Abstract
Free full text

Association of Csk to VE-cadherin and inhibition of cell proliferation
Abstract
Vascular endothelial cadherin (VE-cadherin) mediates contact inhibition of cell growth in quiescent endothelial cell layers. Searching for proteins that could be involved in VE-cadherin signaling, we found the cytosolic C-terminal Src kinase (Csk), a negative regulator of Src family kinases. We show that Csk binds via its SH2 domain to the phosphorylated tyrosine 685 of VE-cadherin. VE-cadherin recruits Csk to cell contacts and both proteins can be co-precipitated from cell lysates of transfected cells and endothelial cells. Association of VE-cadherin and Csk in endothelial cells increased with increasing cell density. CHO cells expressing the tyrosine replacement mutant VE-cadherin-Y685F grow to higher cell densities than cells expressing wild-type VE-cadherin. Overexpression of Csk in these cells under an inducible promoter inhibits cell proliferation in the presence and absence of VE-cadherin, but not in the presence of VE-cadherin-Y685F. Reduction of Csk expression by RNA interference enhances endothelial cell proliferation. Our results suggest that the phosphorylated tyrosine residue 685 of VE-cadherin and probably the binding of Csk to this site are involved in inhibition of cell growth triggered by cell density.
Introduction
Intact endothelium in adult blood vessels has a low turnover rate. If junctions are disrupted, the endothelium is able to proliferate, a process that slows down again if cell–cell contacts are reformed (Schwartz et al, 1981). Thus, stable endothelial cell contacts create signals that counteract cell proliferation.
An adhesion molecule of major importance for the formation and stability of interendothelial cell contacts is the endothelial-specific cadherin called vascular endothelial cadherin (VE-cadherin). Contact inhibition of the growth of endothelial cells was suggested to be mediated by VE-cadherin (Caveda et al, 1996; Rahimi and Kazlauskas, 1999). Like other members of the cadherin family, VE-cadherin binds to cytosolic proteins located at the plasma membrane, the catenins. β-Catenin and γ-catenin (plakoglobin) can link cadherins to α-catenin, which anchors the complex to the actin cytoskeleton. Linkage of the cadherins to the cytoskeleton is thought to be an important requisite for the control of cell contact stability. In addition, β-catenin and, under certain conditions, also γ-catenin can translocate to the nucleus and modulate transcriptional activities (Hecht and Kemler, 2000; Gottardi and Gumbiner, 2001; Giles et al, 2003).
Among the many interesting target genes for the transcription modulating activity of β-catenin, there are several genes important in cell cycle regulation (Polakis, 2000; Conacci-Sorrell et al, 2002), suggesting that β-catenin can act as an oncogene. It is an attractive hypothesis that the binding of cadherins to β-catenin would reduce the amount of cytosolic β-catenin moieties available for signaling to the nucleus and therefore would negatively influence expression of cell cycle-stimulating factors. By such mechanisms, E- and N-cadherin could be implicated in the regulation of cell growth in various tumor cell lines (St Croix et al, 1998; Levenberg et al, 1999; Gottardi et al, 2001; Stockinger et al, 2001). The mechanism was found to involve cell cycle arrest in G1 phase as well as elevation of the cyclin D1-dependent kinase inhibitor p27kip1 and the late reduction of cyclin D1.
Recently, an alternative mechanism for the growth inhibitory activity of VE-cadherin was published (Lampugnani et al, 2003). According to this report, endothelial cells required β-catenin and the density enhanced phosphatase (DEP-1), a receptor-tyrosine protein phosphatase (R-PTP), for contact inhibition of VEGF-stimulated cell growth. VEGF stimulated the association of VEGFR-2 with the VE-cadherin/β-catenin complex in confluent but not in sparse cells, and this association was concluded to counteract growth stimulatory effects of VEGFR-2, possibly via dephosphorylation of the receptor by DEP-1 (Lampugnani et al, 2003).
Src family kinases (SFKs) are involved in VEGFR-2 signaling and have been involved in the regulation of vascular permeability, angiogenesis (Eliceiri et al, 1999; Weis et al, 2004), cell motility and apoptosis (Abu-Ghazaleh et al, 2001). It is less clear whether SFKs are involved in the proliferation of endothelial cells. Yet, it is well established that elevated Src expression and activity are associated with proliferative disorders and a variety of cancers (Frame, 2002). The protein tyrosine kinase (PTK) Csk (C-terminal Src kinase) is a potent negative regulator of SFKs due to its ability to inactivate these kinases by phosphorylating a negative regulatory tyrosine (Latour and Veillette, 2001). The role of SFKs and of Csk in cell proliferation and apoptosis during embryonal development was recently demonstrated in Drosophila (Pedraza et al, 2004). In mice, Csk deficiency causes embryonal lethality (Imamoto and Soriano, 1993). The analysis of chimeric mouse embryos containing wild-type (wt) and Csk−/− cells allowed the detection of defects in angiogenic sprouting and vascular remodeling (Duan et al, 2004).
In this paper, we have identified Csk as a direct binding partner of VE-cadherin. The SH2 domain of Csk is known to interact with few tyrosine-phosphorylated molecules presumed to allow targeting of Csk to sites of SFK activation (Leo and Schraven, 2001). We show here that Csk binds to VE-cadherin and identify the binding sites. Csk expressed in CHO cells under an inducible promoter slowed down cell proliferation independent of the expression of wt-VE-cadherin, yet no such effect was observed in cells expressing the Y685F mutant of VE-cadherin. In addition, CHO cells expressing mutant VE-cadherin grew to higher cell densities than if they expressed wt-VE-cadherin. Importantly, more Csk bound to VE-cadherin with increasing endothelial cell density. Finally, blocking Csk expression in primary endothelial cells by short interfering RNA (siRNA) attenuated contact inhibition of cell proliferation. Our results suggest that recruitment of Csk to tyrosine-phosphorylated VE-cadherin may be involved in VE-cadherin-mediated inhibition of cell proliferation.
Results
Identification of Csk as binding partner of VE-cadherin and determination of tyrosine 685 as the binding site
In order to gain insights into the role of tyrosine phosphorylation of VE-cadherin, we attempted to identify proteins that associate specifically with the tyrosine-phosphorylated cytoplasmic tail of VE-cadherin. To this end, we performed a modified yeast two-hybrid screen (Schaeper et al, 2000; Fujita et al, 2002). To induce tyrosine phosphorylation of the cytoplasmic domain of VE-cadherin in yeast, most of this domain (aa 621–764) was fused to TPR-Met, a constitutively active form of the tyrosine kinase c-Met. Using our LexA-TPR-Met-VE-cadherin fusion protein, we screened a mouse embryonic cDNA library and isolated a cDNA coding for a part of the open reading frame of murine Csk ranging from aa 57 to 204 (Figure 1A). This fragment covered the complete SH2 domain of Csk but lacked most of the SH3 and the kinase domain.
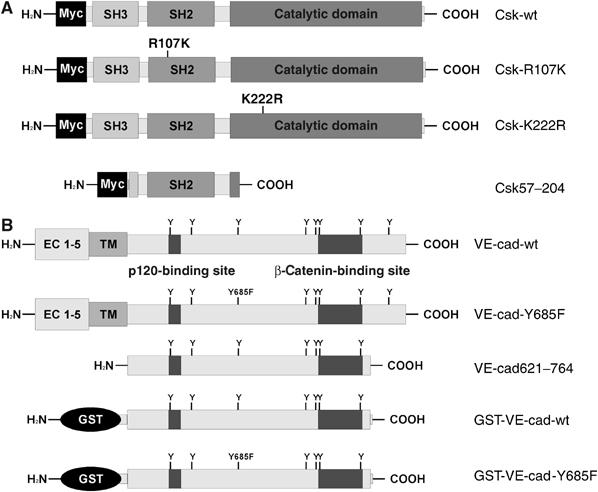
Designation of recombinant forms of Csk and VE-cadherin. (A) All forms of Csk carried a Myc tag at the N-terminus. Besides the wt form (Csk-wt), two mutants were generated, one with arginine 107 in the SH2 domain replaced by lysine (Csk-R107K) and the other with lysine 222 in the kinase domain replaced by an arginine (Csk-K222R). The truncated form Csk57–204 was coded by the cDNA fragment picked in the yeast two-hybrid screen with a VE-cadherin bait construct. (B) The following forms of VE-cadherin are depicted: wt form of VE-cadherin (VE-cad-wt), a tyrosine replacement mutant of full-length VE-cadherin (VE-cad-Y685F), a fragment of the cytoplasmic tail of VE-cadherin (VE-cad621–764), which was inserted in the yeast two-hybrid bait construct (see Materials and methods for details) and two GST fusion proteins containing the same part of the cytoplasmic tail of VE-cadherin as the bait construct. One of the two GST fusion proteins contained the Y685F point mutation. The binding sites for p120 and β-catenin are demarcated in analogy to the binding sites for E-cadherin.
