Abstract
Free full text

New families in the classification of glycosyl hydrolases based on amino acid sequence similarities.
Abstract
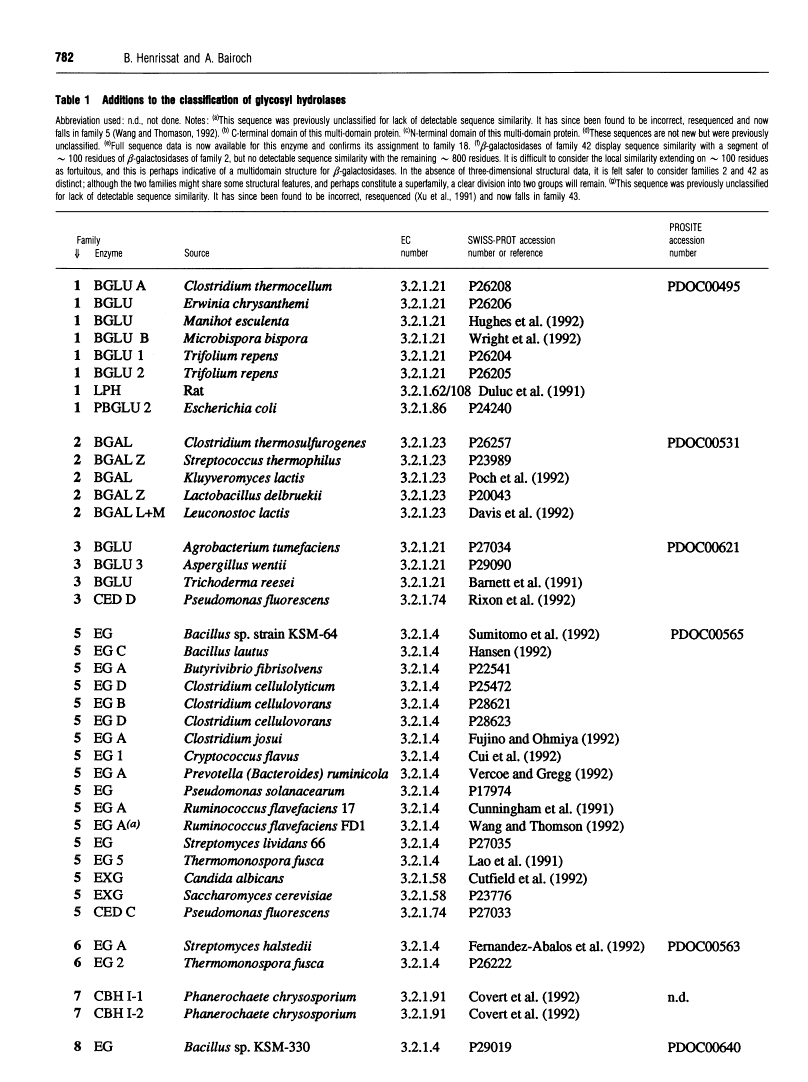
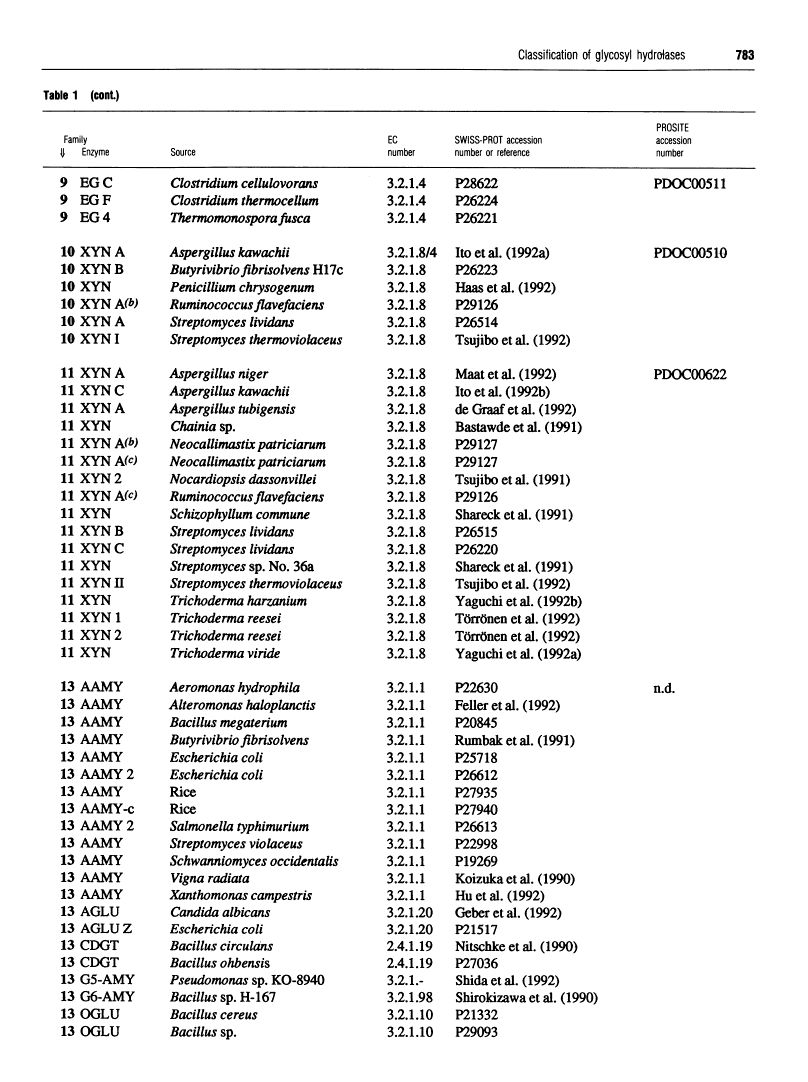
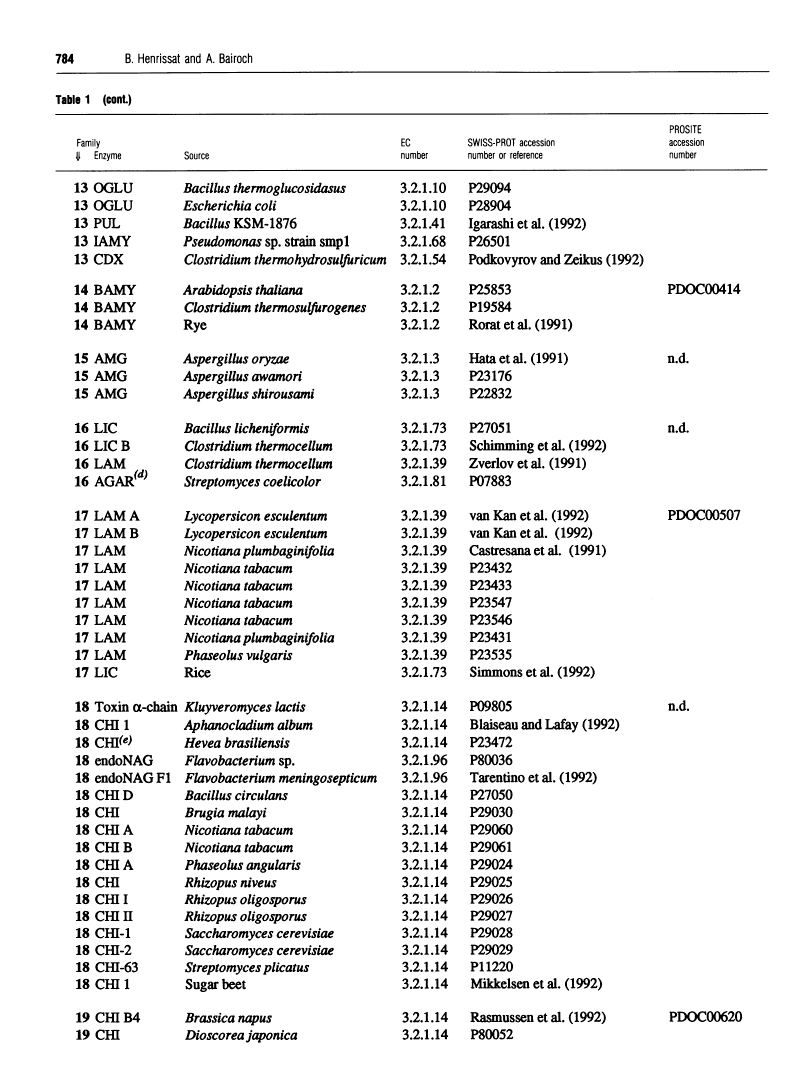
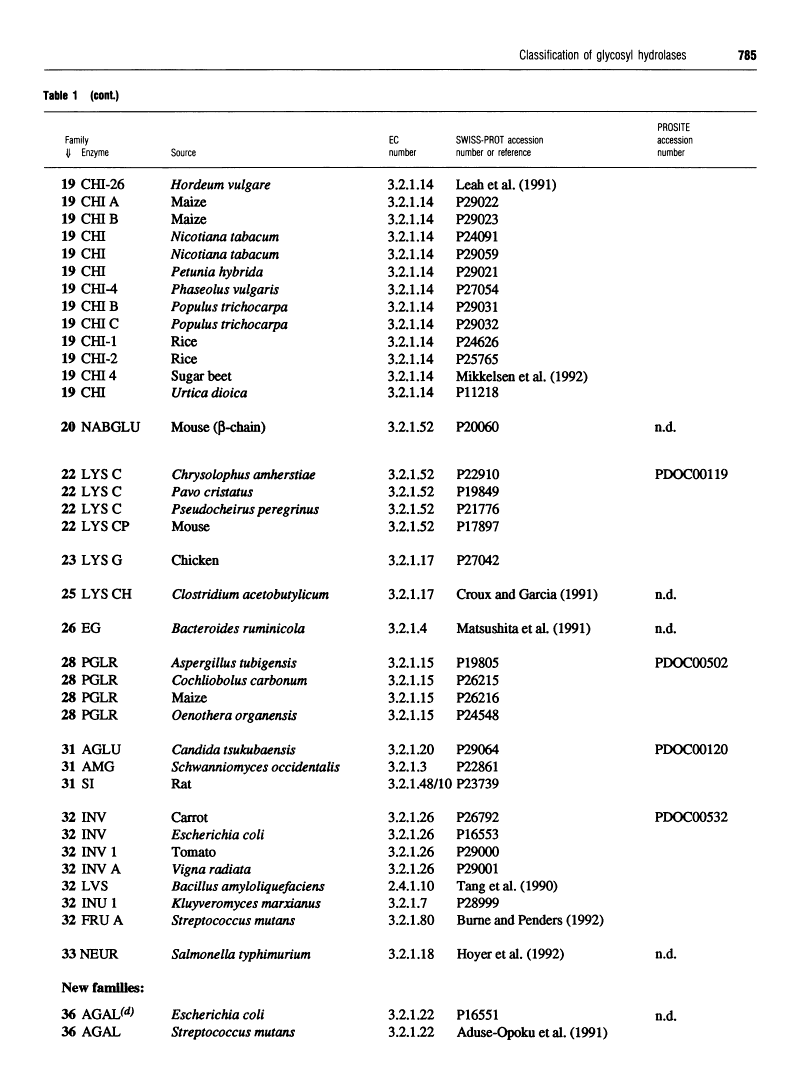
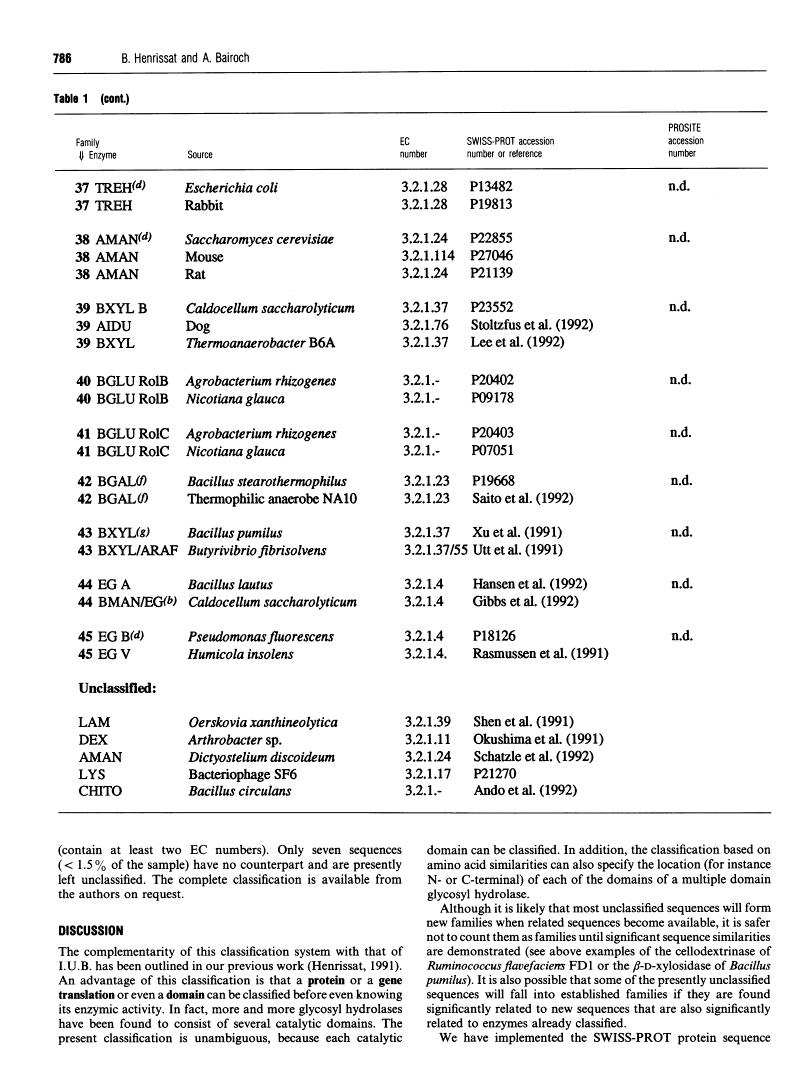
301 glycosyl hydrolases and related enzymes corresponding to 39 EC entries of the I.U.B. classification system have been classified into 35 families on the basis of amino-acid-sequence similarities [Henrissat (1991) Biochem. J. 280, 309-316]. Approximately half of the families were found to be monospecific (containing only one EC number), whereas the other half were found to be polyspecific (containing at least two EC numbers). A > 60% increase in sequence data for glycosyl hydrolases (181 additional enzymes or enzyme domains sequences have since become available) allowed us to update the classification not only by the addition of more members to already identified families, but also by the finding of ten new families. On the basis of a comparison of 482 sequences corresponding to 52 EC entries, 45 families, out of which 22 are polyspecific, can now be defined. This classification has been implemented in the SWISS-PROT protein sequence data bank.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aduse-Opoku J, Tao L, Ferretti JJ, Russell RR. Biochemical and genetic analysis of Streptococcus mutans alpha-galactosidase. J Gen Microbiol. 1991 Apr;137(4):757–764. [Abstract] [Google Scholar]
- Baird SD, Hefford MA, Johnson DA, Sung WL, Yaguchi M, Seligy VL. The Glu residue in the conserved Asn-Glu-Pro sequence of two highly divergent endo-beta-1,4-glucanases is essential for enzymatic activity. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1035–1039. [Abstract] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992 May 11;20 (Suppl):2013–2018. [Europe PMC free article] [Abstract] [Google Scholar]
- Bairoch A, Boeckmann B. The SWISS-PROT protein sequence data bank. Nucleic Acids Res. 1992 May 11;20 (Suppl):2019–2022. [Europe PMC free article] [Abstract] [Google Scholar]
- Barnett CC, Berka RM, Fowler T. Cloning and amplification of the gene encoding an extracellular beta-glucosidase from Trichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates. Biotechnology (N Y) 1991 Jun;9(6):562–567. [Abstract] [Google Scholar]
- Blaiseau PL, Lafay JF. Primary structure of a chitinase-encoding gene (chi1) from the filamentous fungus Aphanocladium album: similarity to bacterial chitinases. Gene. 1992 Oct 21;120(2):243–248. [Abstract] [Google Scholar]
