Abstract
Free full text

Interleukin-12 Is Required for Control of the Growth of Attenuated Aromatic-Compound-Dependent Salmonellae in BALB/c Mice: Role of Gamma Interferon and Macrophage Activation
Abstract
The attenuated S. typhimurium SL3261 (aroA) strain causes mild infections in BALB/c mice. We were able to exacerbate the disease by administering anti-interleukin-12 (IL-12) antibodies, resulting in bacterial counts in the spleens and livers of anti-IL-12-treated mice that were 10- to 100-fold higher than the ones normally observed in premortem mice; yet the animals showed only mild signs of illness. Nevertheless, they eventually died of a slow, progressive disease. Mice infected with salmonellae become hypersusceptible to endotoxin. We found that IL-12 neutralization prevented the death of infected mice following subcutaneous injection of lipopolysaccharide. Granulomatous lesions developed in the spleens and livers of control animals, as opposed to a widespread infiltration of mononuclear cells seen in the organs of anti-IL-12-treated mice. In the latter (heavily infected), salmonellae were seen within mononuclear cells, indicating an impairment of the bactericidal or bacteriostatic ability of the phagocytes in the absence of biologically active IL-12. Gamma interferon (IFN-γ) levels were reduced in the sera and tissue homogenates from anti-IL-12-treated mice compared to those in control animals. Furthermore, fluorescence-activated cell sorter analysis on spleen cells showed that IL-12 neutralization impaired the upregulation of I-Ad/I-Ed antigens on macrophages from infected mice. Inducible nitric oxide synthase and IFN-γ mRNA production was down-regulated in anti-IL-12-treated mice, which also showed an increased production of IL-10 mRNA and a decrease in nitric oxide synthase activity in the tissues. Administration of recombinant IFN-γ to anti-IL-12-treated mice was able to restore host resistance, granuloma formation, and expression of major histocompatibility complex class II antigens in F4/80+ and CD11b+ spleen cells.
Salmonella infections still pose a serious health hazard worldwide, affecting both humans and animals. Salmonella typhi, the agent of human typhoid fever, is not pathogenic for common laboratory animals. Therefore, natural resistance and acquired immunity to Salmonella are studied mainly in the mouse model by using host-adapted salmonellae which cause systemic infections believed to mimic the human disease.
In mice, early bacterial growth in the reticuloendothelial system (RES) is controlled by the innate resistance Nramp (Ity) gene, which is expressed in macrophages (22). In lethal infections, salmonellae rapidly reach large numbers in the tissues and death occurs presumably by endotoxin poisoning when bacterial counts reach levels of ca. 108 CFU per organ (30). In sublethal infections, survival requires a host response that suppresses the exponential growth of the organisms in the RES towards the end of the first week, resulting in a plateau phase (17, 25). The establishment of the plateau phase does not require functional T cells. In fact, nude (T-cell-deficient) mice and mice depleted of T cells by administration of anti-CD4 and anti-CD8 antibodies can still suppress Salmonella growth in infected tissues (17). A bone marrow-dependent influx of radiation-sensitive cells is required for the plateau phase and for the formation of granulomas rich in mononuclear cells (17, 32). Most of the salmonellae in the spleens and livers of the infected animals are localized within the phagocytes present in the focal lesions (38). Tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and nitric oxide (NO) derivatives appear to be required for the suppression of salmonella growth in the RES (27, 28, 32, 36, 37, 48). TNF-α is needed for the recruitment of mononuclear cells in the tissues and for granuloma formation (32); IFN-γ can activate macrophages to kill salmonellae in vitro (20).
The establishment of the plateau phase coincides with the development of hypersusceptibility to the toxic and lethal effects of bacterial lipopolysaccharide (LPS) (29, 33). We have previously shown that mice immunized with a live attenuated aromatic-dependent Salmonella vaccine strain show transient hypersusceptibility to LPS, which can be prevented by treatment with anti-TNF-α antibodies (29). The role of other cytokines in this phenomenon is not known.
Interleukin-12 (IL-12) is a 70-kDa heterodimeric cytokine produced by macrophages, B cells, polymorphonuclear leukocytes, and dendritic cells in response to a variety of stimuli including products of bacterial origin (5, 10). IL-12 mediates resistance to intracellular organisms including Listeria, Toxoplasma, Candida, Leishmania, Mycobacterium tuberculosis, and Brucella abortus (8, 13, 18, 23, 39, 46, 50). IL-12 is generally believed to mediate host resistance by inducing IFN-γ production by NK and T cells as well as by contributing to the establishment of protective Th1 antigen-specific responses (5, 6, 9, 10, 12, 13, 24, 34, 39, 43, 47).
Evidence for IL-12 induction in salmonellosis has been provided. IL-12 and IL-12-specific mRNA have been detected in vivo and in vitro in response to Salmonella. Elicited peritoneal mouse macrophages stimulated with Salmonella dublin express elevated levels of IL-12 p40-specific mRNA (4, 7). Oral infection with virulent or live attenuated S. dublin induces early (6 and 20 h postinfection) production of IL-12-specific mRNA in Peyer’s patches and mesenteric lymph nodes (3); biologically active IL-12 in lymph node homogenates has been documented 36 h after S. dublin infection (21). We and others previously reported that in vivo IL-12 neutralization reduces the ability of the host to suppress the growth of virulent salmonellae in the tissues and impairs IFN-γ production (21, 31). A recent report indicates that a mutation in the IL-12 receptors renders humans more susceptible to salmonellosis (11). Nevertheless, the mechanisms by which IL-12 mediates host resistance to Salmonella are still unclear.
In the present study, we attempted to clarify the mechanisms by which IL-12 contributes to host resistance in mice infected with Salmonella. We investigated the role of IL-12 in survival, granuloma formation, and macrophage activation in mice infected with an attenuated Salmonella strain that normally causes very mild infections in BALB/c mice. We also investigated the involvement of IL-12 in the toxic and lethal effects of high bacterial loads in the tissues as well as in the expression of hypersusceptibility to LPS normally seen in mice infected with salmonellae. We also wished to clarify the involvement of IFN-γ in IL-12-mediated resistance to salmonellosis.
MATERIALS AND METHODS
Animals.
Female BALB/c mice were purchased from Harlan Olac Ltd., Blackthorn, Bicester, United Kingdom, and age-matched groups were used when older than 8 weeks.