To verify the binding directly, we used a bacterial glutathione S-transferase (GST)-based fusion protein containing the cytoplasmic tail of VE-cadherin (aa 621–764) in pull-down experiments with Csk synthesized in reticulocyte lysates by in vitro transcription/translation reactions. As shown in Figure 2, the originally cloned Csk fragment and full-length Csk were pulled down by GST-VE-cadherin only if the fusion protein had been preincubated with recombinant c-Src. No binding was observed if this kinase reaction was omitted (Figure 2).
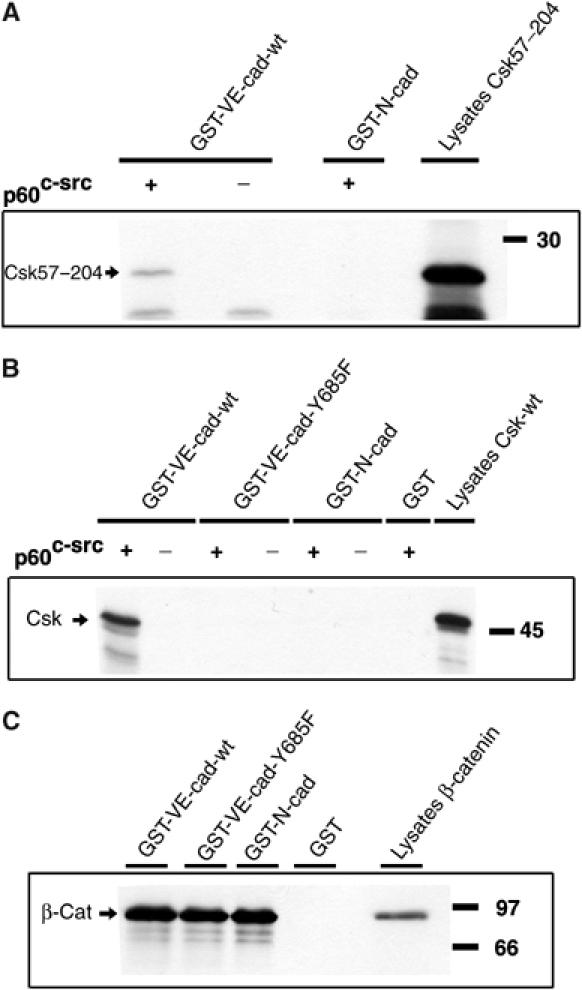
In vitro-translated Csk can be precipitated with a GST-VE-cadherin fusion protein. Either the Csk57–204 fragment identified in the yeast two-hybrid screen (A) or full-length Csk (Csk-wt) (B) was synthesized in a coupled in vitro transcription/translation reaction and precipitation was tested with the indicated GST-VE-cadherin (see Figure 1) and the GST-N-cadherin (GST-N-cad) fusion proteins. An aliquot of the translation reaction is shown in the right lane. The intactness of the GST-cadherin fusion proteins was tested in pull-down experiments with in vitro-translated β-catenin (C). Molecular mass markers are indicated on the right.
The SH2 domain was the only intact domain in the originally identified Csk fragment (Figure 1). Since this domain was suggested to specifically interact with tyrosine residues in the context of the motif pY(T/A/S)X(M/I/V) (Songyang et al, 1994), we searched for this motif in the cytoplasmic tail of VE-cadherin. Out of eight tyrosine residues, tyrosine 685 was the only one embedded in this motif (YTQV). Based on this, we generated a GST-VE-cadherin fusion protein with tyrosine 685 replaced by phenylalanine. This protein did not bind to the Csk57–204 fragment or to full-length Csk even if it was preincubated with c-Src (Figure 2). No binding was seen with recombinant GST alone or with a c-Src preincubated GST-N-cadherin fusion protein containing the complete cytoplasmic tail of N-cadherin. The GST-N-cadherin and GST-VE-cadherin-Y685F were intact and functional, since they could pull down in vitro-translated β-catenin as efficiently as the VE-cad-wt fusion protein (Figure 2). We conclude that Csk binds specifically to tyrosine-phosphorylated VE-cadherin, but not to N-cadherin and that this binding requires tyrosine 685.
Csk and VE-cadherin can be co-precipitated when transfected into CHO cells or endogenously expressed in endothelial cells
To analyze interactions of VE-cadherin with Csk in mammalian cells, we generated two double-transfected CHO cells, each expressing Myc-tagged Csk from an inducible promoter, and one expressing constitutively full-length wt-VE-cadherin and the other full-length VE-cadherin-Y685F. Induction of Csk was based on the activation of a Gal-4-based chimeric transcription factor with the progesterone antagonist mifepristone. Induced Myc-tagged Csk had a slightly higher molecular weight than endogenous Csk (Figure 3A, bottom panel).
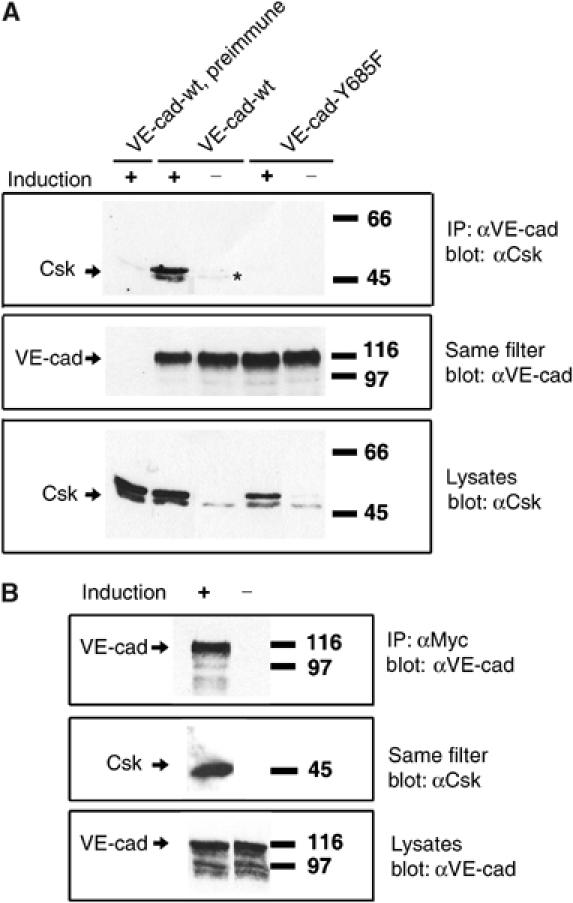
Csk can be co-immunoprecipitated together with VE-cadherin from CHO cell lysates. (A) CHO cells stably cotransfected with inducible Csk and either wt-VE-cadherin or the tyrosine replacement mutant (as indicated above) were subjected to immunoprecipitations with polyclonal preimmune antibodies (preimmune) or affinity-purified anti-VE-cadherin antibodies, and precipitates were blotted with antibodies against Csk (upper panel) or VE-cadherin (middle panel). Aliquots of cell lysates were directly analyzed in immunoblots for expression of Csk (bottom panel). Induction of exogenous Csk expression (Induction) is indicated above. Note that transfected, Myc-tagged Csk has a slightly higher apparent molecular weight than endogenous Csk. The asterisk marks weakly co-precipitated endogenous Csk. (B) CHO cells stably cotransfected with inducible Csk and constitutively expressed wt-VE-cadherin were subjected to immunoprecipitations with anti-Myc-tag antibodies (α-Myc) and precipitates were blotted with antibodies against VE-cadherin (upper panel) or Csk (middle panel). Aliquots of cell lysates were directly analyzed in immunoblots for expression of VE-cadherin (bottom panel). Molecular mass markers are indicated on the right.