- Burne RA, Penders JE. Characterization of the Streptococcus mutans GS-5 fruA gene encoding exo-beta-D-fructosidase. Infect Immun. 1992 Nov;60(11):4621–4632. [Europe PMC free article] [Abstract] [Google Scholar]
- Castresana C, de Carvalho F, Gheysen G, Habets M, Inzé D, Van Montagu M. Tissue-specific and pathogen-induced regulation of a Nicotiana plumbaginifolia beta-1,3-glucanase gene. Plant Cell. 1990 Dec;2(12):1131–1143. [Abstract] [Google Scholar]
- Chothia C. Proteins. One thousand families for the molecular biologist. Nature. 1992 Jun 18;357(6379):543–544. [Abstract] [Google Scholar]
- Claeyssens M, Henrissat B. Specificity mapping of cellulolytic enzymes: classification into families of structurally related proteins confirmed by biochemical analysis. Protein Sci. 1992 Oct;1(10):1293–1297. [Europe PMC free article] [Abstract] [Google Scholar]
- Covert SF, Vanden Wymelenberg A, Cullen D. Structure, organization, and transcription of a cellobiohydrolase gene cluster from Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jul;58(7):2168–2175. [Europe PMC free article] [Abstract] [Google Scholar]
- Croux C, García JL. Sequence of the lyc gene encoding the autolytic lysozyme of Clostridium acetobutylicum ATCC824: comparison with other lytic enzymes. Gene. 1991 Jul 31;104(1):25–31. [Abstract] [Google Scholar]
- Cui Z, Mochizuki D, Matsuno Y, Nakamura T, Liu Y, Hatano T, Fukui S, Miyakawa T. Cloning and molecular analysis of cDNA encoding a carboxymethylcellulase of the yeast Cryptococcus flavus. Biosci Biotechnol Biochem. 1992 Aug;56(8):1230–1235. [Abstract] [Google Scholar]
- Cunningham C, McPherson CA, Martin J, Harris WJ, Flint HJ. Sequence of a cellulase gene from the rumen anaerobe Ruminococcus flavefaciens 17. Mol Gen Genet. 1991 Aug;228(1-2):320–323. [Abstract] [Google Scholar]
- Cutfield S, Brooke G, Sullivan P, Cutfield J. Crystallization of the exo(1,3)-beta-glucanase from Candida albicans. J Mol Biol. 1992 May 5;225(1):217–218. [Abstract] [Google Scholar]
- David S, Stevens H, van Riel M, Simons G, de Vos WM. Leuconostoc lactis beta-galactosidase is encoded by two overlapping genes. J Bacteriol. 1992 Jul;174(13):4475–4481. [Europe PMC free article] [Abstract] [Google Scholar]
- Duluc I, Boukamel R, Mantei N, Semenza G, Raul F, Freund JN. Sequence of the precursor of intestinal lactase-phlorizin hydrolase from fetal rat. Gene. 1991 Jul 22;103(2):275–276. [Abstract] [Google Scholar]