Bacteria.
S. typhimurium SL3261 is an aroA attenuated live vaccine strain with an intravenous (i.v.) 50% lethal dose for BALB/c mice of ca. 107 CFU (15). Bacteria were grown at 37°C as stationary overnight cultures in Luria-Bertani broth (Difco). Aliquots were snap frozen and stored in liquid nitrogen. The inoculum was diluted in phosphate-buffered saline (PBS) and injected in a lateral tail vein. The dose was further checked by pour plating.
Bacterial enumeration in organ homogenates.
Mice were killed by cervical dislocation. The spleens and livers were aseptically removed and homogenized in 5 ml of cold distilled water in a Colworth Stomacher (31). Viable counts were measured by using pour plates of Luria-Bertani agar.
Anti-IL-12 antibodies.
Ascitic fluid containing the neutralizing anti-IL12 monoclonal antibody C.17.8 (49) was purchased from Harlan Bioproducts (Indianapolis, Ind.). Whole globulins were used after 40% ammonium sulfate precipitation and dialysis against PBS. The endotoxin content of the preparation was less than 50 pg/ml as assessed by the Limulus amoebocyte assay (Sigma). The biological activity of the preparation was assessed as the ability to inhibit IFN-γ production from mouse spleen cells stimulated with recombinant IL-12 (rIL-12) (a kind gift of Genetics Institute Inc., Cambridge, Mass.). Single-cell suspensions prepared from mouse spleens were washed once in RPMI 1640 medium (Sigma, Poole, United Kingdom) by centrifugation at 300 × g and then incubated in Gey’s solution to lyse the erythrocytes. Leukocytes were washed twice more before being resuspended in RPMI 1640 supplemented with 100 U of penicillin per ml, 100 μg of streptomycin (Sigma) per ml, 5 mM glutamine (Sigma), 2 × 10−5 M β-mercaptoethanol (Sigma), 1 mM HEPES (Sigma), and 10% heat-inactivated fetal calf serum (Sigma). The cells were dispensed into round-bottom 96-multiwell plates (Corning, New York, N.Y.) at a concentration of 4 × 105 cells in 100 μl. The plates were incubated at 95% humidity in a 5% CO2 atmosphere at 37°C. Threefold dilutions of murine rIL-12 ranging between 1.2 and 0.009 ng/ml were incubated with 10-fold dilutions of the anti-IL-12 preparation for 1 h at 37°C in a final volume of 100 μl before addition to the splenocyte cultures. Culture supernatants were collected after 24 and 48 h. IFN-γ was measured by an enzyme-linked immunosorbent assay (ELISA) with commercially available antibody pairs (PharMingen, Cambridge BioScience, Cambridge, United Kingdom) as described below. An amount of anti-IL-12 globulins of ≤100 ng was found to completely inhibit the ability of 40 pg of rIL-12 to induce IFN-γ from mouse splenocytes.
Before use, anti-IL-12 antibodies were diluted in sterile PBS and 1 mg of globulins was injected in a lateral tail vein at specified times. Normal rat gamma globulins (Sigma) were used as a control.
IFN-γ.
Murine recombinant IFN-γ (rIFN-γ) was a kind gift of G. R. Adolf, Bender, Vienna, Austria. Mice received a daily intraperitoneal (i.p.) injection of 105 U of rIFN-γ in 0.2 ml of endotoxin-free PBS. The endotoxin content of the preparation was less than 66 pg/ml as assessed by the Limulus amoebocyte assay.
Collection of sera and organ homogenates for IFN-γ and IL-10 determinations.
The mice were bled before being sacrificed, and the sera were collected and stored at −70°C. The spleens were homogenized as described above. Aliquots of spleen homogenates from individual mice were clarified by centrifugation, sterilized by filtration through 0.22-μm-pore-size filters, and stored at −70°C until use.
IFN-γ ELISA.
IFN-γ was measured by capture ELISA with antibody pairs and rIFN-γ purchased from PharMingen. The 96-multiwell ELISA plates (Maxisorp Nunc Immuno plate; Nunc, Roskilde, Denmark) were coated at 37°C for 2 h with 50 μl of a capture rat anti-mouse IFN-γ immunoglobulin G1 monoclonal antibody (clone R4-6A2) per well in 0.1 M NaHCO3 buffer (pH 8.2) at 2 μg/ml and incubated overnight at 4°C. After blocking with RPMI 1640 supplemented with 10% fetal calf serum (FCS) (RPMI-FCS) at 37°C for 1 h, samples (diluted 1/2 in RPMI-FCS) were loaded in 50 μl in triplicate and the plates were incubated at 37°C for 2 h. Serial twofold dilutions of rIFN-γ ranging from 20 ng/ml to 40 pg/ml were included as standards. A volume of 100 μl of the biotinylated rat anti-mouse IFNγIgG1 monoclonal antibody (clone XMG1.2) was added per well at 1 μg/ml in PBS–10% FCS (1 h at 37°C) and was followed by 100 μl of peroxidase-labelled streptavidin per well at 2.5 mg/ml (Sigma) in PBS–10% FCS for 45 min at room temperature. o-Phenylenediamine (1 mg/ml in 0.2 M Na2HPO4–0.1 M citrate buffer) in the presence of H2O2 was used to develop the plates. The reaction was stopped by adding 15 μl of 3 M H2SO4 per well. The optical density was read at 490 nm. IFN-γ concentrations (in picograms per milliliter) were determined by comparison with the standard curve. We considered 80 pg/ml to be the lower limit of sensitivity of our ELISA.
IL-10 ELISA.
IL-10 was measured in the sera of infected mice by using the Biotrack IL-10 mouse ELISA system (Amersham Pharmacia Biotech UK Ltd.) as specified by the manufacturer. We considered 100 pg/ml to be the lower limit of sensitivity of our ELISA.
NOS activity.
Nitric oxide synthase (NOS) activity was measured in liver and spleen homogenates by using the citrulline assay (40). Briefly, tissue samples were pooled and homogenized at 1:5 (wt/vol) in 320 mM sucrose–50 mM Tris–1 mM EDTA–1 mM dl-dithiothreitol–10 μg of leupeptin per ml–10 μg of soybean trypsin inhibitor per ml–2 μg of aprotinin, cleared by centrifugation, and kept on ice until used.
