Abstract
Free full text

Proprotein convertase subtilisin/kexin type 9 (PCSK9) as a marker of coronary lesion severity in stable coronary artery disease (CAD) patients
Abstract
Coronary artery disease (CAD) remains a significant global health concern with considerable high morbidity and mortality and its development is influenced by various genetic and environmental factors. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a vital regulator of low-density lipoprotein receptor (LDLR) metabolism, directly impacting serum cholesterol levels. However, its role in development of CAD is not fully understood. The aim of this study was to assess the association between the level of PCSK9 and coronary lesion severity in patients with CAD. A case-control study using consecutive sampling was conducted among CAD patients at H. Adam Malik General Hospital and Murni Teguh Memorial Hospital, Medan, Indonesia. A total of 200 CAD patients were divided into two groups based on the SYNTAX score: control (score ≤22, n=100) and case (score >22, n=100). Plasma PCSK9 levels were measured from venous blood using quantitative sandwich enzyme immunoassay. The Chi-squared test was used to analyze the data. Our data suggested that PCSK9 level was associated with coronary lesion severity (p<0.001) of which high PCSK9 level was associated with severe coronary lesion. We also found that hypertension (p<0.001), smoking (p=0.072), diabetes (p<0.001), dyslipidemia (p<0.001), obesity (p=0.023), and family history (p=0.001) were associated with lesion severity. Using the receiver operating characteristic (ROC) curve analysis, the cut-off 70.35 ng/mL of PCSK9 had sensitivity 75% and specificity 78% to predict severe coronary lesion. This study highlights that PCSK9 level has moderate sensitivity and specificity to predict the coronary lesion severity among CAD patients.
Introduction
Cardiovascular disease incidence continues to increase in developed and developing countries and cardiometabolic, lifestyle, genetic, and environmental risks are major factors that play important role in its increase incidence [1]. Cardiovascular disease is the leading cause of death globally, with men being affected more frequently than women [2]. One in three individuals with heart disease will have impaired quality of life (QoL) compared to the healthy population [2].
One of the cardiovascular disease, coronary artery disease (CAD), has a concerning rise in its incidence. It is not only a health problem but also has impacts on the economy and healthcare systems [2]. The World Health Organization (WHO) data published by the American Heart Association (AHA) in 2020 states that over 17 million people died of cardiovascular disease, around 32% of all deaths [3]. In 2017, 17.8 million deaths worldwide were caused by heart disease, and this increased by 21.1% from 2007 [4]. Based on 2018 Indonesian Basic Health Research (Riskesdas) data, the incidence of cardiovascular disease increases each year and at least 15 out of 1,000 people suffer from heart disease, or approximately 2,784,064 [4]. In Indonesia, CAD is the leading cause of all deaths, making up 26.4% of all deaths and this figure is four times higher than the death rate caused by cancer (6%) [3]. In other words, approximately one in four people who die in Indonesia is due to CAD [5].
Of the entire spectrum of cardiovascular diseases, CAD is responsible for the highest mortality and the mortality rate is estimated to be 30% of all global mortality rate [6]. CAD is a multifactorial disease resulting from the interaction of genetic, environmental, and other risk factors [7]. Various risk factors for atherosclerosis induce CAD including risk factors that can be modified, such as diabetes mellitus (DM), hypertension, smoking, lack of activity, and dyslipidemia, or those that cannot be modified, such as age, gender, and family history and genetics [8]. Atherosclerosis is considered an inflammatory disease because the cells that play a role are macrophages, which originate from monocytes that induced from the inflammatory process. The pathogenesis of atherosclerosis begins when injury occurs due to various risk factors in multiple intensities and different durations of exposure to the arterial endothelium, thereby activating or causing endothelial dysfunction [8]. Endothelial dysfunction is a precursor to atherosclerosis [7].
Some genes that are known to be associated in CAD and the progressive and premature process of atherosclerosis are low-density lipoprotein receptor (LDLR), apolipoprotein B (APOB), and proprotein convertase subtilisin/kexin type 9 (PCSK9) [9-11]. Family history of CAD and the possibility of genetic factors are critical to be assessed [12]. PCSK9 is a gene associated with familial autosomal dominant hypercholesterolemia with an estimated incidence of around 2.3% after the LDLR and APOB gene [13]. The discovery of PCSK9 gene is a major development in the cardiovascular field in the last decade, especially in the regulation of low-density lipoprotein cholesterol (LDL-C) [14]. PCSK9 is a protein molecule to increase the degradation of LDLR, which is secreted mainly by hepatocyte cells [15]. Recent studies found that in patients with stable CAD and patients with acute coronary syndrome (ACS), PCSK9 level was significantly related to the severity of coronary lesions and disease outcomes [15,16].