- Farber GK, Petsko GA. The evolution of alpha/beta barrel enzymes. Trends Biochem Sci. 1990 Jun;15(6):228–234. [Abstract] [Google Scholar]
- Feller G, Lonhienne T, Deroanne C, Libioulle C, Van Beeumen J, Gerday C. Purification, characterization, and nucleotide sequence of the thermolabile alpha-amylase from the antarctic psychrotroph Alteromonas haloplanctis A23. J Biol Chem. 1992 Mar 15;267(8):5217–5221. [Abstract] [Google Scholar]
- Fernández-Abalos JM, Sánchez P, Coll PM, Villanueva JR, Pérez P, Santamaría RI. Cloning and nucleotide sequence of celA1, and endo-beta-1,4-glucanase-encoding gene from Streptomyces halstedii JM8. J Bacteriol. 1992 Oct;174(20):6368–6376. [Europe PMC free article] [Abstract] [Google Scholar]
- Geber A, Williamson PR, Rex JH, Sweeney EC, Bennett JE. Cloning and characterization of a Candida albicans maltase gene involved in sucrose utilization. J Bacteriol. 1992 Nov;174(21):6992–6996. [Europe PMC free article] [Abstract] [Google Scholar]
- Gebler JC, Aebersold R, Withers SG. Glu-537, not Glu-461, is the nucleophile in the active site of (lac Z) beta-galactosidase from Escherichia coli. J Biol Chem. 1992 Jun 5;267(16):11126–11130. [Abstract] [Google Scholar]
- Gebler J, Gilkes NR, Claeyssens M, Wilson DB, Béguin P, Wakarchuk WW, Kilburn DG, Miller RC, Jr, Warren RA, Withers SG. Stereoselective hydrolysis catalyzed by related beta-1,4-glucanases and beta-1,4-xylanases. J Biol Chem. 1992 Jun 25;267(18):12559–12561. [Abstract] [Google Scholar]
- Gibbs MD, Saul DJ, Lüthi E, Bergquist PL. The beta-mannanase from "Caldocellum saccharolyticum" is part of a multidomain enzyme. Appl Environ Microbiol. 1992 Dec;58(12):3864–3867. [Europe PMC free article] [Abstract] [Google Scholar]
- Gilkes NR, Claeyssens M, Aebersold R, Henrissat B, Meinke A, Morrison HD, Kilburn DG, Warren RA, Miller RC., Jr Structural and functional relationships in two families of beta-1,4-glycanases. Eur J Biochem. 1991 Dec 5;202(2):367–377. [Abstract] [Google Scholar]
- Gilkes NR, Henrissat B, Kilburn DG, Miller RC, Jr, Warren RA. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. [Europe PMC free article] [Abstract] [Google Scholar]
- Haas H, Herfurth E, Stöffler G, Redl B. Purification, characterization and partial amino acid sequences of a xylanase produced by Penicillium chrysogenum. Biochim Biophys Acta. 1992 Oct 27;1117(3):279–286. [Abstract] [Google Scholar]
- Hansen CK, Diderichsen B, Jørgensen PL. celA from Bacillus lautus PL236 encodes a novel cellulose-binding endo-beta-1,4-glucanase. J Bacteriol. 1992 Jun;174(11):3522–3531. [Europe PMC free article] [Abstract] [Google Scholar]
