number of tubercle bacilli recovered from LP cultures (HP = 11,420 ± 3,200 and LP = 28,200 ± 3,600 CFU per culture; P < 0.05). These results indicate that protein malnutrition impairs the macrophage-lymphocyte interaction and/or macrophage antimicrobial effector functions.
PPD-driven lymphocyte blastogenesis.
Figure Figure33 illustrates the impact of protein malnutrition on the proliferative response of splenocytes 6 weeks following M. tuberculosis H37Ra vaccination. Lymphocytes derived from LP guinea pigs had a markedly depressed proliferative response to PPD compared with that from HP guinea pigs (P < 0.01). Lymphocyte subpopulation depletions revealed that CD4 T-cell depletion significantly reduced the PPD-induced lymphoproliferation in HP guinea pigs (P < 0.05), while CD8 T-cell depletion did not. In contrast, neither CD4 nor CD8 T-cell depletion significantly altered the suppressed PPD-induced responses of splenocytes from LP guinea pigs. These results suggest that protein malnutrition alters the development and/or proliferative function of antigen-specific lymphocytes.
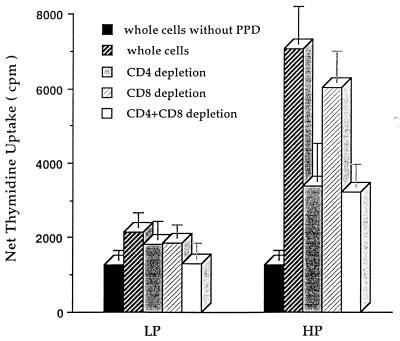
Dietary protein malnutrition depresses the proliferative responses of lymphocyte subpopulations in guinea pigs. Inbred strain 2 guinea pigs maintained on the LP or HP diet were vaccinated with M. tuberculosis H37Ra. Six weeks later, guinea pigs were euthanized and splenocytes were prepared. After no depletion or depletion of CD4 T cells, CD8 T cells, or both, the splenocytes were stimulated with PPD at 15 μg/ml for 3 days and labeled with [3H]thymidine for another 18 h. The cultures were harvested, and proliferation was measured as net thymidine uptake. The means ± standard errors of the means for eight guinea pigs per treatment are shown.
Impact of protein malnutrition on the production of cytokines.
The effect of dietary protein malnutrition on TNF-α production was evaluated by measuring TNF-α bioactivity in the sera of M. tuberculosis H37Ra-vaccinated guinea pigs with or without intravenous injection of PPD and in the culture supernatants of peritoneal macrophages stimulated with PPD or infected with M. tuberculosis H37Rv (Table (Table1).1). Six weeks following vaccination, sera were separated by centrifugation of blood samples and measured for TNF-α bioactivity. Protein malnutrition resulted in a significantly higher serum level of bioactive TNF-α in guinea pigs vaccinated with M. tuberculosis H37Ra. An intravenous injection of PPD boosted remarkably the serum level of bioactive TNF-α in both LP and HP guinea pigs, with the level of TNF-α in sera from LP guinea pigs rising significantly higher than that from HP guinea pigs (P < 0.001). In contrast, peritoneal macrophages derived from HP guinea pigs produced a much higher amount of TNF-α than did those from LP guinea pigs when stimulated in vitro with PPD (P < 0.01) or infected with M. tuberculosis H37Rv for 2 and 4 days (P < 0.001). These results indicate that protein malnutrition impairs the capacity of macrophages to produce TNF-α in response to mycobacteria and their antigens, at least in vitro, and that elevated serum TNF bioactivity in LP animals may not be due to increased production by macrophages.
TABLE 1
Effect of dietary protein deprivation on the production of TNF-α in vivo and in vitro in guinea pigs vaccinated with M. tuberculosis H37Ra
H37Ra
| Source | Treatment | TNF-α concn (pg/ml)a
| P | |
|---|---|---|---|---|
| HP | LP | |||
| Serumb | None | 162.2 ± ± 23.9 23.9 | 395.8 ± ± 45.3 45.3 | <0.001 |
| Intravenous PPD injection | 2,409.5 ± ± 404.6 404.6 | 8,802.5 ± ± 872.5 872.5 | <0.001 | |
| Macrophagec | PPD | 3,188.2 ± ± 426.6 426.6 | 1,238.8 ± ± 148.1 148.1 | <0.01 |
| H37Rv infection (2 days) | 1,322.4 ± ± 242.8 242.8 | 781.2 ± ± 182.5 182.5 | <0.01 | |
| H37Rv infection (4 days) | 1,069.5 ± ± 248.3 248.3 | 502.7 ± ± 99.6 99.6 | <0.001 | |
Figure Figure44 illustrates that vaccinated LP guinea pigs had a significantly higher level of TGF-β in their serum than HP guinea pigs after an intravenous injection of PPD (P < 0.05), while peritoneal macrophages from LP guinea pigs produced significantly less TGF-β in response to PPD in vitro than did those from HP guinea pigs (P < 0.01). In contrast, as seen in Fig. Fig.5,5, peritoneal macrophages from immune LP guinea pigs produced a significantly higher level of TGF-β when infected with M. tuberculosis H37Rv for 4 days than did those from HP guinea pigs (P < 0.01).