The association of VE-cadherin and Csk was tested by immunoprecipitating VE-cadherin from these cells and immunoblotting the precipitate for mouse Csk. Co-precipitation of Csk was strongly detected if Csk was induced (Figure 3A, top panel) and weakly detected when endogenous Csk was analyzed (Figure 3A, third lane). In contrast, immunoprecipitates of VE-cadherin-Y685F did not contain any Csk even when Csk was induced. Co-precipitation of Csk and VE-cadherin could also be demonstrated if Myc-Csk was immunoprecipitated with an anti-Myc mAb and VE-cadherin was detected in immunoblots (Figure 3B).
Co-precipitation of Csk and VE-cadherin was also found with bEnd.3 mouse endothelioma cells and with primary isolated human endothelial cells (HUVECs), endogenously expressing VE-cadherin and Csk (Figure 4). Importantly, association clearly increased with increasing cell density, whereas the total level of VE-cadherin and Csk was unchanged (Figure 4C).
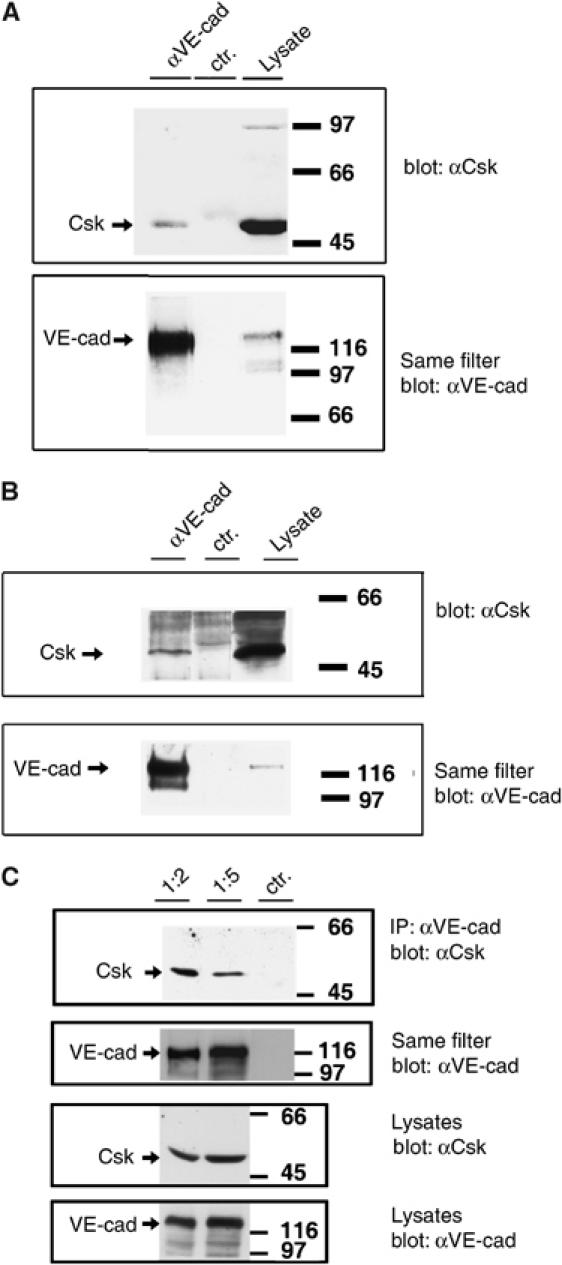
Endogenously expressed Csk can be co-precipitated with VE-cadherin from lysates of endothelial cells and association increases with cell density. (A, C) Mouse bEnd.3 endothelioma cells and (B) HUVECs were subjected to immunoprecipitations with (A, C) affinity-purified polyclonal anti-mouse VE-cadherin antibodies (αVE-cad) and preimmune antibodies (ctr). (B) mAb against human VE-cadherin (αVE-cad) and an irrelevant class-matched mAb (ctr). Precipitates were analyzed in immunoblots with anti-Csk antibodies and the same filters were re-analyzed with anti-VE-cadherin antibodies (as indicated). Expression levels of Csk and VE-cadherin were analyzed by immunoblotting cell lysates (lysates). (C) Two different cell densities were plated by splitting confluent monolayers 1:2 or 1:5. Cell lysates containing identical protein amounts were analyzed. Molecular mass markers are indicated on the right.
Csk enhances tyrosine phosphorylation of VE-cadherin in COS-7 cells: involvement of the SH2 domain but not the kinase domain
We tested whether Csk would affect the tyrosine phosphorylation of VE-cadherin. Indeed, we found that VE-cadherin precipitated from COS7 cells cotransfected with Csk was strongly labeled in immunoblots with a phospho-tyrosine-specific antibody, whereas tyrosine phosphorylation of VE-cadherin was almost undetectable in COS7 cells cotransfected with a vector control (Figure 5A).
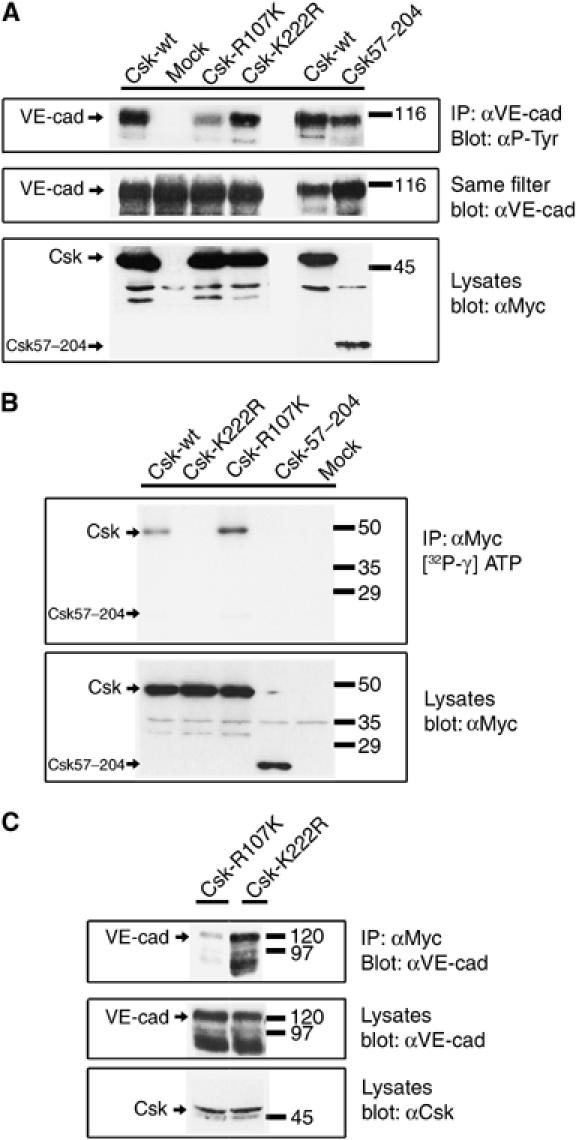
Coexpression of Csk with VE-cadherin in COS-7: upregulation of tyrosine phosphorylation requires SH2 domain, but not kinase domain. (A) COS-7 cells were transiently cotransfected with VE-cadherin and various recombinant forms of Csk as indicated above. Cell lysates were immunoprecipitated with anti-VE-cadherin antibodies and precipitates were analyzed in immunoblots first with anti-phospho-tyrosine antibodies (αP-Tyr) and then (on the same filter) with anti-VE-cadherin antibodies (αVE-cad). Expression of the various forms of Csk was controlled in immunoblots of cell lysates (bottom panel). Note that the Csk-SH2 domain mutant (Csk-R107K) caused strongly reduced tyrosine phosphorylation of VE-cadherin, whereas the Csk kinase mutant (Csk-K222R) was not impaired in its ability to stimulate tyrosine phosphorylation of VE-cadherin. (B) Analysis of the kinase activity of Csk mutants. Various recombinant forms of Csk were expressed in COS-7 cells, immunoprecipitated with anti-Myc-tag antibodies and the kinase activity of the precipitated proteins was analyzed by incubation with [32P-γ]ATP, followed by gel electrophoresis and autoradiography (upper panel). Efficiency of immunoprecipitations was controlled by analyzing immunoblots of equivalent aliquots of the precipitates with anti-Myc-tag antibodies (lower panel). Note that no kinase activity was detectable for the kinase point mutation Csk-K222R. (C) Analysis of the association of the two Csk point mutants with VE-cadherin: COS-7 cells were transfected with VE-cadherin and in addition either with the Csk-R107K SH2 domain mutant or with the Csk-K222R kinase mutant and cell lysates were immunoprecipitated with anti-Myc-tag antibodies. Precipitates were analyzed in immunoblots with anti-VE-cadherin antibodies (upper panel). Expression levels of VE-cadherin and Csk were tested in immunoblots of cell lysates (middle and bottom panels). Note that binding of the SH2 domain mutant to VE-cadherin was strongly but not completely inhibited. Molecular mass markers are indicated on the right.