- Hata Y, Kitamoto K, Gomi K, Kumagai C, Tamura G, Hara S. The glucoamylase cDNA from Aspergillus oryzae: its cloning, nucleotide sequence, and expression in Saccharomyces cerevisiae. Agric Biol Chem. 1991 Apr;55(4):941–949. [Abstract] [Google Scholar]
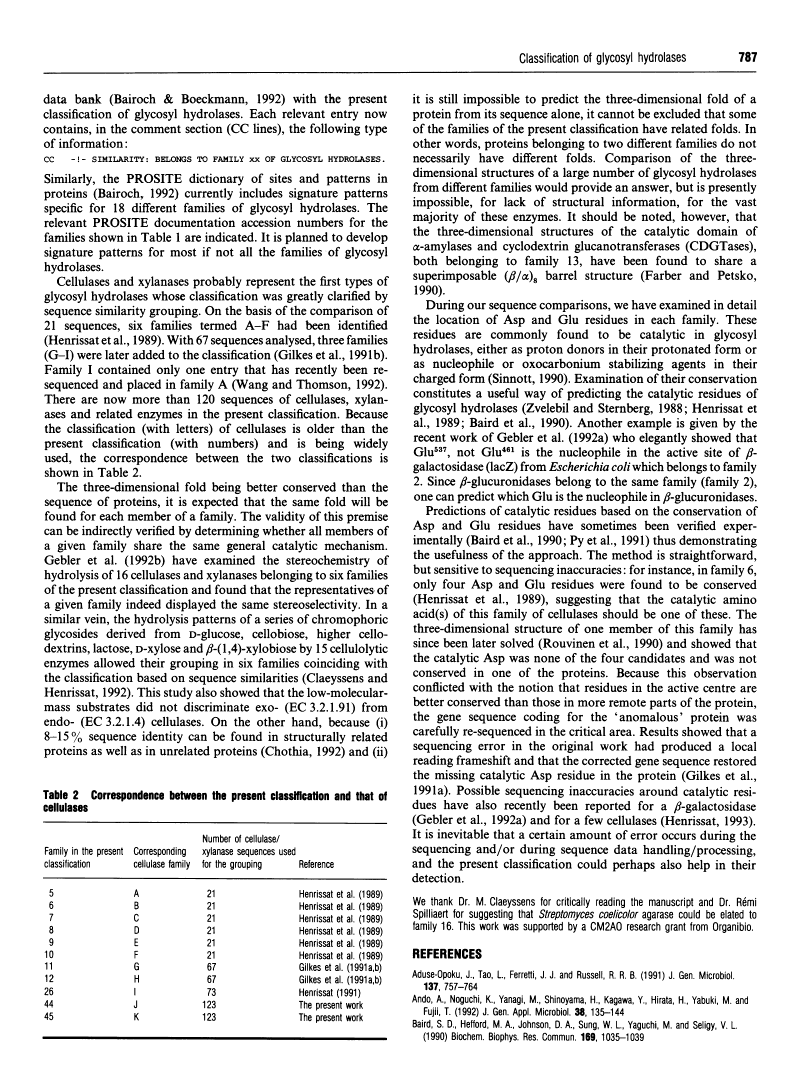
- Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1991 Dec 1;280(Pt 2):309–316. [Europe PMC free article] [Abstract] [Google Scholar]
- Henrissat B. Hidden domains and active site residues in beta-glycanase-encoding gene sequences? Gene. 1993 Mar 30;125(2):199–204. [Abstract] [Google Scholar]
- Henrissat B, Claeyssens M, Tomme P, Lemesle L, Mornon JP. Cellulase families revealed by hydrophobic cluster analysis. Gene. 1989 Sep 1;81(1):83–95. [Abstract] [Google Scholar]
- Hoyer LL, Hamilton AC, Steenbergen SM, Vimr ER. Cloning, sequencing and distribution of the Salmonella typhimurium LT2 sialidase gene, nanH, provides evidence for interspecies gene transfer. Mol Microbiol. 1992 Apr;6(7):873–884. [Abstract] [Google Scholar]
- Hu NT, Hung MN, Huang AM, Tsai HF, Yang BY, Chow TY, Tseng YH. Molecular cloning, characterization and nucleotide sequence of the gene for secreted alpha-amylase from Xanthomonas campestris pv. campestris. J Gen Microbiol. 1992 Aug;138(Pt 8):1647–1655. [Abstract] [Google Scholar]
- Hughes MA, Brown K, Pancoro A, Murray BS, Oxtoby E, Hughes J. A molecular and biochemical analysis of the structure of the cyanogenic beta-glucosidase (linamarase) from cassava (Manihot esculenta Cranz). Arch Biochem Biophys. 1992 Jun;295(2):273–279. [Abstract] [Google Scholar]
- Igarashi K, Ara K, Saeki K, Ozaki K, Kawai S, Ito S. Nucleotide sequence of the gene that encodes a neopullulanase from an alkalophilic Bacillus. Biosci Biotechnol Biochem. 1992 Mar;56(3):514–516. [Abstract] [Google Scholar]
- Ito K, Ikemasu T, Ishikawa T. Cloning and sequencing of the xynA gene encoding xylanase A of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992 Jun;56(6):906–912. [Abstract] [Google Scholar]
- Ito K, Iwashita K, Iwano K. Cloning and sequencing of the xynC gene encoding acid xylanase of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992 Aug;56(8):1338–1340. [Abstract] [Google Scholar]
- Koizuka N, Tanaka Y, Morohashi Y. Isolation of a cDNA Clone for alpha-Amylase in Mung Bean Cotyledons : Analysis of alpha-Amylase mRNA Levels in Cotyledons during and following Germination of Mung Bean Seeds. Plant Physiol. 1990 Nov;94(3):1488–1491. [Abstract] [Google Scholar]