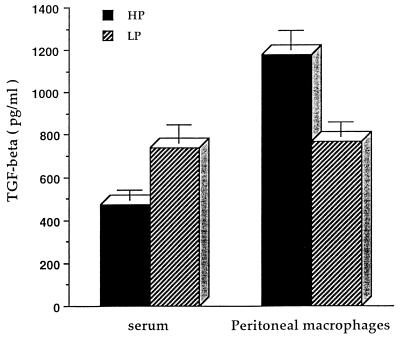
Dietary protein malnutrition alters TGF-β production in guinea pigs vaccinated with M. tuberculosis H37Ra. Outbred Hartley strain guinea pigs were vaccinated with M. tuberculosis H37Ra and maintained on the LP or HP diet for 6 weeks. Sera were collected following an intravenous injection of 1 mg of PPD/kg of body weight 2 h prior to euthanization. Peritoneal macrophages were stimulated with PPD at 15 μg/ml for 48 h. Cell-free supernatants were collected and measured for TGF-β bioactivity. Data for six guinea pigs per treatment are expressed as mean ± standard error of the mean.
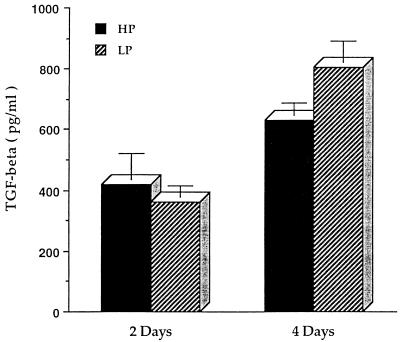
Dietary protein malnutrition enhances the production of TGF-β by peritoneal macrophages infected with M. tuberculosis H37Rv in guinea pigs. Peritoneal macrophages derived from outbred Hartley strain guinea pigs vaccinated with M. tuberculosis H37Ra and maintained on the LP or HP diet were infected with live, dispersed M. tuberculosis H37Rv for 2 to 4 h and then cultured for 2 and 4 days. Cell-free supernatants were collected and measured for TGF-β bioactivity. Data for six guinea pigs per treatment are expressed as mean ± standard error of the mean.
The production of bioactive IFN by splenocytes from vaccinated guinea pigs in response to PPD was suppressed by protein malnutrition. Immune splenocytes from HP guinea pigs produced a significantly higher amount of bioactive IFN than did those from LP guinea pigs (HP = 326.4 ± 37 and LP = 194.8 ± 24.5 U/ml; P < 0.01) following culture for 48 h with 15 μg of PPD per ml.
DISCUSSION
The detrimental effects of nutritional deficiencies on tuberculosis could result from alterations in the individual protective functions of, or the interaction between, T lymphocytes and macrophages, which are the major cell types mediating antimycobacterial immunity (15, 22, 39). In the present study, we demonstrated that splenocytes derived from inbred strain 2 protein-malnourished guinea pigs vaccinated previously with M. tuberculosis H37Ra were able to cooperate with syngeneic macrophages from normally nourished donors to control the accumulation of virulent tubercle bacilli inside infected macrophages (Figure (Figure2)2) but not with autologous macrophages from the same protein-deficient environment. The fact that lymphocytes from LP guinea pigs could activate macrophages from normally nourished guinea pigs suggests that protein deficiency did not alter this activity, at least in vitro. On the other hand, the inability of LP lymphocytes to activate LP macrophages provides evidence that protein malnutrition impairs the productive interaction between macrophages and T lymphocytes and/or the acquisition of mycobactericidal-mycobacteriostatic activity by LP macrophages in the presence of adequate activation signals.