SYNTAX score (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) is an angiographic scoring system that assesses the complexity of coronary artery lesions based on coronary anatomy and lesion characteristics to help clinicians decide on revascularization methods in patients with CAD [17-19]. A study in China reported there was association between PCSK9 level and the severity of coronary artery lesions calculated based on the SYNTAX score [16]. An increased rate of acute major cardiovascular events was also found in patients with higher PCSK9 level [20].
In addition, high level of PCSK9 was also found in patients with peripheral arterial disease, especially in groups with more severe coronary lesions [21,22]. Similarly, another study found a relationship between serum PCSK9 level and plaque necrotic cores obtained from intravascular ultrasound (IVUS) examination [23]. In another study in China with a greater variety of ethnicities found that patients with elevated PCSK9, LDL and triglyceride levels were likely to have severe coronary lesions as assessed by the Gensini score [24]. Platelet distribution and reactivity was also higher among individual with elevated PCSK9 level [16,23, 25]. However, study assessing the role of PCSK9 level on severity of coronary lesions in CAD patients in Indonesia is limited. The aim of this study was to determine the association between PCSK9 level and degree severity of coronary lesion in patients with stable CAD in Indonesian.
Methods
Study design and setting
This study was an analytical study with a case-control study design assessing the association between PCSK9 levels and coronary lesion severity in the control group (SYNTAX score ≤22) and the case group (SYNTAX score >22). This study was conducted on patients with stable CAD who underwent coronary angiography examinations at the H. Adam Malik General Hospital and Murni Teguh Memorial Hospital located in Medan, Indonesia from September 2022 until the sample size was met according to the sample size formula.
Sample size and patients
The control in this study was 100 patients with a SYNTAX score of ≤22 and 100 patients with a SYNTAX score of >22 as cases. The patients were all stable CAD patients. The inclusion criteria for the patients to be included were: (1) patients with definitive clinical evidence of atherosclerotic lesions leading to a diagnosis of CAD and (2) patients did not used statin, fibrate or other dyslipidemia drugs in the last three months. All patients with a history of transient ischemic attack (TIA), stroke, peripheral arterial disease, blood disorders (e.g., coagulation disorder, polycythemia, myelodysplastic syndromes, hemoglobinopathies, and others), malignancies, infection, thyroid function disorders; decreased kidney function or liver function; having a history of percutaneous coronary intervention (PCI) or cardiac surgery or previous installation of intracardiac implants or pacemakers and/or prosthetic valves; and having acute coronary syndrome (ACS) were excluded.
The consecutive sampling method was used to collect samples, where each stable CAD patient who met the inclusion criteria was included in the study. We included 100 patients in the case group and 100 patients in the control group.
Variables and data collection
Clinical history, anthropometric characteristics and standard cardiovascular risk factors were collected by direct interview or during the clinical assessment to the patients. They were also interviewed to ask the lifestyle behaviors, such as smoking. Hypertension and diabetes were defined as being present when a health professional diagnosed of these conditions or were using associated drugs. All of the participants were asked for consent after explanation regarding the purpose of the study, angiography examination procedure and PCSK9 plasma examination.
Coronary angiography and laboratory examination
Each CAD patient was included to case or control based on the complexity of coronary artery lesion assessed using coronary angiography and scored using SYNTAX score. SYNTAX score was calculated using a computer program containing interrelated questions. This algorithm consists of 12 questions [18,26] and the results were calculated and grouped into a low score (mild) ≤22 and medium-high score (moderate-high) >22. A low SYNTAX score was categorized as a mild coronary lesion, while a medium-high SYNTAX score was classified as severe coronary lesion.
To measure the PCSK9 level, venous blood samples were collected from each patient who had fasted for 12 hours overnight. All plasma samples were collected into EDTA containing tube and were store at -80°C until the analysis. The plasma PCSK9 level was measured using a high sensitivity, quantitative sandwich enzyme immunoassay (Quantikine ELISA, R&D systems, Minneapolis, MN, USA).