- Lao G, Ghangas GS, Jung ED, Wilson DB. DNA sequences of three beta-1,4-endoglucanase genes from Thermomonospora fusca. J Bacteriol. 1991 Jun;173(11):3397–3407. [Europe PMC free article] [Abstract] [Google Scholar]
- Leah R, Tommerup H, Svendsen I, Mundy J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem. 1991 Jan 25;266(3):1564–1573. [Abstract] [Google Scholar]
- Matsushita O, Russell JB, Wilson DB. A Bacteroides ruminicola 1,4-beta-D-endoglucanase is encoded in two reading frames. J Bacteriol. 1991 Nov;173(21):6919–6926. [Europe PMC free article] [Abstract] [Google Scholar]
- Nitschke L, Heeger K, Bender H, Schulz GE. Molecular cloning, nucleotide sequence and expression in Escherichia coli of the beta-cyclodextrin glycosyltransferase gene from Bacillus circulans strain no. 8. Appl Microbiol Biotechnol. 1990 Aug;33(5):542–546. [Abstract] [Google Scholar]
- Okushima M, Sugino D, Kouno Y, Nakano S, Miyahara J, Toda H, Kubo S, Matsushiro A. Molecular cloning and nucleotide sequencing of the Arthrobacter dextranase gene and its expression in Escherichia coli and Streptococcus sanguis. Jpn J Genet. 1991 Apr;66(2):173–187. [Abstract] [Google Scholar]
- Poch O, L'Hôte H, Dallery V, Debeaux F, Fleer R, Sodoyer R. Sequence of the Kluyveromyces lactis beta-galactosidase: comparison with prokaryotic enzymes and secondary structure analysis. Gene. 1992 Sep 1;118(1):55–63. [Abstract] [Google Scholar]
- Podkovyrov SM, Zeikus JG. Structure of the gene encoding cyclomaltodextrinase from Clostridium thermohydrosulfuricum 39E and characterization of the enzyme purified from Escherichia coli. J Bacteriol. 1992 Aug;174(16):5400–5405. [Europe PMC free article] [Abstract] [Google Scholar]
- Py B, Bortoli-German I, Haiech J, Chippaux M, Barras F. Cellulase EGZ of Erwinia chrysanthemi: structural organization and importance of His98 and Glu133 residues for catalysis. Protein Eng. 1991 Feb;4(3):325–333. [Abstract] [Google Scholar]
- Rasmussen U, Bojsen K, Collinge DB. Cloning and characterization of a pathogen-induced chitinase in Brassica napus. Plant Mol Biol. 1992 Oct;20(2):277–287. [Abstract] [Google Scholar]
- Rixon JE, Ferreira LM, Durrant AJ, Laurie JI, Hazlewood GP, Gilbert HJ. Characterization of the gene celD and its encoded product 1,4-beta-D-glucan glucohydrolase D from Pseudomonas fluorescens subsp. cellulosa. Biochem J. 1992 Aug 1;285(Pt 3):947–955. [Europe PMC free article] [Abstract] [Google Scholar]