A 1.2-μl volume of 100 mM EGTA was added to one set of tubes, and 1.2 μl of 100 mM l-N-monomethyl arginine (l-NMMA) was added to another set of tubes, and blank tubes contained distilled water (H2O). A 100-μl volume of reaction mixture (50 mM K2HPO4, 60 mM l-valine, 120 μM NADPH, 1.2 mM l-citrulline, 24 μM l-arginine and l-[U-14C]arginine, 1.2 mM MgCl2, 0.24 mM CaCl2) was added to the three sets of tubes. Aliquots (18 μl) of liver homogenate were added to the appropriate tubes followed by 1.5 ml of Dowex resin (Sigma) and 5 ml of distilled H2O. After a 5-min incubation, the disintegrations per minute were counted for 5 min after the addition of scintillation fluid. Counts from tubes containing l-NMMA or EGTA were used to eliminate background reading and Ca2+-dependent NOS activity respectively. Ca2+-independent inducible NOS (iNOS) activity was expressed as picomoles per minute per milligram of protein.
Histologic testing.
Sections (8 μm thick) were prepared from tissues fixed by immersion in 10% formal saline and stained with hematoxylin-eosin. Sections (1 μm thick) were prepared from similarly fixed tissue embedded in epoxy resin (T.A.A.B. Lab. Equipment Ltd., Aldermaston, United Kingdom) and stained with toluidine blue.
Staining for flow cytometry analysis.
Mouse splenocytes were prepared as described above, with the only exception that Dulbecco’s PBS (Ca2+ and Mg2+ free) was used instead of RPMI. Cells were suspended at a final concentration of 107/ml in PBS containing 1% FCS, 0.1% NaN3, and 2 mM EDTA. All further steps were performed on ice. The antibodies for fluorescence-activated cell sorter analysis were purchased from PharMingen and used at a concentration of 1 μg/106 cells, with the exception of the anti-F4/80 antibody, which was obtained from Serotec (Kidlington, United Kingdom) and used at 0.6 μg/106 cells. FcγIII/II receptors were blocked by incubating the cells with rat anti-mouse CD16/CD32 (clone 2.4G2) monoclonal antibody for 10 min. The following antibodies were used: fluorescein isothiocyanate anti-F4/80 (clone CI:A3-1), fluorescein isothiocyanate anti-CD11b (clone M1/70), and R-phycoerythrin anti-mouse I-Ad/I-Ed (clone 2G9). Similarly conjugated isotype standards were used as controls. The cells were stained for 30 min with the relevant antibodies, fixed with 1% paraformaldehyde, and kept in the dark until use. A total of 105 events were acquired on a Becton Dickinson FACScan apparatus. The cells were double stained for the expression of F4/80+ or CD11b+ and I-Ad/I-Ed. F4/80+ or CD11b+ mononuclear cells (gated by forward scatter and side scatter) were gated by FL1 (green fluorescence), and the results of I-Ad/I-Ed expression are shown as FL2 (red fluorescence) histograms and/or as geometric mean of fluorescence (FL2) intensity. The geometric mean of fluorescence intensity when staining with conjugated isotype control antibodies was less than 10 for all experimental groups of mice (uninfected mice, infected mice, infected and anti-IL-12-treated mice, and infected and anti-IL-12+rIFN-γ-treated mice).
Isolation of mRNA and detection of specific mRNA by RT-PCR.
Spleens and livers from groups of five mice were individually stored at −70°C. Poly(A) mRNA was purified from pooled spleen and liver tissue by using the FastTrack 2.0 isolation kit (Invitrogen, San Diego, Calif.) as specified by the manufacturer. Reverse transcription and PCR amplification of mRNA was performed with the GeneAmp RNA PCR Kit (Perkin-Elmer, Branchburg, N.J.). The amount of mRNA from different samples to be added to the reverse transcriptase-PCR (RT-PCR) mixture was equalized on the detection of the β-actin PCR product in the logarithmic phase of amplification (determined by repeated sampling every three cycles starting from cycle 23). Sense and antisense primers were synthesized by Perkin-Elmer. The primer sequences were 5′-TGG-AAT-CCT-GTG-GCA-TCC-ATG-AAA-C-3′ and
and 5′-AAC-GCA-GCT-CAG-TAA-CAG-TCC-GCC-TA-3′ for β-actin (345 bp), 5′-CCC-TTC-CGA-AGT-TTC-TGG-CAG-C-3′
5′-AAC-GCA-GCT-CAG-TAA-CAG-TCC-GCC-TA-3′ for β-actin (345 bp), 5′-CCC-TTC-CGA-AGT-TTC-TGG-CAG-C-3′ and
and 5′-GGC-TGT-CAG-AGC-CTC-GTG-GCT-TTG-G-3′
5′-GGC-TGT-CAG-AGC-CTC-GTG-GCT-TTG-G-3′ for iNOS (420 bp), 5′-ACC-TGG-TAG-AAG-TGA-TGC-CCA-GGC-A-3′ and 5′-CTA-TGC-AGT-TGA-TGA-AGA-TGT-CAA-A-3′
for iNOS (420 bp), 5′-ACC-TGG-TAG-AAG-TGA-TGC-CCA-GGC-A-3′ and 5′-CTA-TGC-AGT-TGA-TGA-AGA-TGT-CAA-A-3′ for
for IL-10
IL-10 (237
(237 bp), 5′-GAA-AGC-CTA-GAA-AGT-CTG-AAT-AAC-T-3′
bp), 5′-GAA-AGC-CTA-GAA-AGT-CTG-AAT-AAC-T-3′ and
and 5′-ATC-AGC-AGC-GAC-TCC-TTT-TCC-GCT-T-3′ for IFN-γ (388 bp), and 5′-ACA-AAA-ATC-ACT-TGA-GAG-AGA-TCA-T-3′ and 5′-AGT-AAT-CCA-TTT-GCA-TGA-TGC-TCT-T-3′ for IL-4 (351 bp). Cycling conditions were 1 min at 96°C, 2 min at 55°C, and 2 min at 72°C for β-actin, iNOS, transforming growth factor β, and IL-4 (35 cycles) and 1 min at 96°C, 2 min at 60°C, and 2 min at 72°C for IFN-γ and IL-10 (35 cycles). Each RT-PCR was repeated three times with similar results. Amplified DNAs were resolved by 2% agarose gel electrophoresis and stained with ethidium bromide for visual evaluation.