Adoptive transfer experiments conducted previously in this laboratory demonstrated that immune lymphocytes derived from protein-deficient guinea pigs have a relatively low ability to protect normally nourished recipients from respiratory challenge with virulent M. tuberculosis H37Rv, indicating that protein deficiency prevented guinea pigs from generating a population of antigen-specific immune lymphocytes and/or impaired the proliferative capacity of these cells (27). In this study, we demonstrated that immune lymphocytes derived from protein-deficient guinea pigs responded poorly to PPD (Fig. (Fig.3)3) and were unable to control the accumulation of virulent tubercle bacilli in the cocultures with autologous macrophages. These findings could be interpreted to result from a defective generation of antigen-specific immune lymphocytes caused by protein deficiency because little evidence for macrophage defects in protein-deficient guinea pigs was found in previous studies (24, 31, 33). This interpretation is compatible with the results of adoptive transfer experiments described above (27). Further experiments will be carried out to verify if there is a defective generation of antigen-specific immune lymphocytes in LP guinea pigs or if the defect is in cell-to-cell communication. The observation that immune lymphocytes from protein-deficient guinea pigs were able to activate macrophages from normally nourished guinea pigs to control the growth of virulent mycobacteria in vitro could be explained by the relatively high number of immune lymphocytes concentrated in the cocultures and the closeness of contact between lymphocytes and macrophages, which may facilitate the generation of adequate activation signals for macrophages and thus compensate for the functional alteration of these cells.
Both CD4 and CD8 T cells have been shown to be required in the control of mycobacterial infection in mice (14, 25, 36). However, the precise role of each of these cell types has not been fully elucidated, and no data on the role of T-cell subsets in the guinea pig have been published. In our in vitro macrophage coculture system, CD4 T cells were demonstrated to be the major effector cells in containing the growth of virulent M. tuberculosis H37Rv, while the depletion of CD8 T cells did not alter the outcome (Figure (Figure2).2). This result suggests that CD8 T cells contribute little, if any, to the growth control of virulent tubercle bacilli either by mediating target cell lysis or by cytokine production in this coculture system. Immunization with M. tuberculosis H37Ra, which is attenuated and thought to reside exclusively in the phagosome (39), may not effectively activate major histocompatibility complex class I-restricted CD8 T cells. Therefore, one reasonable explanation for our result is that very few, if any, antigen-reactive CD8 T cells are present in the cocultures.
Cytokines play a central role in mediating antimycobacterial immunity. IL-2 is required to initiate and amplify immune responses. IFN-γ and TNF-α are important macrophage-activating cytokines and crucial in the immune response to tuberculosis (10, 13). Depressed production of IL-2 (21, 45) and IFN-γ (21, 40) in patients with chronic pulmonary tuberculosis has been reported. Similarly, IL-2 production was depressed in chronically protein-deficient guinea pigs vaccinated with M. bovis BCG (29), and TNF-α and IFN-γ expression was reportedly decreased in protein calorie-malnourished mice infected intravenously with a lethal dose of the virulent M. tuberculosis Erdman strain (6). In this study, the capacity of splenocytes and macrophages to produce total bioactive IFN and TNF-α in vitro (Table (Table1)1) was found to be suppressed. The decreased production of these cytokines at the site of immune inflammatory responses may be responsible for the impairment of overall host antimycobacterial resistance in protein-deficient guinea pigs. IFN-γ is a key cytokine in the development of Th1-type immune responses which are required for the elimination of intracellular pathogens, including mycobacteria (41, 48). IFN-γ induces major histocompatibility class II expression and thus increases antigen processing and presentation by monocytes-macrophages to CD4 T lymphocytes (2). It also induces differentiation and activation of monocyte-macrophages and enhances their intracellular microbicidal activity (6). Therefore, IFN-γ is crucial in the immune response to mycobacterial infection (9, 13). The present study revealed that total bioactive IFN produced by immune splenocytes in protein-malnourished guinea pigs was depressed. This decrease is most likely due to the depressed production of IFN-γ because protein malnutrition has been observed to cause marked depletion of T lymphocytes (23, 28) and profound depression of NK cell activity (20) in the spleen, resulting in IFN-γ-secreting cell loss and functional defect. Moreover, IL-2 production, upon which IFN-γ production is dependent, was depressed in earlier studies with protein-malnourished guinea pigs (29). All of these alterations induced by protein malnutrition may contribute to the depressed production of IFN-γ.
Dietary protein malnutrition impaired TNF-α production by peritoneal macrophages. In contrast, intravenous injection of PPD triggered a higher level of TNF-α in the serum of LP guinea pigs (Table (Table1).1). One possible explanation is that vaccination with M. tuberculosis H37Ra may trigger inflammatory and immune responses in guinea pigs on the LP diet different from those in guinea pigs on the HP diet. It is also possible that protein deficiency results in leakage of lipopolysaccharide from the gut, which primes macrophages to respond more dramatically to PPD in terms of TNF-α production.