Statistical analysis
The normality test was carried out for each data using the Kolmogorov-Smirnov test. Continuous data was shown as the mean ± standard deviation (SD) for normally distributed data or minimum and maximum values for data that was not normally distributed. The Chi-squared test was used to assessed factor associated with degree severity of coronary lesion. The association was considered statistically significant if the p-value less than 0.05. The cut off point for PCSK9 level was obtained through receiver operating characteristic (ROC), namely the PCSK9 level value found based on the severity of the coronary lesion.
Results
Characteristics of patients
A total of 200 CAD patients included in this study and their demographic, clinical and laboratory characteristics are presented in Table 1. Out of total, 160 (80%) patients were men with a mean age of 57.3±9.5 (range 34–82 years). Some comorbid identified included 145 (72.5%) patients with obesity, 83 (41.5%) with hypertension, 92 (46%) with dyslipidemia, and 63 (31.5%) with diabetes mellitus. A total of 77 (38.5%) patients had a family history of coronary atherosclerosis. There were 138 (69%) patients who were smokers. Of all study patients, 100 (50%) had a mild degree of coronary lesion, while 100 (50%) patients had a severe degree of coronary lesion severity. A total of 106 (53%) patients had PCSK9 levels <70.35 ng/mL, and 94 (47%) patients had PCSK9 levels ≥70.35 ng/mL. The cut of 70.35 ng/mL was based on ROC curve analysis. Several laboratory parameters were examined in this study, including HbA1c, total cholesterol, LDL, high-density lipoprotein (HDL) and triglyceride levels. The mean of HbA1c 6.71%, total cholesterol 211.7 mg/dL, HDL 38.57 mg/dL, LDL ±71.5 mg/dL, and triglyceride 131.71 mg/dL (Table 1).
Table 1.
| Baseline characteristics | Frequency (%) |
|---|---|
| Male | 160 (80.0) |
| Age, mean (ranges) | 77 (50–100) |
| Comorbidities | |
| Obesity | 145 (72.5) |
| Hypertension | 83 (41.5) |
| Dyslipidemia | 92 (46.0) |
| Diabetes mellitus | 63 (31.5) |
| Family history of coronary atherosclerosis | 77 (38.5) |
| Smoking | 138 (69.0) |
| Coronary lesion severity | |
| Mild | 100 (50.0) |
| Severe | 100 (50.0) |
| Proprotein convertase subtilisin/kexin type 9 (PCSK9) level (ng/dL) | |
| ≤70.35 | 106 (53.0) |
| >70.35 | 94 (47-0) |
| Laboratoiies (mean±SD) | |
| HbAic (%) | 6.71±o.9 |
| Total cholesterol (mg/dL) | 211.71±88.6 |
| High-density lipoprotein (mg/dL) | 38.57±14.2 |
| Low-density lipoprotein (mg/dL) | 134.29±71.5 |
| Triglyceride (mg/dL) | 131.71.i30.6 |
Association of PCSK9 level and other factors on coronary lesion severity
Our data indicated that PCSK9 level and was associated with coronary lesion severity significantly with p<0.001. In addition, comorbidities such as hypertension (p<0.001), smoking (p=0.072), diabetes (p<0.001), dyslipidemia (p<0.001), obesity (p=0.023), and family history (p=0.001) were associated with coronary lesion severity (Table 2). There was no association between age and gender with coronary lesion severity.
Table 3.