To address the question of whether the enhanced phosphorylation of VE-cadherin was due to the kinase activity of Csk or simply due to the binding of Csk covering and protecting phosphate groups from removal by phosphatases, we constructed two different mutant forms of Csk, one of them carrying a mutation in the kinase domain (Csk-K222R) and the other a mutation in the SH2 domain (Csk-R107K). The effect of these mutations on the function of the respective domains was tested in kinase and binding assays. Indeed, the kinase mutant, but not the SH2 mutant, had lost its autophosphorylation activity (Figure 5B). Likewise, the SH2 mutant but not the kinase mutant was strongly reduced in its binding activity, as tested in co-precipitation experiments (Figure 5C). The strong but not complete reduction of the binding capacity of the SH2 mutant is in line with a previous report (Lemay et al, 2000).
We then assessed the ability of the two Csk mutants to enhance phosphorylation of VE-cadherin upon coexpression in COS-7 cells. As shown in Figure 5A, the kinase mutant stimulated tyrosine phosphorylation of VE-cadherin as well as wt-Csk, whereas the SH2 mutant caused a much weaker increase in phosphorylation (Figure 5A). Truncated Csk57–204 containing the SH2 domain as the only intact domain and lacking the kinase domain (see Figure 1) could efficiently stimulate phosphorylation of VE-cadherin. We conclude that the stimulatory effect of Csk on tyrosine phosphorylation of VE-cadherin is not based on the kinase activity of Csk, but rather depends on the protection of the phosphorylation of VE-cadherin due to the binding of Csk.
VE-cadherin recruits Csk to cell contacts
The interaction between VE-cadherin and Csk prompted us to determine the subcellular distribution of Csk. We found Csk strongly stained at cell contacts in CHO cells expressing wt-VE-cadherin, whereas much less was detected in cells expressing VE-cadherin-Y685F (Figure 6), indicating recruitment of Csk by VE-cadherin. Residual staining of Csk was probably due to other Csk-binding membrane proteins. No staining was seen if pervanadate (PV) treatment of cells was omitted (not shown), arguing for a very short half-life of tyrosine 685 phosphorylation. Expression levels of inducible Csk in both CHO transfectants were similar (Supplementary Figure 1).
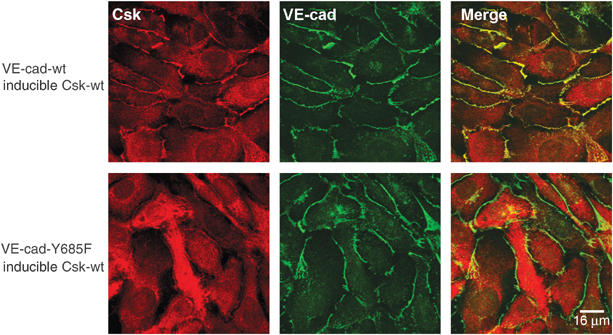
VE-cadherin recruits Csk to cell contacts. CHO cells stably transfected with Csk under an inducible promoter and either coexpressing wt-VE-cadherin (upper row) or the tyrosine mutant VE-cad-Y685F (bottom row) were analyzed by indirect immunofluorescence staining for the localization of Csk (red, left column) and VE-cadherin (green, middle column). The merge is shown in the right column. In all cases, Csk expression was induced. Bar=16 μm.
Induction of Csk inhibits proliferation in CHO cells, but not if VE-cadherin-Y685F is present
Since VE-cadherin is able to inhibit proliferation, we tested whether the binding site for Csk on VE-cadherin was involved. We analyzed whether induction of Csk in our double transfectants expressing either wt- or mt-VE-cadherin, and in CHO cells not expressing VE-cadherin would affect incorporation of [3H]thymidine. Two independent clones for each transfectant were analyzed. Proliferation was reduced by Csk induction in cells expressing or lacking wt-VE-cadherin, whereas no reduction was seen with cells expressing mt-VE-cadherin-Y685F (Figure 7). Since VE-cadherin recruits Src in endothelial cells (Lambeng et al, 2005), a result that we confirmed (not shown), it is conceivable that Src molecules recruited to VE-cadherin are not accessible to Csk, if tyrosine 685 in VE-cadherin is mutated. Indeed, we have found that induction of Csk phosphorylates (deactivates) Src in cells lacking or expressing wt-VE-cadherin, but not in cells expressing mt-VE-cadherin (Supplementary Figure 2). Induction of the kinase mutant Csk-K222R in VE-cadherin-expressing double transfectants did not reduce proliferation (Figure 7). We conclude that VE-cadherin lacking its Csk-binding site interferes with the proliferation inhibitory effect of Csk and that this effect requires the kinase activity of Csk.
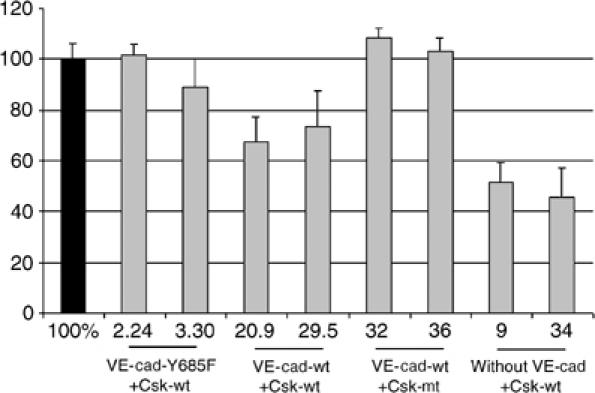
Induction of Csk in transfected CHO cells slows down cell proliferation only if wt-VE-cadherin is coexpressed. CHO cells expressing inducible wt form of Csk (Csk-wt) or the kinase mutant of Csk (Csk-mt) were cotransfected with wt form of VE-cadherin (VE-cad-wt), or VE-cadherin-Y685F (VE-cad-Y685F) or without VE-cadherin (without VE-cad), and were analyzed for [3H]thymidine incorporation. In each case, two different transfected clones were analyzed. For each clone, [3H]thymidine incorporation of cells not induced for Csk expression was set as 100% (black bar). Measurements were carried out with cells at early confluence, resembling the stage between 72 and 96 h growth in Figure 8. At this stage, [3H]thymidine incorporation of wt-VE-cadherin and mt-VE-cadherin cells in the absence of Csk induction was similar (within a range of 15%, not shown), in agreement with the results in Figure 8.
VE-cadherin-mediated contact inhibition in CHO cells requires tyrosine 685
If the binding of Csk to VE-cadherin was relevant for VE-cadherin-mediated contact inhibition of growth, cells expressing wt-VE-cadherin-Y685F should stop growing at lower cell densities than cells expressing mt-VE-cadherin. To test this, we plated identical cell numbers of two transfected CHO clones of each of the wt-VE-cadherin- and the mt-VE-cadherin-expressing transfectants without inducing Csk and determined cell numbers after various time intervals. As shown in Figure 8, at very high densities, cell numbers increased further only if cells expressed mt-VE-cadherin whereas they stagnated if cells expressed wt-VE-cadherin. Induction of Csk slowed down the proliferation rate in wt-VE-cadherin cells, resulting in a reduction of cell numbers (1.8-fold at 72 h, 2.3-fold at 96 h and 3.1-fold at 120 h). No inhibition of cell growth was seen by inducing Csk in mt-VE-cadherin cells at any time point. We conclude that the binding site for Csk in the cytoplasmic portion of VE-cadherin is involved in the cell density-dependent growth inhibitory activity of VE-cadherin.