- Rouvinen J, Bergfors T, Teeri T, Knowles JK, Jones TA. Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990 Jul 27;249(4967):380–386. [Abstract] [Google Scholar]
- Rumbak E, Rawlings DE, Lindsey GG, Woods DR. Cloning, nucleotide sequence, and enzymatic characterization of an alpha-amylase from the ruminal bacterium Butyrivibrio fibrisolvens H17c. J Bacteriol. 1991 Jul;173(13):4203–4211. [Europe PMC free article] [Abstract] [Google Scholar]
- Schatzle J, Bush J, Cardelli J. Molecular cloning and characterization of the structural gene coding for the developmentally regulated lysosomal enzyme, alpha-mannosidase, in Dictyostelium discoideum. J Biol Chem. 1992 Feb 25;267(6):4000–4007. [Abstract] [Google Scholar]
- Schimming S, Schwarz WH, Staudenbauer WL. Structure of the Clostridium thermocellum gene licB and the encoded beta-1,3-1,4-glucanase. A catalytic region homologous to Bacillus lichenases joined to the reiterated domain of clostridial cellulases. Eur J Biochem. 1992 Feb 15;204(1):13–19. [Abstract] [Google Scholar]
- Shareck F, Roy C, Yaguchi M, Morosoli R, Kluepfel D. Sequences of three genes specifying xylanases in Streptomyces lividans. Gene. 1991 Oct 30;107(1):75–82. [Abstract] [Google Scholar]
- Shen SH, Chrétien P, Bastien L, Slilaty SN. Primary sequence of the glucanase gene from Oerskovia xanthineolytica. Expression and purification of the enzyme from Escherichia coli. J Biol Chem. 1991 Jan 15;266(2):1058–1063. [Abstract] [Google Scholar]
- Shida O, Takano T, Takagi H, Kadowaki K, Kobayashi S. Cloning and nucleotide sequence of the maltopentaose-forming amylase gene from Pseudomonas sp. KO-8940. Biosci Biotechnol Biochem. 1992 Jan;56(1):76–80. [Abstract] [Google Scholar]
- Shirokizawa O, Akiba T, Horikoshi K. Nucleotide sequence of the G6-amylase gene from alkalophilic Bacillus sp. H-167. FEMS Microbiol Lett. 1990 Jul;58(2):131–135. [Abstract] [Google Scholar]
- Simmons CR, Litts JC, Huang N, Rodriguez RL. Structure of a rice beta-glucanase gene regulated by ethylene, cytokinin, wounding, salicylic acid and fungal elicitors. Plant Mol Biol. 1992 Jan;18(1):33–45. [Abstract] [Google Scholar]
- Stoltzfus LJ, Sosa-Pineda B, Moskowitz SM, Menon KP, Dlott B, Hooper L, Teplow DB, Shull RM, Neufeld EF. Cloning and characterization of cDNA encoding canine alpha-L-iduronidase. mRNA deficiency in mucopolysaccharidosis I dog. J Biol Chem. 1992 Apr 5;267(10):6570–6575. [Abstract] [Google Scholar]