| SYNTAX Score | PCSK9 level cut off (ng/dL) | |||
|---|---|---|---|---|
| >70.35 | ≤70.35 | |||
| Severe (>22) | 74 | 78.7% | 26 | 24.5% |
| Mild (≤22) | 20 | 21.3% | 80 | 75.5% |
| Total | 94 | 100% | 106 | 100% |
Sensitivity and specificity of PCSK9 level as a predictor of coronary lesion severity
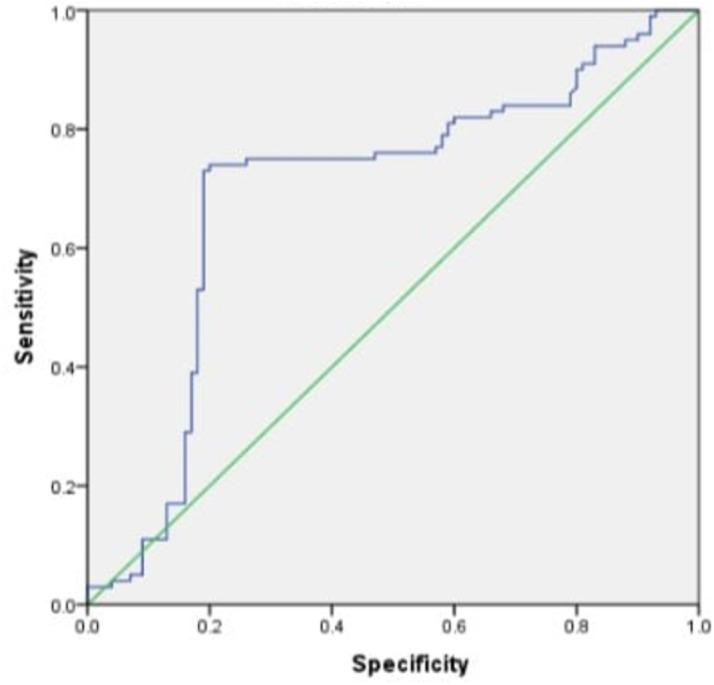
ROC curve analysis showed that the diagnostic cut off value of PCSK9 level was 70.35 ng/dL, resulting in an area under curve (AUC) of 0.693 (95% confidence interval (CI), sensitivity 75%, and specificity 78% (Figure 1). The positive predictive value was 0.8 with and the negative predictive value was 0.74. The diagnostic test table can be found in Table 3.
Table 2.
| Variable | Degree of lesion | severity, n (%) | p-value |
|---|---|---|---|
| Mild, n (%) | Severe, n (%) | ||
| Hypertension | |||
| Yes | 19 (19.0) | 64 (64.0) | <0.001** |
| No | 81 (81.0) | 36 (36.0) | |
| Obesity | |||
| Yes | 68 (68.0) | 77 (77.0) | 0.023* |
| No | 32 (32.0) | 23 (23.0) | |
| Dyslipidemia | |||
| Yes | 13 (13.0) | 79 (79.0) | <0.001** |
| No | 87 (87.0) | 21 (21.0) | |
| Diabetes mellitus | |||
| Yes | 11 (11.0) | 52 (52.0) | <0.001** |
| No | 89 (89.0) | 48 (48.0) | |
| Family history of coronary atherosclerosis | |||
| Yes | 26 (26.0) | 51 (51.0) | 0.001* |
| No | 74 (74.0) | 49 (49.0) | |
| Smoking | |||
| Yes | 62 (62.0) | 76 (76.0) | 0.072* |
| No | 38 (38.0) | 24 (24.0) | |
| Age (year) | |||
| <45 | 12 (12.0) | 27 (27.0) | 0.122 |
| ≥45 | 88 (88.0) | 73 (73.0) | |
| Gender | |||
| Male | 74 (74.0) | 86 (86.0) | 0.098 |
| Female | 26 (26.0) | 14(14.0) | |
| Proprotein convertase subtilisin/kexin type 9 (ng/dL) | |||
| ≤70.35 | 80 (80.0) | 26 (26.0) | <0.001** |
| >70.35 | 20 (20.0) | 74 (74.0) |
Discussion
There were 200 patients in this study; the majority of patients, 157.0 (78.5%), were men with an average age of 57.3 years. Patients in this study also had several comorbidities such as obesity (27.5%), hypertension (40.5%), dyslipidemia (45.5%), and DM (31%). These results are similar with previous study the CAD population was also predominantly male, with comorbidities such as hypertension, diabetes, obesity, and dyslipidemia [27]. A previous study also found something similar, where comorbidities such as hypertension, diabetes, and obesity were also found in the same population [29]. According to the Framingham Heart Study, there were five modifiable CAD risk factors that operate independently, including hypertension, dyslipidemia, glucose intolerance, smoking, and left ventricular hypertrophy. In addition, there was a correlation between hypertension and body mass index, and both were strongly correlated with CAD. Thus, CAD and hypertension, obesity, and diabetes often occur together due to the same risk factors, pathophysiological mechanisms, and complex interactions [28,29]. In this study 38% had a family history of coronary atherosclerosis, and 68.5% of patients smoked. This finding was in accordance with a previous study where in a population of CAD patients, it was found that 8.2% of patients had a family history of premature coronary heart disease, and 32.4% had a history of coronary heart disease that occurred at any time [30]. In this study, 45% of patients had a mild degree of severity of coronary lesions. In contrast, the majority of patients, namely 55%, had a severe degree of severity of coronary lesions. This was in accordance with a previous study where patients with comorbidities such as diabetes, dyslipidemia and risk factors such as smoking had more severe coronary lesions [31]. Patients in this study also tended to had high HbA1c at 6.71%. The patients also had high total cholesterol, low HDL, and high LDL (211.71 mg/dL, 38.57 mg/dL, and 134.29 mg/dL respectively). These results was in accordance with a previous study where in patients with the same population, abnormal total cholesterol, LDL, and HDL values were found [30].