TGF-β production could be influenced by many factors, including the type and state of activation of cells, external stimuli, and the presence of other cytokines. A study by Chandrasekar et al. revealed that restricting dietary calories significantly increased mRNA and protein expression of TGF-β and decreased those of proinflammatory cytokines, including IL-6 and TNF-α, in autoimmune prone mice (8). Bermudez reported that TGF-β production by human monocyte-derived macrophages infected in vitro with M. avium isolates was bacterial strain dependent and was more pronounced in macrophages infected with a virulent strain (3). In this study, we found that the serum of protein-deprived guinea pigs had higher levels of bioactive TGF-β, while peritoneal macrophages from protein-deficient guinea pigs had a depressed ability to produce TGF-β in response to PPD (Fig. (Fig.4).4). Decreased TGF-β production in response to PPD may reflect impairment of the ability of peritoneal macrophages from protein-deficient guinea pigs to generate stimulating components for TGF-β production. In fact, peritoneal macrophages from protein-deficient guinea pigs produced significantly more bioactive TGF-β when infected with virulent M. tuberculosis H37Rv in vitro for 4 days compared to peritoneal macrophages from control diet guinea pigs (Fig. (Fig.5).5). There was no difference between the diet treatment groups at 2 days (Fig. (Fig.5).5). Given the slow replication rate of the bacilli, it is not surprising that time in culture might be an important variable. One possible explanation for the discrepancy between the effect of diet on the production of TGF-β by macrophages exposed to PPD, in contrast to viable M. tuberculosis, is that peritoneal macrophages from protein-deficient guinea pigs cannot control the intracellular multiplication of virulent mycobacteria as successfully as those from control diet-fed animals, and thus mycobacteria grow faster and secrete more proteins inside LP peritoneal macrophages. Some proteins secreted by viable mycobacteria which are not necessarily found in PPD could trigger TGF-β release from these infected macrophages.
Taken together, the results of this study demonstrate that protein malnutrition has multiple detrimental effects on host antimycobacterial resistance. First, dietary protein malnutrition impairs macrophage functions, including TNF-α production and cooperation with T lymphocytes to control mycobacterial growth in vitro; second, it causes remarkable depression of T-lymphocyte functions, as indicated by reductions in PPD-driven proliferation and Th1 cytokine (IFN-γ) production; third, protein malnutrition potentiates live M. tuberculosis H37Rv-infected monocytes-macrophages to produce higher levels of TGF-β, which is a likely mediator of immunosuppression and immunopathogenesis in tuberculosis (16, 17, 46).
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.66.8.3562-3568.1998
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/66/8/3562.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/content/full/66/8/3562
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/66/8/3562
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/66/8/3562
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.66.8.3562-3568.1998
Article citations
The Tolerance Model of Non-Inflammatory Immune Competence in Acute Pediatric Malnutrition: Origins, Evidence, Test of Fitness and Growth Potential.
Nutrients, 15(23):4922, 25 Nov 2023
Cited by: 0 articles | PMID: 38068780 | PMCID: PMC10707886
Review Free full text in Europe PMC
Tuberculosis in an Aging World.
Pathogens, 11(10):1101, 26 Sep 2022
Cited by: 14 articles | PMID: 36297158 | PMCID: PMC9611089
Review Free full text in Europe PMC
Severe undernutrition in children affects tuberculin skin test performance in Southern India.
PLoS One, 16(7):e0250304, 16 Jul 2021
Cited by: 2 articles | PMID: 34270546 | PMCID: PMC8284816
Leptin Deficiency, Caused by Malnutrition, Makes You Susceptible to SARS-CoV-2 Infection but Could Offer Protection from Severe COVID-19.
mSphere, 6(3):e00031-21, 12 May 2021
Cited by: 6 articles | PMID: 33980671 | PMCID: PMC8125045
Risk Factors for False-Negative Interferon-γ Release Assay Results in Culture-Confirmed Childhood TB.
Am J Trop Med Hyg, 101(6):1303-1307, 01 Dec 2019
Cited by: 7 articles | PMID: 31674295 | PMCID: PMC6896862
Go to all (58) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Expression of interferon-gamma and tumour necrosis factor-alpha messenger RNA does not correlate with protection in guinea pigs challenged with virulent Mycobacterium tuberculosis by the respiratory route.
Immunology, 128(1 suppl):e296-305, 07 Nov 2008
Cited by: 12 articles | PMID: 19016908
Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects.
J Immunol, 160(5):2408-2417, 01 Mar 1998
Cited by: 71 articles | PMID: 9498784
Lung macrophages from bacille Calmette-Guérin-vaccinated guinea pigs suppress T cell proliferation but restrict intracellular growth of M. tuberculosis after recombinant guinea pig interferon-gamma activation.
Clin Exp Immunol, 149(2):387-398, 12 Jun 2007
Cited by: 15 articles | PMID: 17565610
Functions of T-cell subsets and cytokines in mycobacterial infections.
Eur Respir J Suppl, 20:668s-675s, 01 Sep 1995
Cited by: 41 articles | PMID: 8590567
Review
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R01 AI015495
Grant ID: AI-15495





