Abstract
Free full text

Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide.
Abstract
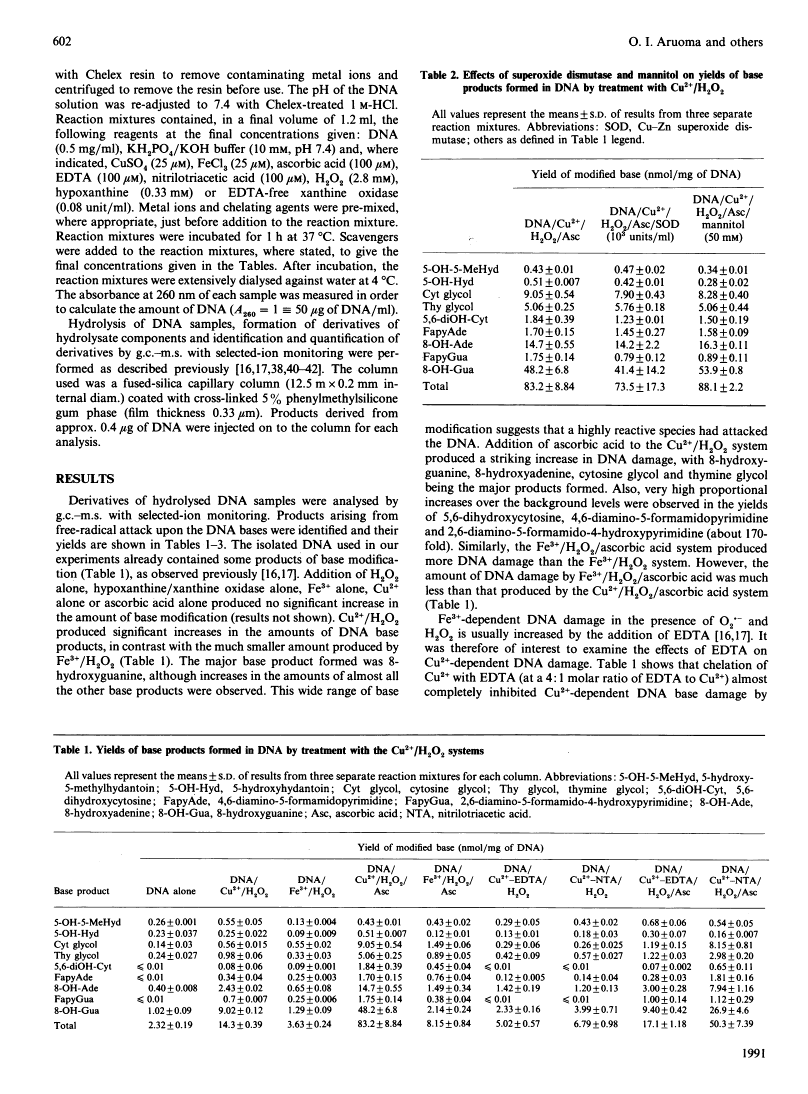
Mixtures of Cu2+ and H2O2 at pH 7.4 caused damage to the bases in DNA greater than that caused by mixtures of Fe3+ and H2O2. Addition of ascorbic acid to the Cu2+/H2O2 system caused a very large increase in base damage, much greater than that produced by the Fe3+/H2O2/ascorbic acid system. The products of base damage in the presence of Cu2+ were typical products that have been shown to result from attack of hydroxyl radicals upon the DNA bases. Cytosine glycol, thymine glycol, 8-hydroxyadenine and especially 8-hydroxyguanine were the major products in both the Cu2+/H2O2 and the Cu2+/H2O2/ascorbic acid systems. Base damage in DNA by these systems was inhibited by the chelating agents EDTA and nitrilotriacetic acid and by catalase, but not by superoxide dismutase, nor by the hydroxyl-radical scavenger mannitol. It is proposed that Cu2+ ions bound to the DNA react with H2O2 and ascorbic acid to generate hydroxyl radicals, which then immediately attack the DNA bases in a site-specific manner. A hypoxanthine/xanthine oxidase system also caused damage to the DNA bases in the presence of Cu2+ ions. This was inhibited by superoxide dismutase and catalase. The high activity of Cu2+ ions, when compared with Fe3- ions, in causing hydroxyl-radical-dependent damage to DNA and to other biomolecules, means that the availability of Cu2+ ions in vivo must be carefully controlled.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (782K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. [Abstract] [Google Scholar]
- Schraufstätter I, Hyslop PA, Jackson JH, Cochrane CG. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. [Europe PMC free article] [Abstract] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [Abstract] [Google Scholar]
- Moody CS, Hassan HM. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982 May;79(9):2855–2859. [Europe PMC free article] [Abstract] [Google Scholar]
- Brawn MK, Fridovich I. Increased superoxide radical production evokes inducible DNA repair in Escherichia coli. J Biol Chem. 1985 Jan 25;260(2):922–925. [Abstract] [Google Scholar]
- Weitzman SA, Weitberg AB, Clark EP, Stossel TP. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. [Abstract] [Google Scholar]
- Cerutti PA. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. [Abstract] [Google Scholar]
- Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. [Abstract] [Google Scholar]
- McConkey DJ, Hartzell P, Nicotera P, Wyllie AH, Orrenius S. Stimulation of endogenous endonuclease activity in hepatocytes exposed to oxidative stress. Toxicol Lett. 1988 Aug;42(2):123–130. [Abstract] [Google Scholar]
- Nakayama T, Kaneko M, Kodama M, Nagata C. Cigarette smoke induces DNA single-strand breaks in human cells. Nature. 1985 Apr 4;314(6010):462–464. [Abstract] [Google Scholar]