- Sumitomo N, Ozaki K, Kawai S, Ito S. Nucleotide sequence of the gene for an alkaline endoglucanase from an alkalophilic Bacillus and its expression in Escherichia coli and Bacillus subtilis. Biosci Biotechnol Biochem. 1992 Jun;56(6):872–877. [Abstract] [Google Scholar]
- Tang LB, Lenstra R, Borchert TV, Nagarajan V. Isolation and characterization of levansucrase-encoding gene from Bacillus amyloliquefaciens. Gene. 1990 Nov 30;96(1):89–93. [Abstract] [Google Scholar]
- Tarentino AL, Quinones G, Schrader WP, Changchien LM, Plummer TH., Jr Multiple endoglycosidase (Endo) F activities expressed by Flavobacterium meningosepticum. Endo F1: molecular cloning, primary sequence, and structural relationship to Endo H. J Biol Chem. 1992 Feb 25;267(6):3868–3872. [Abstract] [Google Scholar]
- Tsujibo H, Sakamoto T, Miyamoto K, Hasegawa T, Fujimoto M, Inamori Y. Amino acid compositions and partial sequences of xylanases from a new subspecies, Nocardiopsis dassonvillei subsp. alba OPC-18. Agric Biol Chem. 1991 Aug;55(8):2173–2174. [Abstract] [Google Scholar]
- Tsujibo H, Miyamoto K, Kuda T, Minami K, Sakamoto T, Hasegawa T, Inamori Y. Purification, properties, and partial amino acid sequences of thermostable xylanases from Streptomyces thermoviolaceus OPC-520. Appl Environ Microbiol. 1992 Jan;58(1):371–375. [Europe PMC free article] [Abstract] [Google Scholar]
- Utt EA, Eddy CK, Keshav KF, Ingram LO. Sequencing and expression of the Butyrivibrio fibrisolvens xylB gene encoding a novel bifunctional protein with beta-D-xylosidase and alpha-L-arabinofuranosidase activities. Appl Environ Microbiol. 1991 Apr;57(4):1227–1234. [Europe PMC free article] [Abstract] [Google Scholar]
- van Kan JA, Joosten MH, Wagemakers CA, van den Berg-Velthuis GC, de Wit PJ. Differential accumulation of mRNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol Biol. 1992 Nov;20(3):513–527. [Abstract] [Google Scholar]
- Vercoe PE, Gregg K. DNA sequence and transcription of an endoglucanase gene from Prevotella (Bacteroides) ruminicola AR20. Mol Gen Genet. 1992 May;233(1-2):284–292. [Abstract] [Google Scholar]
- Wright RM, Yablonsky MD, Shalita ZP, Goyal AK, Eveleigh DE. Cloning, characterization, and nucleotide sequence of a gene encoding Microbispora bispora BglB, a thermostable beta-glucosidase expressed in Escherichia coli. Appl Environ Microbiol. 1992 Nov;58(11):3455–3465. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu WZ, Shima Y, Negoro S, Urabe I. Sequence and properties of beta-xylosidase from Bacillus pumilus IPO. Contradiction of the previous nucleotide sequence. Eur J Biochem. 1991 Dec 18;202(3):1197–1203. [Abstract] [Google Scholar]