Our study found significant association between obesity, hypertension, dyslipidemia, DM, family history, and smoking with the severity of coronary artery lesion. A previous study also found a significant relationship was found with the severity of the lesion as represented by the SYNTAX score and risk factors such as hypertension, dyslipidemia, DM, and smoking [31]. It is estimated that CAD is more complex in patients with several risk factors than in patients without risk factors. DM and hypertension are common risk factors for atherosclerosis.
There are conflicting results regarding hypertension and its association with coronary lesions. The findings from the study by Karabag et al. [32] are in accordance with the findings of this present study. It was found that hypertension, LDL-C, and hs-CRP/albumin ratio were significant predictors of higher SYNTAX scores [32]. However, another study found that hypertension was not significantly associated with higher SYNTAX scores in patients with CAD [33]. Nevertheless, it was accepted that hypertension disrupts the endothelial system, thereby increasing the risk of coronary artery disease, thus representing a significant risk factor for the development of atherosclerotic disease [34].
Previous studies suggest a relationship between DM, CAD, and the complexity of coronary lesions [31,35]. A study reported that DM was significantly associated with higher SYNTAX scores in patients with CAD [35]. Another study found similar results, where DM was associated with CAD complexity and was an independent risk factor for CAD complexity [31]. In DM patients, compared with patients without DM, CAD tends to be more widespread, complex, and associated with increased morbidity and mortality due to cardiovascular disease [35]. The mechanism of CAD pathogenesis in patients with DM is related to epigenetic, genetic, and cell signaling problems in interrelated metabolic and inflammatory pathways [38]. Hyperglycemia causes chronic damage to the endothelium, and the effects of inflammatory cytokines on the endothelium contribute to the development of atherosclerosis and arterial stiffness and have an essential role in plaque stability [38]. Hyperglycemia induces hyperacetylation of histone H3K9/K14 in the endothelium, which leads to the expression of metalloproteinases such as matrix metalloproteinase (MMP) protein-10, cysteine/glutamate transporter (SLC7A11), and MMP1 [38]. Metalloproteinases participate in vascular remodeling, particularly in plaque development and instability [36].
In a previous study it was found that dyslipidemia, including LDL-C, total cholesterol, and total cholesterol/HDL-C ratio, was associated with higher SYNTAX scores in stable CAD patients [39]. LDL-C levels are associated with the development of atherogenesis. Oxidation of LDL-C due to increased oxidative stress plays a role in the progression of atherosclerosis [37].
No association was found between age and gender with lesion severity in this present study. However, there was a tendency for older patients to had more severe lesions. These results contradict findings in a previous study, where aging was a significant independent risk factor for CAD complexity and high SYNTAX score results [33]. It was known that vascular endothelial function decreases, and arterial atherosclerosis progresses with age. Thus, advanced age is an independent risk factor for the complexity of CAD [33]. Similarly, in a previous study, gender was not associated with higher SYNTAX scores or more complex lesions [31]. In accordance with the results of previous study, there was no relationship between gender and complexity of coronary lesions [33]. Previously, it was thought that CAD in men was more complex and severe than in women [40]. However, another study reported that women with CAD have smaller coronary artery diameters and often have diffuse stenosis unsuitable for PCI and coronary calcifications [41]. Previously, it was known that estrogen has anti-arteriosclerotic effects, so women often experience CAD after menopause [42]. However, current findings show that the pathogenesis of CAD is very similar in men and women [31].