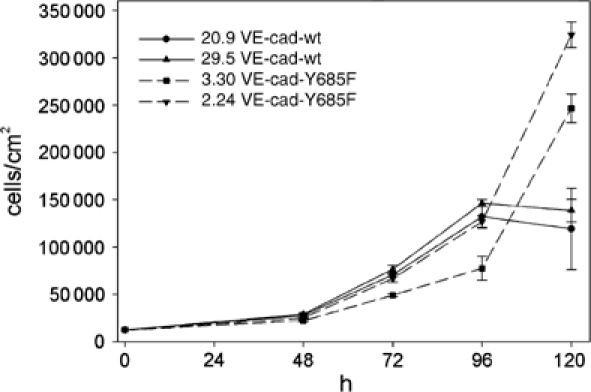
CHO cells expressing mutant VE-cadherin-Y685-F grow to higher cell densities than CHO cells expressing wt-VE-cadherin. Identical cell numbers of the two CHO clones 20.9 and 29.5 each transfected with wt-VE-cadherin and inducible Csk and the two CHO clones 3.30 and 2.24 each transfected with mutant VE-cadherin-Y685-F and inducible Csk were plated and cells were harvested and counted at the indicated time points (in hours). Cell densities are given on the left as cells per cm2. Note that these cells were not induced for exogenous Csk expression and the effects shown are based on endogenous Csk expression levels.
Inhibition of Csk expression in HUVECs by siRNA enhances cell proliferation
In order to determine whether Csk is involved in growth control of endothelial cells, we inhibited the expression of Csk in primary isolated HUVECs. Transfection of HUVECs with siRNA targeting Csk strongly reduced expression of Csk (Figure 9A), whereas no effect was seen with two negative control siRNAs, of which only one is shown in Figure 9A. Reduction of Csk expression with the Csk-directed siRNA was analyzed by immunoblotting at 24 h and at 48 h after transfection.
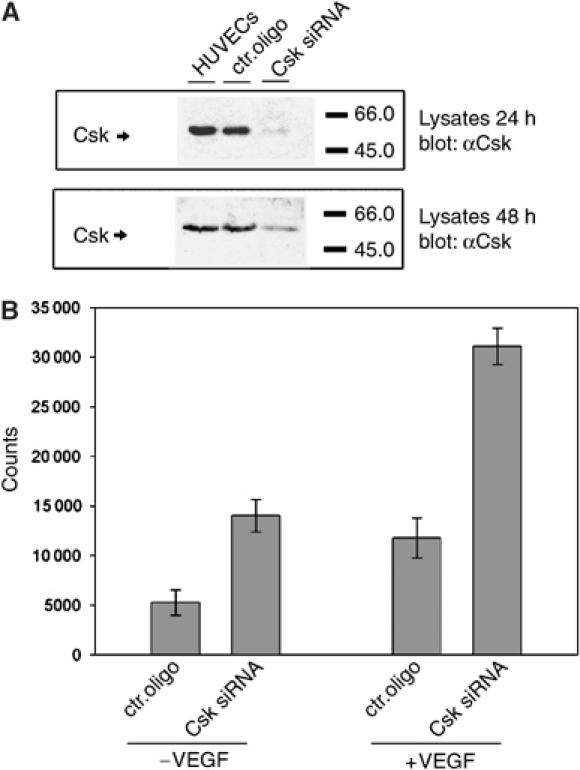
Downregulation of Csk by siRNA increases cell proliferation of HUVECs. (A) Immunoblot analysis for Csk expression of lysates of HUVECs that had been transfected with siRNA against an irrelevant mouse antigen (ctr. oligo) or with siRNA targeting Csk (Csk siRNA) or not transfected (HUVECs). Results are shown at 24 and 48 h after transfection (as indicated). (B) HUVECs were either transfected with siRNA against an irrelevant mouse antigen (ctr. oligo) or with a Csk-specific siRNA (Csk siRNA) and incorporation of [3H]thymidine into cells was measured either in the absence (−VEGF) or presence (+VEGF) of added VEGF.
Proliferation of transfected HUVECs was analyzed by determining incorporation of [3H]thymidine in confluent cell monolayers. We found that inhibition of the expression of Csk by siRNA enhanced proliferation of HUVECs in comparison to two different negative control siRNA, of which only one is shown in Figure 9B. This increase was observed with cells deprived of growth factors and with VEGF-stimulated cells.
Discussion
We have found the cytosolic kinase Csk as a specific binding partner of tyrosine-phosphorylated VE-cadherin. Identification of tyrosine residue 685 as the binding site for Csk on VE-cadherin allowed us to analyze VE-cadherin-dependent functions of Csk. Induction of Csk in CHO cells slowed down cell proliferation in the presence or absence of wt-VE-cadherin, but not if the cells expressed the Y685F mutant. In addition, CHO cells expressing mutant VE-cadherin grew to higher cell densities than cells expressing wt-VE-cadherin, demonstrating that tyrosine 685 in VE-cadherin is necessary for the growth inhibitory effect of VE-cadherin in these cells. Although we cannot formally rule out other binding partners for tyrosine 685 in VE-cadherin, it is likely that it is the association of Csk to this site that is involved in contact inhibition. Furthermore, inhibiting the expression of endogenous Csk in primary human endothelial cells by siRNA enhanced cell proliferation. Finally, association of VE-cadherin with Csk increased with higher cell density. Together, our results demonstrate that Csk interacts with tyrosine 685-phosphorylated VE-cadherin in cells and suggest that this interaction is involved in contact inhibition of endothelial cell growth.
Cadherin-driven inhibition of cell proliferation was already described for E- and N-cadherin in tumor cells (St Croix et al, 1998; Levenberg et al, 1999; Gottardi et al, 2001; Stockinger et al, 2001), involving elevation of p27kip1 and late reduction of cyclin D1, leading to cell cycle arrest at G1 phase. Since β-catenin can stimulate transcription of cell proliferation-supporting proteins, a possible mechanism for the growth-retarding function of E- and N-cadherin would be to sequester β-catenin from the nucleus. Whether the disengagement of cadherin–cadherin contacts would increase the β-catenin pool available for nuclear functions is less clear. In fact, no dissociation of the E-cadherin–β-catenin complex was found upon blocking cell contacts with anti-E-cadherin antibodies, although the antibodies restored cell growth (St Croix et al, 1998). Similarly, antibodies against VE-cadherin blocked cell contacts and abolished contact inhibition of cell growth, yet this did not lead to dissociation of β-catenin from VE-cadherin (Caveda et al, 1996; Corada et al, 1999).
Recently, an alternative mechanism for VE-cadherin-mediated inhibition of cell proliferation was found, and this requires the association of VE-cadherin with β-catenin, but not the transcriptional activity of β-catenin (Lampugnani et al, 2003). The growth inhibitory effect of VE-cadherin and its association with VEGFR-2 were abolished if the endothelial cells lacked β-catenin or if VE-cadherin lacked its β-catenin-binding site. Since VE-cadherin-mediated growth inhibition was dependent on cell confluence and VEGF induced association of VEGFR-2 with VE-cadherin only in confluent cells, it was suggested that β-catenin-dependent clustering of VE-cadherin at firmly established cell contacts was necessary for the growth inhibitory effect of VE-cadherin.
It has been shown before that CHO cells transfected with VE-cadherin are contact inhibited in cell growth at higher cell densities compared to CHO cells lacking VE-cadherin (Caveda et al, 1996). We show here that tyrosine 685 in VE-cadherin is involved in this effect and that phosphorylation of this residue allows Csk to bind. Importantly, we found that higher cell densities stimulated the binding of Csk to VE-cadherin without affecting the overall expression level of Csk. Therefore, we speculate that VE-cadherin clustering, which accompanies formation of tight cell contacts, may be involved in phosphorylation of tyrosine 685 and Csk binding. In this context, it will be interesting to test whether the binding of VE-cadherin to β-catenin is involved in the association with Csk.