5′-ATC-AGC-AGC-GAC-TCC-TTT-TCC-GCT-T-3′ for IFN-γ (388 bp), and 5′-ACA-AAA-ATC-ACT-TGA-GAG-AGA-TCA-T-3′ and 5′-AGT-AAT-CCA-TTT-GCA-TGA-TGC-TCT-T-3′ for IL-4 (351 bp). Cycling conditions were 1 min at 96°C, 2 min at 55°C, and 2 min at 72°C for β-actin, iNOS, transforming growth factor β, and IL-4 (35 cycles) and 1 min at 96°C, 2 min at 60°C, and 2 min at 72°C for IFN-γ and IL-10 (35 cycles). Each RT-PCR was repeated three times with similar results. Amplified DNAs were resolved by 2% agarose gel electrophoresis and stained with ethidium bromide for visual evaluation.
Statistical analysis.
Student’s t test was used to determine the significance of differences between controls and experimental groups. Differences between experimental groups were considered significant for P < 0.05.
RESULTS
In vivo administration of anti-IL-12 antibodies exacerbates the course of an infection with attenuated salmonellae.
BALB/c mice were infected i.v. with 5 × 105 CFU of the attenuated S. typhimurium SL3261 strain. One group of mice received i.v. 1 mg of neutralizing anti-IL-12 antibodies on days 0, 4, 8, 12, and 16 of the infection; control animals received a similar amount of normal rat globulins (NRG). No mice in the control group died during a 60-day observation period. In contrast, all the anti-IL-12-treated mice succumbed within 21 days after infection, with scattered deaths starting on day 13 (Fig. (Fig.1).1).
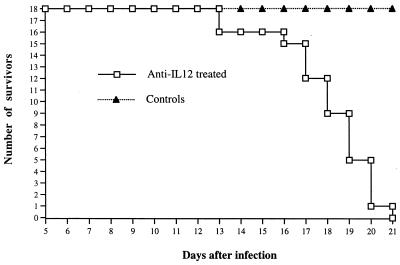
Effect of administration of anti-IL-12 antibodies on the survival of mice infected with S. typhimurium SL3261. A total of 36 BALB/c mice were infected i.v. with 5 × 105 CFU of the attenuated S. typhimurium SL3261 strain. Eighteen mice received 1 mg of neutralizing anti-IL-12 antibodies i.v. on days 0, 4, 8, 12, and 16 of the infection (anti-IL-12 treated); the remaining control animals received a similar amount of NRG.
In a similar experiment, bacterial counts were performed on groups of five controls and five anti-IL-12-treated mice on days 1, 3, 7, and 13 after infection. On days 1 and 3, spleen and liver counts were similar in controls and anti-IL-12-treated mice. Day 7 liver counts were significantly higher in anti-IL-12-treated animals than in control animals, while viable counts in the spleens were not significantly different in the two groups of mice. On day 13, very high bacterial numbers were found in both spleens and livers of anti-IL-12-treated mice, in contrast to control animals (Fig. (Fig.2).2). Mice infected with S. typhimurium SL3261 and treated with anti-IL-12 antibodies showed no signs of illness on day 7 and were only moderately ill on day 13 of the infection, despite the unusually high bacterial loads observed in the tissues (ca. 10-fold higher than the counts usually observed in premortem mice). Similar results were obtained in a repeat experiment (data not shown).
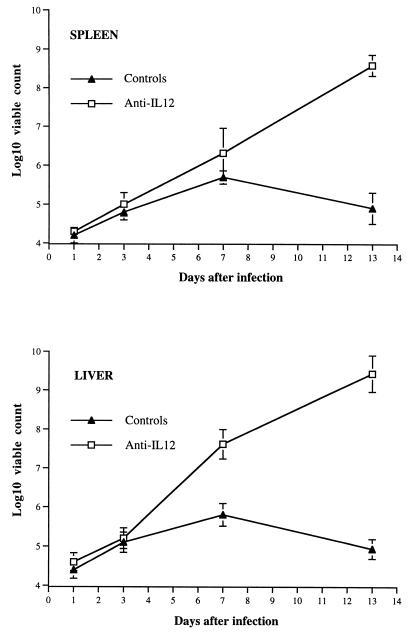
Effect of administration of anti-IL-12 antibodies on the growth of S. typhimurium SL3261 in the spleens and livers of BALB/c mice. Mice were infected and treated as in Fig. Fig.1.1. Viable counts were performed on the spleens and livers of controls and anti-IL-12-treated mice on days 1, 3, 7, and 13 postchallenge. The results are expressed as log10 viable counts ± standard deviations from groups of five mice per point.
Thus, IL-12 neutralization abrogates host resistance to infection with an attenuated aroA Salmonella strain, causing the death of all the infected mice. A clear discrepancy between the symptoms of infection and the level of bacterial counts can be observed in the absence of biologically active IL-12.
IL-12 is involved in the expression of hypersusceptibility to LPS seen in mice infected with Salmonella.
BALB/c mice were infected with S. typhimurium SL3261 as above. On day 10, groups of five mice received a single i.v. dose of 1 mg of anti-IL-12 antibodies or NRG 2 h before being given a subcutaneous injection (in the footpad) with decreasing doses of LPS (phenol-extracted LPS [Sigma no. L6511]) ranging between 100 and 25 μg per mouse. None of the infected mice pretreated with NRG survived when injected with 100 or 50 μg of LPS, and only one mouse from this group survived the LPS dose of 25 μg. Conversely, no deaths were observed in anti-IL-12- treated mice upon LPS injection (Table (Table1).1). Five normal (not infected) mice survived the administration of 100 μg of LPS.
TABLE 1
Effect of IL-12 neutralization on the expression of hypersusceptibility to LPS seen in mice infected with Salmonellaa
Salmonellaa
| Mouse group | No. of survivors/total no. after LPS dose of:
| ||
|---|---|---|---|
| 100 μg | 50 μg | 25 μg | |
| Normal | 5/5 | NDb | ND |
| NRG treated | 0/5 | 0/5 | 1/5 |
| Anti-IL-12 treated | 5/5 | 5/5 | 5/5 |
Thus, IL-12 neutralization protects Salmonella-infected mice against the lethal effects of LPS administration.