- Zvelebil MJ, Sternberg MJ. Analysis and prediction of the location of catalytic residues in enzymes. Protein Eng. 1988 Jul;2(2):127–138. [Abstract] [Google Scholar]
- Zverlov VV, Laptev DA, Tishkov VI, Velikodvorskaja GA. Nucleotide sequence of the Clostridium thermocellum laminarinase gene. Biochem Biophys Res Commun. 1991 Dec 16;181(2):507–512. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2930781
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1134435?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Structural studies of β-glucosidase from the thermophilic bacterium Caldicellulosiruptor saccharolyticus.
Acta Crystallogr D Struct Biol, 80(pt 10):733-743, 01 Oct 2024
Cited by: 0 articles | PMID: 39361356 | PMCID: PMC11448918
Probing the function of C-terminal region of recombinant α-amylase BmaN1 from <i>Bacillus megaterium</i> NL3.
Microbiol Spectr, 12(10):e0335123, 30 Aug 2024
Cited by: 0 articles | PMID: 39212453 | PMCID: PMC11448133
Genome-Wide Identification of a Maize Chitinase Gene Family and the Induction of Its Expression by Fusarium verticillioides (Sacc.) Nirenberg (1976) Infection.
Genes (Basel), 15(8):1087, 17 Aug 2024
Cited by: 1 article | PMID: 39202446 | PMCID: PMC11353892
Dual functionality of pathogenesis-related proteins: defensive role in plants versus immunosuppressive role in pathogens.
Front Plant Sci, 15:1368467, 02 Aug 2024
Cited by: 0 articles | PMID: 39157512 | PMCID: PMC11327054
Fluorinated indeno-quinoxaline bearing thiazole moieties as hypoglycaemic agents targeting α-amylase, and α-glucosidase: synthesis, molecular docking, and ADMET studies.
J Enzyme Inhib Med Chem, 39(1):2367128, 24 Jun 2024
Cited by: 1 article | PMID: 38913598 | PMCID: PMC467095
Go to all (975) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A classification of glycosyl hydrolases based on amino acid sequence similarities.
Biochem J, 280 ( Pt 2):309-316, 01 Dec 1991
Cited by: 1517 articles | PMID: 1747104 | PMCID: PMC1130547
Structural and sequence-based classification of glycoside hydrolases.
Curr Opin Struct Biol, 7(5):637-644, 01 Oct 1997
Cited by: 829 articles | PMID: 9345621
Review
Updating the sequence-based classification of glycosyl hydrolases.
Biochem J, 316 ( Pt 2):695-696, 01 Jun 1996
Cited by: 637 articles | PMID: 8687420 | PMCID: PMC1217404
beta-fructosidase superfamily: homology with some alpha-L-arabinases and beta-D-xylosidases.
Proteins, 42(1):66-76, 01 Jan 2001
Cited by: 28 articles | PMID: 11093261