Induced overexpression of Csk in transfected CHO cells inhibited proliferation independent of VE-cadherin, probably because other Csk-binding proteins were able to recruit sufficient quantities of Csk, if it is overexpressed. However, at endogenous Csk expression levels, only cells expressing wt-VE-cadherin stopped proliferating at certain cell densities, but not cells expressing mt-VE-cadherin (Figure 8) or no VE-cadherin (Caveda et al, 1996). This and the fact that increasing cell density stimulates the binding of Csk to VE-cadherin in endothelial cells argues for VE-cadherin as a major membrane anchor recruiting Csk as inhibitor of cell proliferation. Intriguingly, the Y685F mutant of VE-cadherin counteracted even the inhibitory effect of overexpressed Csk on cell proliferation. We believe that this can be explained by the fact that VE-cadherin recruits Src (Lambeng et al, 2005), a result we confirmed by co-immunoprecipitation (not shown), and that VE-cadherin-associated Src molecules may not be accessible to Csk, if tyrosine 685 in VE-cadherin is mutated. Indeed, we have found that induction of Csk phosphorylates (deactivates) Src in cells lacking or expressing wt-VE-cadherin, but not in cells expressing mt-VE-cadherin (Supplementary Figure 2). Furthermore, the kinase activity of Csk was necessary for its antiproliferative function (Figure 7).
Cadherins contain between eight and nine mostly conserved tyrosine residues in their cytoplasmic domains. Tyrosine 685 is the only VE-cadherin tyrosine for which no correlate is found in other cadherins (except for the last C-terminal amino acid that is a tyrosine in human but not in mouse VE-cadherin). In addition, this VE-cadherin-specific tyrosine is the only one among cadherin tyrosine residues that is found within a consensus pY(T/A/S)X(M/I/V) described to favor Csk binding (Songyang et al, 1994). Thus, the Csk-based mechanism of contact inhibition of cell growth, which we present here, is likely to be specific for endothelial cells.
Kinase substrates for Csk are the SFK members. Yet, the theoretical possibility that VE-cadherin could itself be a kinase substrate had to be analyzed. Indeed coexpression of VE-cadherin with Csk in COS-7 cells caused phosphorylation of VE-cadherin. However, this did not require the kinase activity of Csk and was based on protection of tyrosine phosphate groups via binding of Csk. An alternative possibility that Csk binding indirectly triggered VE-cadherin phosphorylation by a still elusive kinase can formally not be ruled out.
PECAM-1 is another endothelial membrane protein that was found to be tyrosine phosphorylated upon cotransfection together with Csk in COS-1 cells (Cao et al, 1998). Whether Csk binds to PECAM-1 or whether the Csk kinase domain is involved in PECAM-1 phosphorylation is unknown. Whereas PECAM-1 represents an interesting candidate as mediator of SFK/Csk functions in endothelial cells, a role as regulator of contact inhibition of growth has been ruled out (Caveda et al, 1996; Halama et al, 1999).
SFKs are important mediators of VEGFR-2 signaling and angiogenesis, yet involvement of SFKs in endothelial cell proliferation has not yet been clearly demonstrated (Cross et al, 2003). In fact, most evidence for a role of SFKs in cell proliferation is based on experiments with transformed cells. Therefore, it was important to show that inhibition of Csk expression in primary isolated HUVECs caused enhanced cell proliferation, suggesting that SFKs are indeed involved in the proliferation of primary endothelial cells.
Other functions for Src and SFKs in endothelial cells have been demonstrated. Src is clearly involved in VEGF-induced phosphorylation of VE-cadherin and VEGF-induced vascular permeability (Esser et al, 1998; Eliceiri et al, 1999; Weis et al, 2004) as well as in VEGF-stimulated phosphorylation of focal adhesion kinase (FAK), increase in cell motility and antiapoptotic signaling (Abu-Ghazaleh et al, 2001). Overexpression of Csk in endothelial cells was found to reduce VEGF-stimulated tyrosine phosphorylation of VE-cadherin and in vitro tube formation (Lin et al, 2003). In addition, the analysis of chimeric mouse embryos containing both wt and Csk−/− cells suggests that Csk is required for angiogenic sprouting and vascular remodeling (Duan et al, 2004). It will be interesting to test whether the binding of Csk to VE-cadherin is involved in these processes. We have tried intensively to detect an effect of the induction of Csk on VE-cadherin-mediated adhesive functions in our double-transfected CHO cells. However, neither cell aggregation nor the permeability of a monolayer of these cells for 40 kDa dextran was affected by Csk (not shown), whereas the induction of the VE-cadherin-associated phosphatase VE-PTP did enhance VE-cadherin function (Nawroth et al, 2002).
It is generally believed that instead of regulating the kinase activity of Csk, the cell regulates its local concentration by recruiting cytosolic Csk to sites of SFK activity. Not many membrane proteins have been described that can function as targeting anchor for the SH2 domain of Csk and they have been best studied so far in the hematopoietic system (Chow and Veillette, 1995; Leo and Schraven, 2001). With VE-cadherin, we have found a novel targeting substrate for Csk, establishing VE-cadherin as a potentially important regulator of SFK function in endothelial cells.
Taken together, we have found that VE-cadherin binds to Csk and that this binding is mediated by the Csk-SH2 domain and phosphorylated tyrosine residue 685 of VE-cadherin. We demonstrated that VE-cadherin and Csk associate in endothelial cells and that this binding depends on cell density. Contact inhibition of growth in VE-cadherin-transfected CHO cells required tyrosine residue 685 in the cytoplasmic tail of VE-cadherin. And finally, inhibition of Csk expression in primary isolated human endothelial cells enhanced cell proliferation. This suggests Csk as a mediator of contact-inhibited growth in endothelial cells and proposes a new signaling step in this VE-cadherin-driven process. It will be important to find out which signaling steps and which kinase trigger the phosphorylation of tyrosine 685 and the binding of Csk.
Materials and methods
Cell culture and transfections
COS7 cells and CHO DUKX B1 (ATCC, Rockville) and mouse endothelioma bEnd.3 were cultured as described (Nawroth et al, 2002). bEnd.3 cells were changed to MCBB131 medium (GIBCO) 2 days prior to immunoprecipitations as described for HUVECs (Zanetti et al, 2002). Transient transfections of COS7 were performed using the GeneJammer transfection reagent (Stratagene Europe, Amsterdam, The Netherlands). Cells were analyzed 36 h after transfection. Stable transfection and selection of CHO cells expressing multiple genes were performed as described (Nawroth et al, 2002) and inducible expression of Csk was achieved with the regulated expression system GeneSwitch™ (Invitrogen, zeocin and hygromycin resistance). VE-cadherin was expressed by the vector pcDNA3 (Invitrogen, G418 resistance).
Antibodies
Rabbit antiserum C5 and rat mAb 11D4.1 against mouse VE-cadherin have been described previously (Gotsch et al, 1997). Antibodies against the following antigens were commercially obtained: recombinant phospho-tyrosine (RC20H, BD Transduction Laboratories, Bluegrass-Lexington, KY), human VE-cadherin (CD144, BD PharMingen), Csk (Csk, BD Transduction Laboratories; C20, Santa Cruz Biotech. Inc., CA), Myc tag (9E10, BD PharMingen). Secondary antibodies against rat and mouse and rabbit IgG and fluorophore-conjugated reagents were purchased from Dianova (Hamburg, Germany).
Generation of DNA constructs
In the course of construction, re-sequencing of the original cDNA of mouse VE-cadherin (Breier et al, 1996) revealed a difference to the publication. Instead of the published sequence GTG (bp200) - - A AAG ATC -AG TCC AAC, we found GTG (bp200) GGA AAG ATC AAG TCC AAC. This new sequence was verified by a newly isolated VE-cadherin cDNA, cloned by RT–PCR from bEnd.3 cells. This change in nucleotide sequence resulted in a change of the published protein sequence V-KDQSN (aa 66–71) to the new sequence VGKIKSN (aa 66–72). Due to the one additional amino acid (glycine at position 67), numbering of the following amino-acid residues was changed accordingly.