Effect of IL-12 neutralization on IFN-γ and IL-10 production and iNOS activity in mice infected with attenuated salmonellae.
IFN-γ was measured in sera and spleen homogenates from the experiment in Fig. Fig.2.2. IFN-γ was undetectable (≤160 pg/ml) either in the circulation or in the organs of infected mice on day 1 after challenge but was present in the sera and in the spleen homogenates from NRG-treated control mice on days 3, 7, and 13 postinfection. At all points assayed, the cytokine levels were significantly lower in the samples taken from anti-IL-12-treated mice than in the samples from mice receiving NRG (Fig. (Fig.3).3).

Effect of administration of anti-IL-12 antibodies on endogenous IFN-γ levels and IFN-γ levels in serum in mice infected with S. typhimurium SL3261. The figure shows IFN-γ levels (as measured by ELISA) in spleen homogenates and sera from the controls and anti-IL-12-treated mice described in the legend to Fig. Fig.2.2. The results are expressed as the means ± standard deviations of IFN-γ levels on days 3, 7, and 13 postchallenge from groups of five mice per point.
IL-10 was measured in the sera of infected mice in an experiment similar to the one in Fig. Fig.2.2. On days 7 and 12 after infection, IL-10 levels were significantly higher in the samples taken from anti-IL-12-treated mice than in the samples from mice receiving NRG (Fig. (Fig.4).4).

Effect of administration of anti-IL-12 antibodies on IL-10 levels in serum of mice infected with S. typhimurium SL3261. The mice were infected and treated as in Fig. Fig.2.2. The figure shows IL-10 levels (as measured by ELISA) in sera from control and anti-IL-12-treated mice on days 1, 7, and 12 of the infection. The results are expressed as the means ± standard deviations of IL-10 levels from groups of five mice per point.
iNOS activity was measured in liver and spleen homogenates from control and anti-IL-12-treated mice infected with S. typhimurium SL3261 by using the citrulline assay. On days 3 and 8 after infection, iNOS activity was lower in the tissue homogenates from anti-IL-12-treated mice than in those from NRG-treated animals (Table (Table2).2).
TABLE 2
iNOS activity in the tissues of control and anti-IL-12-treated micea
micea
| Mouse group | iNOS activity (pmol/min/mg of protein)b
| |||
|---|---|---|---|---|
| Liver
| Spleen
| |||
| Day 3 | Day 8 | Day 3 | Day 8 | |
| NRG treated | 1.8 ± ± 0.15 0.15 | 1.3 ± ± 0.08 0.08 | 1.5 ± ± 0.11 0.11 | 1 ± ± 0.09 0.09 |
| Anti-IL-12 treated | 0.9 ± ± 0.08 0.08 | 0.8 ± ± 0.06 0.06 | 0.9 ± ± 0.06 0.06 | 0.6 ± ± 0.04 0.04 |
Thus, neutralization of IL-12 causes a dramatic decrease in local and systemic IFN-γ levels, a reduction in iNOS activity in the tissues, and an increase in the IL-10 levels in serum in mice infected with attenuated salmonellae.
Effect of in vivo IL-12 neutralization on the histopathology of salmonellosis.
BALB/c mice were infected and treated as in the experiment in Fig. Fig.2.2. Control mice (receiving NRG) and anti-IL-12-treated mice infected with S. typhimurium SL3261 showed enlarged spleens and livers on days 7 and 13 of the infection, in contrast to normal uninfected mice. Hepatosplenomegaly was more pronounced in anti-IL-12-treated mice on day 13 of the infection than in similarly infected controls.
Histologic testing on day 7 revealed well-defined granulomas in the spleens and livers of infected control mice (data not shown). The granulomas were surrounded by areas of normal tissue. At this time (day 7), no granulomas were visible in the spleens of anti-IL-12-treated mice and the overall appearance of the organs did not appear significantly different from that of organs of noninfected mice. On day 7, lesions were present in the livers of anti-IL-12-treated animals; these lesions consisted of a few cells with an abundance of polymorphonuclear leukocytes and no clear granulomatous appearance (data not shown).
On day 13 of the infection, well-defined granulomas were still present in both spleens and livers of infected control mice (Fig. (Fig.5A5A and B). Conversely, the organs of anti-IL-12-treated mice showed a widespread infiltration of mononuclear cells with no evidence of well-established granulomas and with a major disruption of the normal structure of the organs (Fig. (Fig.5C5C and D). In the spleen, white pulp follicles were no longer visible and the red pulp areas appeared to be evenly infiltrated by mononuclear cells. In the liver, hepatocyte damage was evident and wide areas of the organ were densely infiltrated by cells with a mononuclear appearance.
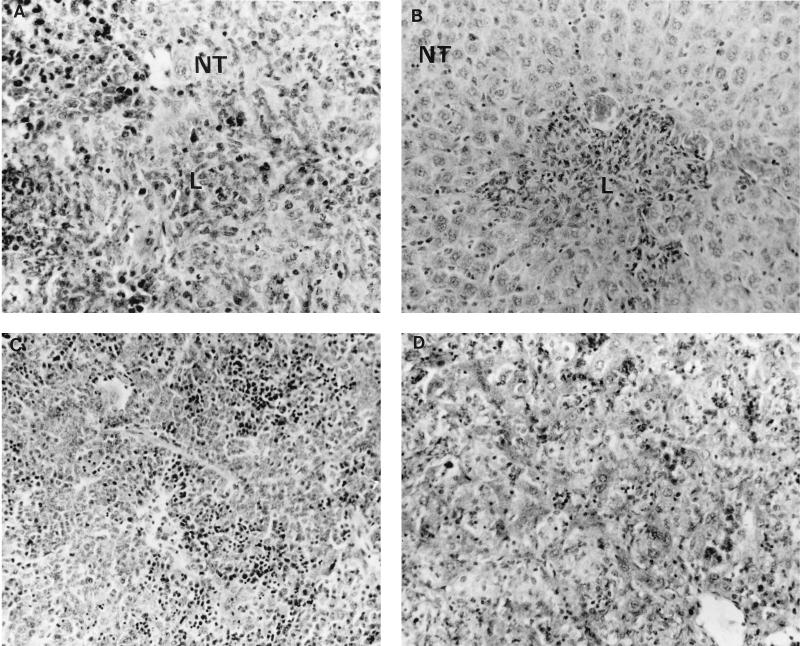
Histopathology of spleens and livers from control and anti-IL-12-treated mice on day 13 of an infection with S. typhimurium SL3261. Sections 8 μm thick were stained with hematoxylin and eosin. L, lesion; NT, normal tissue. Magnifications, ×448 (A) and ×280 (B to D).