This study found significant relationship between smoking and the severity of coronary lesions. A previous study also found that patients who smoked had higher SYNTAX scores than patients who did not smoke [37]. According to another study exposure to cigarette smoke was significant cause of cardiovascular morbidity and mortality. Active or passive exposure to cigarette smoke causes vasomotor dysfunction, atherogenesis, and endothelial thrombosis [31]. A systematic review of the effects of smoking on coronary arteries, it was found that smoking was not significantly associated with the amount of coronary artery damage in CAD patients; however, these findings were only reported in two studies [38]. The failure to find a relationship could be caused by several things, including differences between the duration of smoking, smoking dose, and time of disease in each individual, which can cause more severe and complex coronary lesions. A previous study of 928 patients, a negative correlation was found between high-risk coronary lesions and obesity, while DM and dyslipidemia correlated with the severity of CAD [39]. The relationship between obesity or increased BMI and coronary atherosclerosis has always been controversial. Although it seems reasonable that obese patients are more susceptible to severe CAD, some studies have shown a paradoxical relationship. The reason may be the inability of BMI to precisely identify dangerous fatty deposits, such as in the coronary arteries [40].
In this study, there was a relationship between the severity of coronary lesions and PCSK9 levels. A previous study also found a correlation between serum PCKS9 levels and the severity of coronary artery lesions in CAD patients of which moderate and high PCSK9 levels indicated increased coronary severity (SYNTAX score >21.5) in CAD patients [41]. In contrast, another study found that PCSK9 levels were not associated with the severity of coronary lesions in the acute coronary syndrome population [42]. Many confounding factors can cause variations in serum PCSK9 levels. Differences in days of blood sampling to measure PCSK9 levels can cause variations in PCSK9. In the present study, ROC curve analysis was carried out. ROC curve analysis showed that the diagnostic cutoff value for PCSK9 levels was 70.35, resulting with a sensitivity of 75% and a specificity of 78%.
A potential limitation of this study is its cross-sectional design, which only provides a snapshot of the relationship between PCSK9 levels, comorbidities, and coronary lesion severity at a specific point in time. This design does not allow for the establishment of causality or the assessment of how these factors change over time. Longitudinal or prospective studies would be needed to better understand the temporal aspects of these relationships and draw more definitive conclusions.
Conclusion
There has been no study linking PCSK9 level with the severity of coronary lesions in Indonesia. In this present study, we found association between PCSK9 level, hypertension, smoking, DM, obesity, and dyslipidemia with lesion severity of coronary in CAD patients. Moreover, we found that the level of PCSK9 was more specific than sensitive as diagnostic marker for coronary lesion severity. Further study is needed to explore associations between PCSK9 and lipid profile, age and gender. It is also necessary to conduct a more comprehensive assessment related to patient comorbidities that can affect the severity of coronary lesion in CAD patients in the future.
Ethics approval
This research was approved by the Health Ethics Committee, University of Sumatera Utara prior to conducting the study with approval number 1050/KEPK/USU/2022.
Competing interests
The authors declare that there is no conflict of interest.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Andra CA, Rambe AS, Hasan R, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) as a marker of coronary lesion severity in stable coronary artery disease (CAD) patients. Narra J 2023; 3 (3): e409 - http://doi.org/10.52225/narra.v3i3.409.
References
Articles from Narra J are provided here courtesy of Narra Sains Indonesia
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Increased sortilin and its independent effect on circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) in statin-naive patients with coronary artery disease.
Int J Cardiol, 227:61-65, 08 Nov 2016
Cited by: 19 articles | PMID: 27846466
Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease.
Ann Med, 47(5):386-393, 10 Jul 2015
Cited by: 51 articles | PMID: 26153823
Relation of resistin to proprotein convertase subtilisin-kexin type 9 levels in coronary artery disease patients with different nutritional status.
J Endocrinol Invest, 38(12):1291-1299, 24 May 2015
Cited by: 8 articles | PMID: 26003826
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.
J Am Coll Cardiol, 62(16):1401-1408, 21 Aug 2013
Cited by: 151 articles | PMID: 23973703
Review