Yeast expression vectors were constructed in pBTM116 (bait plasmids), thereby generating fusion proteins between LexA and VE-cadherin. Phosphorylation of the cytoplasmic tail of VE-cadherin was achieved by using a bait vector containing a TPR-Met kinase cassette (pBTM116-TPR-Met, kindly provided by Dr Walter Birchmeier, MDC-Berlin) (Schaeper et al, 2000; Fujita et al, 2002). A VE-cadherin cDNA fragment coding for a sequence covering aa 621–764 was PCR amplified from cloned mouse VE-cadherin (Breier et al, 1996) using the forward primer 5′-GCTGATGCGGCCGCGGAGGCGGATCCGGAAG- 3′ and the reverse primer 5′-CTCAACGACTGGGGAGTCGACTTC-3′, generating the bait vector pBTM116-TPR-Met-VE-cadherin621–764.
Eukaryotic expression was achieved with vectors pKE081myc-#2 (Ebnet et al, 2000), containing an N-terminal Myc tag and a Kozak consensus sequence, and vectors pcDNA3 (Invitrogen), pEF6 (Invitrogen) and pGene/V5-HisA (Invitrogen). The Csk fragment isolated from the yeast two-hybrid library was subcloned into pKE081myc#2. Mouse full-length Csk was PCR amplified from bEND.3 cDNA using the forward primer 5′-GCTGATGCGGCCGCATGTCGGCTATACAGGCC -3′ and the reverse primer 5′-GCTGATGCTGACTCACAGGTGCAGCTCATG-3′ and cloned into pKE081myc-#2. Myc-Cskwt was subcloned into pGeneV5/His. Point mutations in VE-cadherin and Csk were generated with the QuikChange™ Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands) using the following primers: for Csk mutation R107K, forward primer 5′-GGCCTGTTCCTCGTGAAGGAAAGCACCAACTA C-3′ and reverse primer 5′-GTAGTTGGTGCTTTCCTTCACGAGGAACAGGC C-3′; for Csk mutation K222R, forward primer 5′-GCAACAAAGTTGCAGTCAGGTGCATCAAGAAT GACGC-3′ and reverse primer 5′-GCGTCATTCTTGATGCACCTGACTGCAACTTT GTTGC-3′; for VE-cadherin mutation Y685F, forward primer 5′-GGCCGGCAGTGTTCACGCAGGTGCAGAAGC-3′ and reverse primer 5′-GCTTCTGCACCTGCGTGAACACTGCCGGCC-3′. The β-catenin cDNA (pcDNA3) was kindly provided by Dr Jürgen Behrens (MDC, Berlin, Germany); mouse VEGF-R2 full-length cDNA was in pEF6.
The prokaryotic expression constructs encoding GST-VE-cadherinwt and GST-VE-cadherinY685F (aa 621–764 for both constructs) were generated by amplifying the respective VE-cadherin fragments with the forward primer 5′-CGGAATTCCGGAGGCGGATCCGGAAG-3′ and reverse primer 5′-CTCAACGACTGGGGAGTCGACTTC-3′. GST-N-cadherin (covering N-cadherin amino acids 747–906) was generated by PCR with mouse N-cadherin cDNA (Miyatani et al, 1989) using the forward primer 5′-CGGAATTCAAACGGCGGGATAAAGAG-3′ and the reverse primer 5′-GCTGATGCGGCCGCTCAGTCGTCACCACCGCC -3′. Constructs were cloned into pGEX-4T-1 (Amersham Bioscience Europe, Freiburg, Germany).
Yeast two-hybrid screen
Two-hybrid screening experiments were performed essentially as described (Ebnet et al, 2000) screening a mouse embryonic cDNA library (Hollenberg et al, 1995).
In vitro binding experiments
GST-VE-cadherinwt, GST-VE-cadherinY685F, GST-N-cadherin and GST were expressed in Escherichia coli BL21 (Amersham Biosciences) and purified and used in pull-downs as described (Ebnet et al, 2000). Equal amounts of GST proteins were incubated with and without p60c-src (Upstate Biotechnology, NY) for 20 min at 30°C (in 20 mM HEPES pH 7.4, 10 mM MgCl2, 2 mM MnCl2, 10 mM rATP), stopping the reaction with 13 mM EDTA.
Immunoprecipitation and immunoblots
Myc-Csk expression in transfected CHO cells was induced with 1 × 10−8 M mifepristone for 16 h. To block phosphatases, all analyzed cells were treated for the last 7 min before extraction with a combination of vanadate (100 μM; Sigma) and hydrogen peroxide (200 μM; Sigma), premixed before to generate PV. The same treatment has been used before, analyzing VE-cadherin and VEGFR-2 phosphorylation and association (Lampugnani et al, 1997; Zanetti et al, 2002; Lampugnani et al, 2003). As reported by this group, we found that PV treatment allowed the detection of higher tyrosine phosphorylation levels of VE-cadherin, yet did not interfere with the overall downregulation of VE-cadherin tyrosine phosphorylation that can be observed with increasing cell density (data not shown). Cells were lysed and subjected to immunoprecipitations and immunoblots as described (Ebnet et al, 2000), except for using 20 μM orthovanadate in the lysis buffer. For analyzing the association of Csk and VE-cadherin at different cell densities, confluent bEnd.3 cells were split 1:2 and 1:5 2 days prior to immunoprecipitations.
Immunofluorescence
Csk was induced in the respective CHO clones for 7.5 h with mifepristone and cells were treated for 5 min prior to fixation with PV as described above. Fixation was carried out with methanol for 5 min at −20°C. Cells were analyzed by immunofluorescence as described (Ebnet et al, 2000). Experiments shown in Supplementary Figure 1A were carried out without PV treatment.
RNA interference
RNA interference of Csk expression was induced with siRNA directed against Csk. A 21-nucleotide siRNA (5′-AGUACCCAGCAAAUGGGCATT-3′) (Ambion, Austin, TX) targeted human Csk sequence at nucleotide 2123 downstream of the start codon. As negative control, two nonblocking siRNAs of similar length against two unrelated mouse antigens were used, which did not inhibit expression of the respective antigens. HUVECs were passaged 2 days before transfection, and harvested at 90% confluency. Cells (1 × 106) were transfected with 5 μg siRNA via nucleofection (Amaxa Biosystems, Köln, Germany) following the manufacturer's instructions.
Proliferation assay
CHO cells were plated at a density of 4 × 104 cells/well onto 96-well plates. After 12 h, cells were stimulated with mifepristone for 8 h followed by labeling with 0.075 MBq/ml (in 125 μl) [methyl-3H]thymidine (Amersham Biosciences) for 20 h. HUVECs were plated after nucleofection at a density of 5 × 104 cells/well in 96-well plates for 24 h followed by labeling with 0.13 MBq/ml (in 150 μl) [methyl-3H]thymidine for another 24 h. In some cases, HUVEC medium was replaced 12 h after plating by medium lacking fetal calf serum followed by addition of 80 ng/ml VEGF (Preprotech, NJ) 12 h later for a period of 24 h. Labeled cells were harvested and incorporated radioactivity was transferred to filters with a Standard-Harvester ICH-110-96 (Inotech) and measured with a Liquid Scintillation-Counter LS6500 (Beckmann).
In vitro phosphorylation experiments
To determine the kinase activity of Csk and various Csk mutants by autophosphorylation, these proteins were expressed in COS-7 cells and immunoprecipitated. Precipitated proteins were washed two times in immunoprecipitation lysis buffer, two times in TNE (50 mM Tris–HCl pH 8, 140 mM NaCl, 5 mM EDTA pH 8) and once in kinase buffer (50 mM Tris–HCl pH 7.5, 7 mM MgCl2, 5 mM MnCl2, 1 mM DTT). Autophosphorylation was allowed in the presence of 5.55 MBq/ml [γ-32P]ATP (Amersham Biosciences) at 30°C for 30 min, followed by washing two times with TNE, and analysis by SDS–PAGE and subsequent fluorography.
Flow cytometry
Flow cytometry was performed as described previously (Borges et al, 1997).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
We thank Dr M Takeichi for generously providing mouse N-cadherin cDNA, Dr J Behrens for the gift of β-catenin cDNA and Dr W Birchmeier for the valuable bait vector. We are grateful to Dr F Kiefer for valuable advice and for critically reading the manuscript.