In the spleens of the anti-IL-12-treated mice (heavily infected), salmonellae were seen exclusively within mononuclear cells; the vast majority (>85%) of the bacteria observed in the livers were seen within macrophages (Fig. (Fig.6).6).

Intracellular location of salmonellae in spleen and liver macrophages of anti-IL-12-treated mice on day 13 of an infection with S. typhimurium SL3261. (A) Bacteria within a spleen macrophage. (B) Bacteria within a liver macrophage. A 1-μm-thick section stained with toluidine blue is shown. The arrow indicates salmonellae (S) contained in vacuoles. H, hepatocyte. Magnifications, ×2,000.
Thus, IL-12 neutralization prevents granuloma formation and causes a massive influx into the RES of mononuclear cells, which are unable to control the growth of an attenuated Salmonella strain.
Expression of I-Ad/I-Ed antigens on F4/80+ and CD11b+ cells.
Spleen cells expressing the unique macrophage marker F4/80+ or the shared CD11b+ marker were analyzed for the expression of major histocompatibility complex (MHC) class II antigens as a marker of cell activation.
Mice were infected and treated as described for the experiment in Fig. Fig.2.2. On days 7 and 13 of the infection, single-cell suspensions were prepared from the spleens of groups of five infected NRG-treated control mice and five infected anti-IL-12-treated animals. Spleen cells from age- and sex-matched normal (uninfected) mice were included in the experiment. The cells were double stained for F4/80 and I-Ad/I-Ed as well as for CD11b and I-Ad/I-Ed.
Table Table33 shows that on day 7, the expression of I-Ad/I-Ed molecules was highly upregulated on both F4/80+ and CD11b+ cells from infected controls compared to cells from naive mice. I-Ad/I-Ed levels on F4/80+ and CD11b+ cells from anti-IL-12- treated mice were significantly lower than on cells from control animals but still significantly higher than on cells from naive mice. On day 13, I-Ad/I-Ed expression was further increased on F4/80+ and CD11b+ cells from infected controls. Conversely, at this time, the level of surface I-Ad/I-Ed was similar in cells from naive and anti-IL-12-treated mice.
TABLE 3
Expression of I-Ad/I-Ed molecules on F4/80+ or CD11b+ spleen cells from normal uninfected mice and from infected mice treated with NRG or anti-IL-12 antibodies
antibodies
| Mouse group | Mean fluorescence intensity (FL2) of I-Ad/I-Ed ona:
| |||
|---|---|---|---|---|
| F4/80+ cells
| CD11b+ cells
| |||
| Day 7 | Day 13 | Day 7 | Day 13 | |
| Normal | 91 ± ± 13 13 | 82 ± ± 5 5 | 24 ± ± 2 2 | 20 ± ± 1 1 |
| NRG treated | 750 ± ± 50 50 | 1,163 ± ± 233 233 | 421 ± ± 45 45 | 615 ± ± 76 76 |
| Anti-IL-12 treated | 146 ± ± 13 13 | 75 ± ± 16 16 | 61 ± ± 7 7 | 19 ± ± 9 9 |
Thus, the upregulation of MHC class II antigens observed in F4/80+ and CD11b+ spleen cells of mice infected with salmonellae is dramatically reduced as a consequence of IL-12 neutralization.
Effect of IL-12 neutralization on iNOS and cytokine mRNA expression.
Mice were infected and treated as described for the experiment in Fig. Fig.2.2. On day 13 of the infection, tissue samples were removed for semiquantitative RT-PCR. Samples from normal (not infected) mice were also included. Figure Figure77 shows significant expression of iNOS mRNA in the tissues of infected mice, which is greatly downregulated by the anti-IL-12 treatment. IFN-γ mRNA expression was also lower in the absence of IL-12, while a clear upregulation of IL-10-specific mRNA occurred in anti-IL-12-treated mice compared to infected control animals. IL-12 neutralization had no or little effect on the expression of transforming growth factor β (TGF-β)-specific mRNA (data not shown). No IL-4 mRNA could be detected in either group of mice (data not shown).

Expression of iNOS, IFN-γ, and IL-10 mRNAs in the tissues of normal mice (lane A), infected anti-IL-12-treated mice (lane B), and infected control mice treated with NRG (lane C). The mice were infected and treated as described for the experiment in Fig. Fig.2.2. The mRNA samples were obtained from five mice in each group.
Thus, IL-12 neutralization downregulates the expression of iNOS and IFN-γ mRNA in the tissues of Salmonella-infected mice. The treatment also causes a clear upregulation of IL-10-specific mRNA.
Effect of administration of rIFN-γ on the course of the infection in anti-IL-12-treated mice.
Groups of four BALB/c mice were infected with 5 × 105 CFU of S. typhimurium SL3261. One group of mice received 1 mg of anti-IL-12 antibodies on days 0, 4, 7, and 10. A second group received a similar dose of NRG. A third group of mice received the anti-IL-12 treatment combined with a daily i.p. dose of 105 U of murine rIFN-γ.
As expected, day 12 spleen and liver bacterial counts were significantly higher in anti-IL-12-treated mice than in NRG-treated controls. Conversely, bacterial counts in mice treated with both anti-IL-12 antibodies and rIFN-γ were significantly lower than those in mice treated with anti-IL-12 globulins alone, indicating that IFN-γ can restore host resistance in the absence of biologically active IL-12 (Fig. (Fig.8).8). The restoration was not complete, since a statistically significant difference was still detected between the bacterial loads in NRG-treated animals and in mice treated with both anti-IL-12 and rIFN-γ.