References
- Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I (2001) Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J 360: 255–264 [Europe PMC free article] [Abstract] [Google Scholar]
- Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D (1997) P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med 185: 573–578 [Europe PMC free article] [Abstract] [Google Scholar]
- Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E (1996) Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood 87: 630–641 [Abstract] [Google Scholar]
- Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A (1998) Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem 273: 15765–15772 [Abstract] [Google Scholar]
- Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E (1996) Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin). J Clin Invest 98: 886–893 [Europe PMC free article] [Abstract] [Google Scholar]
- Chow LML, Veillette A (1995) The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol 7: 207–226 [Abstract] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A (2002) The cadherin–catenin adhesion system in signaling and cancer. J Clin Invest 109: 987–991 [Europe PMC free article] [Abstract] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E (1999) Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA 96: 9815–9820 [Europe PMC free article] [Abstract] [Google Scholar]
- Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L (2003) VEGF-receptor signal transduction. Trends Biochem Sci 28: 488–494 [Abstract] [Google Scholar]
- Duan LJ, Imamoto A, Fong GH (2004) Dual roles of the C-terminal Src kinase (Csk) during developmental vascularization. Blood 103: 1370–1372 [Abstract] [Google Scholar]
- Ebnet K, Schulz CU, Meyer-zu-Brickwedde M-K, Pendl GG, Vestweber D (2000) Junctional Adhesion Molecule (JAM) interacts with the PDZ domain containing proteins AF-6 and ZO-1. J Biol Chem 275: 27979–27988 [Abstract] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA (1999) Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924 [Abstract] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W (1998) Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111: 1853–1865 [Abstract] [Google Scholar]
- Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602: 114–130 [Abstract] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Molina Leddy HE, Behrens J, Sommer T, Birchmeier W (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4: 222–231 [Abstract] [Google Scholar]
- Giles RH, van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1–24 [Abstract] [Google Scholar]
- Gotsch U, Borges E, Bosse R, Böggemeyer E, Simon M, Mossmann H, Vestweber D (1997) VE-cadherin antibody accelerates neutrophil recruiment in vivo. J Cell Sci 110: 583–588 [Abstract] [Google Scholar]
- Gottardi CJ, Gumbiner BM (2001) Adhesion signaling: how β-catenin interacts with its partners. Curr Biol 11: R792–R794 [Abstract] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner MB (2001) E-cadherin suppresses cellular transformation by inhibiting beta-catenin signalling in an adhesion-independent manner. J Cell Biol 153: 1049–1059 [Europe PMC free article] [Abstract] [Google Scholar]
- Halama T, Staffler G, Hoch S, Stockinger H, Wolff K, Petzelbauer P (1999) Vascular-endothelial cadherin (CD144)- but not PECAM-1 (CD31)-based cell-to-cell contacts convey the maintenance of a quiescent endothelial monolayer. Int Arch Allergy Immunol 120: 237–244 [Abstract] [Google Scholar]
- Hecht A, Kemler R (2000) Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep 1: 24–28 [Europe PMC free article] [Abstract] [Google Scholar]
- Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H (1995) Identification of a new family of tissue-specific basic helix–loop–helix proteins with a two-hybrid system. Mol Cell Biol 15: 3813–3822 [Europe PMC free article] [Abstract] [Google Scholar]
- Imamoto A, Soriano P (1993) Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell 73: 1117–1124 [Abstract] [Google Scholar]
- Lambeng N, Wallez Y, Rampon C, Cand F, Christe G, Gulino-Debrac D, Vilgrain I, Huber P (2005) Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ Res 96: 384–391 [Europe PMC free article] [Abstract] [Google Scholar]
- Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E (1997) Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci 110: 2065–2077 [Abstract] [Google Scholar]
- Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E (2003) Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 161: 793–804 [Europe PMC free article] [Abstract] [Google Scholar]
- Latour S, Veillette A (2001) Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol 13: 299–306 [Abstract] [Google Scholar]
- Lemay S, Davidson D, Latour S, Veillette A (2000) Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol 20: 2743–2754 [Europe PMC free article] [Abstract] [Google Scholar]
- Leo A, Schraven B (2001) Adapters in lymphocyte signalling. Curr Opin Immunol 13: 307–316 [Abstract] [Google Scholar]
- Levenberg S, Yarden A, Kam Z, Geiger B (1999) p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene 18: 869–876 [Abstract] [Google Scholar]
- Lin MT, Yen ML, Lin CY, Kuo ML (2003) Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol 64: 1029–1036 [Abstract] [Google Scholar]
- Miyatani S, Shimamura K, Hatta M, Nagafuchi A, Nose A, Matsunaga M, Hatta K, Takeichi M (1989) Neural cadherin: role in selective cell–cell adhesion. Science 245: 631–635 [Abstract] [Google Scholar]
- Nawroth R, Poell G, Ranft A, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D (2002) VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 21: 4885–4895 [Europe PMC free article] [Abstract] [Google Scholar]
- Pedraza LG, Stewart RA, Li DM, Xu T (2004) Drosophila Src-family kinases function with Csk to regulate cell proliferation and apoptosis. Oncogene 23: 4754–4762 [Abstract] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [Abstract] [Google Scholar]
- Rahimi N, Kazlauskas A (1999) A role for cadherin-5 in regulation of vascular endothelial growth factor receptor 2 activity in endothelial cells. Mol Biol Cell 10: 3401–3407 [Europe PMC free article] [Abstract] [Google Scholar]
- Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W (2000) Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol 149: 1419–1432 [Europe PMC free article] [Abstract] [Google Scholar]
- Schwartz SM, Gajdusek CM, Selden SC (1981) Vascular wall growth control: the role of the endothelium. Artherosclerosis 1: 107–126 [Abstract] [Google Scholar]
- Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T (1994) Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol 14: 2777–2785 [Europe PMC free article] [Abstract] [Google Scholar]
- St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS (1998) E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J Cell Biol 142: 557–571 [Europe PMC free article] [Abstract] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R (2001) E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol 154: 1185–1196 [Europe PMC free article] [Abstract] [Google Scholar]
- Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D (2004) Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest 113: 885–894 [Europe PMC free article] [Abstract] [Google Scholar]
- Zanetti A, Lampugnani MG, Balconi G, Breviario F, Corada M, Lanfrancone L, Dejana E (2002) Vascular endothelial growth factor induces shc association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling. Arterioscler Thromb Vasc Biol 22: 617–622 [Abstract] [Google Scholar]
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/sj.emboj.7600647
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1038/sj.emboj.7600647
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/sj.emboj.7600647
Article citations
Identification of oxidative phosphorylation-related genes in moyamoya disease by combining bulk RNA-sequencing analysis and machine learning.
Front Genet, 15:1417329, 10 Jun 2024
Cited by: 0 articles | PMID: 38919950 | PMCID: PMC11197386
CSK-mediated signalling by integrins in cancer.
Front Cell Dev Biol, 11:1214787, 07 Jul 2023
Cited by: 4 articles | PMID: 37519303 | PMCID: PMC10382208
Review Free full text in Europe PMC
IL-33 via PKCμ/PRKD1 Mediated α-Catenin Phosphorylation Regulates Endothelial Cell-Barrier Integrity and Ischemia-Induced Vascular Leakage.
Cells, 12(5):703, 23 Feb 2023
Cited by: 1 article | PMID: 36899839 | PMCID: PMC10001418
Dialogue between VE-Cadherin and Sphingosine 1 Phosphate Receptor1 (S1PR1) for Protecting Endothelial Functions.
Int J Mol Sci, 24(4):4018, 16 Feb 2023
Cited by: 1 article | PMID: 36835432 | PMCID: PMC9959973
Review Free full text in Europe PMC
Apoptosis regulation by the tyrosine-protein kinase CSK.
Front Cell Dev Biol, 10:1078180, 12 Dec 2022
Cited by: 3 articles | PMID: 36578781 | PMCID: PMC9792154
Review Free full text in Europe PMC
Go to all (86) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site.
Oncogene, 26(7):1067-1077, 14 Aug 2006
Cited by: 143 articles | PMID: 16909109
Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation.
Mol Pharmacol, 64(5):1029-1036, 01 Nov 2003
Cited by: 116 articles | PMID: 14573751
Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148.
J Cell Biol, 161(4):793-804, 01 May 2003
Cited by: 259 articles | PMID: 12771128 | PMCID: PMC2199373
Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers.
J Biol Chem, 285(10):7045-7055, 04 Jan 2010
Cited by: 90 articles | PMID: 20048167 | PMCID: PMC2844154