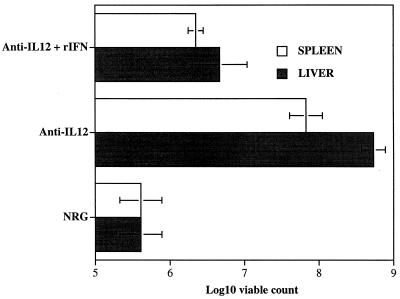
Effect of administration of rIFN-γ on the course of an infection with S. typhimurium SL3261 in anti-IL-12-treated mice. Mice were infected with 5 × 105 CFU of S. typhimurium SL3261. The figure shows spleen and liver bacterial counts from mice receiving NRG on days 0, 4, 7, and 10; anti-IL-12 antibodies at similar intervals; or anti-IL-12 antibodies combined with a daily i.p. dose of 105 U of murine rIFN-γ. The results are expressed as log10 viable counts ± standard deviations from groups of four mice per point on day 12 of the infection.
Spleen and liver histologic testing revealed the expected widespread infiltration of mononuclear cells in the organs of anti-IL-12-treated mice; conversely, the organs of mice injected with rIFN-γ displayed localized granulomas surrounded by normal tissue, similar to the situation observed in NRG-treated controls (Fig. (Fig.5A5A and B).
Fluorescence-activated cell sorter analysis performed on spleen cells from the three groups of mice showed the expected reduction in I-Ad/I-Ed expression on F4/80+ and CD11b+ cells in anti-IL-12-treated mice compared to NRG-treated controls. Administration of rIFN-γ proved to be able to restore MHC class II expression on F4/80+ and CD11b+ cells (Fig. (Fig.9).9).

Effect of administration of rIFN-γ on I-Ad/I-Ed expression on F4/80+ and CD11b+ cells in anti-IL-12-treated mice. Mice were infected and treated as for the experiment in Fig. Fig.7.7. I-Ad/I-Ed was measured by FL2. The results are shown as FL2 histograms of F4/80+ (A) and CD11b+ (B) cells previously gated by FL1. The numbers in brackets indicate the geometric means of FL2 intensity relative to each histogram. A repeat experiment gave similar results.
Thus, administration of rIFN-γ to anti-IL-12-treated mice restores host resistance to infection, granuloma formation, and expression of MHC class II antigens on F4/80+ and CD11b+ cells.
DISCUSSION
In the present report, we show that mice treated with anti-IL-12 antibodies are unable to control an infection with an attenuated aromatic-dependent S. typhimurium strain, to form granulomas in the tissues, and to upregulate MHC class II antigens on spleen cells. IFN-γ and iNOS mRNA production was down-regulated in IL-12-treated mice, which also showed an increased production of IL-10-specific mRNA. IFN-γ levels were reduced in sera and spleen homogenates from anti-IL-12-treated mice, and there was a significant increase in circulating IL-10 levels. iNOS activity was reduced in anti-IL-12- treated mice. Administration of recombinant IFN-γ reversed the effects of IL-12 neutralization. We also show that IL-12 is responsible for the toxic effects caused by high bacterial loads in the tissues and for the expression of hypersusceptibility to LPS seen in Salmonella-infected mice.
Survival of Salmonella-infected mice requires the suppression of bacterial growth in the tissues and the establishment of a plateau phase, with bacterial counts remaining at constant levels, until clearance is eventually accomplished (17, 30). The host defense mechanisms that operate in the plateau phase are complex and as yet not fully understood. The suppression of bacterial growth in mouse typhoid coincides with hepatosplenomegaly and with the formation of granulomas rich in mononuclear cells, which contain most of the organisms seen in the tissues (32, 38); both events require an influx of bone marrow-derived radiation-sensitive cells (17) in addition to IFN-γ and TNF-α (27, 28, 32, 36, 37). Inflammatory macrophages appear to be needed, since T-cell-depleted but not macrophage-depleted immune spleen cells harvested in the plateau phase can transfer resistance against infection to naive recipients (25).
In the present report, we show that IL-12 is absolutely required for the establishment of the plateau phase. In the absence of biologically active IL-12, mice cannot control the growth of the attenuated S. typhimurium SL3261 vaccine strain, which normally causes very mild infections in BALB/c mice. In anti-IL-12-treated mice, the plateau phase is missing and bacterial growth proceeds at a slow but constant rate, reaching high levels. Clearly, host resistance is abrogated in the absence of IL-12. A recent report has shown that a mutation in the IL-12 receptor gene can render humans more susceptible to salmonellosis (11). Therefore, a thorough evaluation of live attenuated salmonella strains in IL-12-deficient mice is essential (in addition to other known immunodeficiency models) for the preliminary assessment of the safety of candidate vaccines.
Most of the salmonellae present in the tissues of anti-IL-12-treated mice are seen within recruited macrophages (which are abundant in both the liver and the spleen), indicating that the influx of inflammatory cells in the tissues is not sufficient for the expression of host resistance to Salmonella. The recruited cells must be activated to kill intracellular bacteria; in the absence of biologically active IL-12, such activation appears to be lacking, as shown both by the abundance of intracellular bacteria in macrophages and by the deficient upregulation of MHC class II molecules on cells expressing the macrophage markers F4/80 and CD11b. iNOS mRNA expression and NOS activity were lower in the tissues of anti-IL-12-treated mice than in those of control mice, further indicating that macrophage activation was deficient in the absence of IL-12. Others have previously reported reduced nitrite production in vitro from splenocytes of anti-IL-12-treated mice infected with salmonellae (41). Noticeably, nitric oxide has been shown to be important in host resistance to Salmonella and in the formation of granulomatous lesions in the RES (48).
Our results show a clear upregulation of IL-10-specific mRNA and increased levels of circulating IL-10 in anti-IL-12-treated mice, suggesting that in the absence of IL-12, the enhanced (unregulated) production of IL-10 combined with decreased IFN-γ release (discussed below) might contribute to the inability of the phagocytes to restrain bacterial growth. Noticeably, IL-10 is known to suppress several macrophage functions (35), and in vivo neutralization of IL-10 has been reported to enhance host resistance to Salmonella, to upregulate IFN-γ production, and to increase the expression of MHC class II antigens on macrophages from mice infected with Salmonella (1).
In the organs of anti-IL-12-treated mice, early granuloma formation is inefficient and a massive infiltration of mononuclear cells occurs in the later stages of the infection. These findings indicate that IL-12 is needed for the formation of granulomas but not for the recruitment of mononuclear cells in the tissues. We have previously observed that TNF-α is also required for the control of a Salmonella infection in mice and for the formation of granulomatous lesions in the liver, spleen, and lymph nodes (26, 32). Nevertheless, in the absence of TNF-α, little macrophage infiltration was observed in the tissues and a marked cellular depletion was seen late in the course of the infection. Taken together, these findings indicate that granuloma formation requires both cellular recruitment and activation of the infiltrating cells. Both IL-12 and TNF-α are required for the formation of granulomas in the tissues; TNF-α seems to be involved in macrophage recruitment, while IL-12 is also involved in cellular activation.
IL-12 appears to exert its effects (host resistance, granuloma formation, and macrophage activation) via the induction of IFN-γ. In fact, in the present paper, we show that IL-12 is required both for IFN-γ production and for the activation of the bactericidal/bacteriostatic activity of recruited mononuclear cells. Furthermore, exogenous administration of rIFN-γ restored, at least in part, host resistance, granuloma formation, and MHC class II expression (a well-accepted marker of macrophage activation [2]) in anti-IL-12-treated mice. Taken together, these findings indicate that IFN-γ is one of the mediators of the effects of IL-12. Our results obtained in vivo are in line with the in vitro observation that IFN-γ can increase the ability of cultured macrophages to kill intracellular salmonellae (48) and that in the absence of IFN-γ mice develop progressive infections despite the abundance of macrophages infiltrating the tissues (references 14 and 26 and our unpublished observations).
In the present study, we observed that anti-IL-12-treated mice can carry high bacterial loads (10 to 100 times higher than the loads usually seen in premortem mice) and yet show very mild symptoms of infection. The latter results indicate that IL-12 is involved in the toxic effects exerted by large bacterial numbers in the tissues; this is probably one of the factors that led to the death of heavily infected animals. Noticeably, mice infected with salmonellae can be killed by low doses of LPS, since they become hypersusceptible to endotoxin (29, 33), and in the present study we found that a single injection of anti-IL-12 antibodies protected infected mice from death caused by LPS administration. These data are in line with recent observations indicating a role for IL-12 and IFN-γ in hypersusceptibility to LPS induced by Mycobacterium bovis BCG infection (49). We have previously reported that TNF-α neutralization in Salmonella-infected mice protects mice from death due to exogenous LPS injection (29); nevertheless, in slowly evolving infections, we never observed either unusually large bacterial numbers in the tissues of anti-TNF-α-treated mice or a discrepancy between bacterial numbers and symptoms or survival of the anti-TNF-α-treated mice. Therefore, our results suggest that different mechanisms operate in toxicity induced by high bacterial loads during a slowly progressive infection and in toxicity and death in response to LPS administration to infected mice.
The increased production of IL-10 in the tissues of infected anti-IL-12-treated mice might also account for the reduced sensitivity to LPS and large bacterial numbers observed by us. In fact, IL-10 has been shown to confer protection against toxic effects induced by products of microbial origin (reviewed in reference 45).
Attenuated aromatic-dependent salmonellae were initially believed to be completely safe by virtue of their low virulence in immunocompromised animals (reviewed in reference 16). This was thought to be due to their inability to synthesize p-aminobenzoic acid (PABA) (15). Unfortunately, most of the early data were obtained over very short observation periods (19, 44). In the present paper, we show that BALB/c mice treated with anti-IL-12 globulins cannot control the growth of an attenuated aroA Salmonella strain. We and others have also recently shown that S. typhimurium live aro vaccines cause progressive, lethal infections in nu/nu (T-cell-deficient) mice and in gene-targeted mice deficient in CD4+ T-cell receptor β−/− or IFN-γ receptor (14, 42). This is probably due to the presence of sufficient levels of PABA in the pelleted diet feed used by others and us (5 mg of PABA per kg; Special Diets Services, Wiltham, United Kingdom) (42).
It is now clear that aro vaccines can cause severe infections but only in animals with serious and persistent immunological defects. Therefore, a search for additional attenuating mutations, in combination with further studies on the fundamental mechanisms of immunity to Salmonella, is needed.
ACKNOWLEDGMENTS
This work was supported by grants from EEC and Wellcome Trust.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.66.10.4767-4776.1998
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/66/10/4767.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/content/full/66/10/4767
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/66/10/4767
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/66/10/4767
Citations & impact
Impact metrics
Citations of article over time
Article citations
Changes in monocyte subsets in volunteers who received an oral wild-type Salmonella Typhi challenge and reached typhoid diagnosis criteria.
Front Immunol, 15:1454857, 27 Aug 2024
Cited by: 0 articles | PMID: 39263222 | PMCID: PMC11388309
New Approaches to Tackling Intractable Issues in Infectious Disease.
Microorganisms, 12(3):421, 20 Feb 2024
Cited by: 1 article | PMID: 38543472 | PMCID: PMC10971794
Review Free full text in Europe PMC
Nitrate Utilization Promotes Systemic Infection of Salmonella Typhimurium in Mice.
Int J Mol Sci, 23(13):7220, 29 Jun 2022
Cited by: 5 articles | PMID: 35806223 | PMCID: PMC9266322
Revisiting Persistent Salmonella Infection and the Carrier State: What Do We Know?
Pathogens, 10(10):1299, 09 Oct 2021
Cited by: 24 articles | PMID: 34684248 | PMCID: PMC8537056
Review Free full text in Europe PMC
Salmonella Virulence and Immune Escape.
Microorganisms, 8(3):E407, 13 Mar 2020
Cited by: 49 articles | PMID: 32183199 | PMCID: PMC7143636
Review Free full text in Europe PMC
Go to all (96) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid.
Infect Immun, 64(1):189-196, 01 Jan 1996
Cited by: 77 articles | PMID: 8557339 | PMCID: PMC173745
Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro- Salmonella vaccines.
Microb Pathog, 13(6):477-491, 01 Dec 1992
Cited by: 121 articles | PMID: 1363824
Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections.
Infect Immun, 67(12):6242-6248, 01 Dec 1999
Cited by: 26 articles | PMID: 10569733 | PMCID: PMC97025
[TH1 response in the experimental infection with Trypanosoma cruzi].
Medicina (B Aires), 59 Suppl 2:84-90, 01 Jan 1999
Cited by: 10 articles | PMID: 10668248
Funding
Funders who supported this work.