- Lesko SA, Lorentzen RJ, Ts'o PO. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980 Jun 24;19(13):3023–3028. [Abstract] [Google Scholar]
- Brawn K, Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. [Abstract] [Google Scholar]
- Rowley DA, Halliwell B. DNA damage by superoxide-generating systems in relation to the mechanism of action of the anti-tumour antibiotic adriamycin. Biochim Biophys Acta. 1983 Nov 22;761(1):86–93. [Abstract] [Google Scholar]
- Aruoma OI, Halliwell B, Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [Abstract] [Google Scholar]
- Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [Abstract] [Google Scholar]
- Mello Filho AC, Hoffmann ME, Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. [Europe PMC free article] [Abstract] [Google Scholar]
- Nassi-Calò L, Mello-Filho C, Meneghini R. o-phenanthroline protects mammalian cells from hydrogen peroxide-induced gene mutation and morphological transformation. Carcinogenesis. 1989 Jun;10(6):1055–1057. [Abstract] [Google Scholar]
- Kyle ME, Nakae D, Sakaida I, Miccadei S, Farber JL. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [Abstract] [Google Scholar]
- Sagripanti JL, Kraemer KH. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem. 1989 Jan 25;264(3):1729–1734. [Abstract] [Google Scholar]
- Feldberg RS, Carew JA, Paradise R. Probing Cu(II)/H2O2 damage in DNA with a damage-specific DNA binding protein. J Free Radic Biol Med. 1985;1(5-6):459–466. [Abstract] [Google Scholar]
- Stoewe R, Prütz WA. Copper-catalyzed DNA damage by ascorbate and hydrogen peroxide: kinetics and yield. Free Radic Biol Med. 1987;3(2):97–105. [Abstract] [Google Scholar]
- Chiou SH. DNA- and protein-scission activities of ascorbate in the presence of copper ion and a copper-peptide complex. J Biochem. 1983 Oct;94(4):1259–1267. [Abstract] [Google Scholar]
- Fujimoto S, Adachi Y, Ishimitsu S, Ohara A. Release of bases from deoxyribonucleic acid by ascorbic acid in the presence of Cu2+. Chem Pharm Bull (Tokyo) 1986 Nov;34(11):4848–4851. [Abstract] [Google Scholar]
- Reed CJ, Douglas KT. Single-strand cleavage of DNA by Cu(II) and thiols: a powerful chemical DNA-cleaving system. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1111–1117. [Abstract] [Google Scholar]
- Tachon P. Ferric and cupric ions requirement for DNA single-strand breakage by H2O2. Free Radic Res Commun. 1989;7(1):1–10. [Abstract] [Google Scholar]
- Rowley DA, Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals in the presence of copper salts: a physiologically significant reaction? Arch Biochem Biophys. 1983 Aug;225(1):279–284. [Abstract] [Google Scholar]
- Sutton HC, Winterbourn CC. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6(1):53–60. [Abstract] [Google Scholar]
- Czapski G, Goldstein S, Meyerstein D. What is unique about superoxide toxicity as compared to other biological reductants? A hypothesis. Free Radic Res Commun. 1988;4(4):231–236. [Abstract] [Google Scholar]
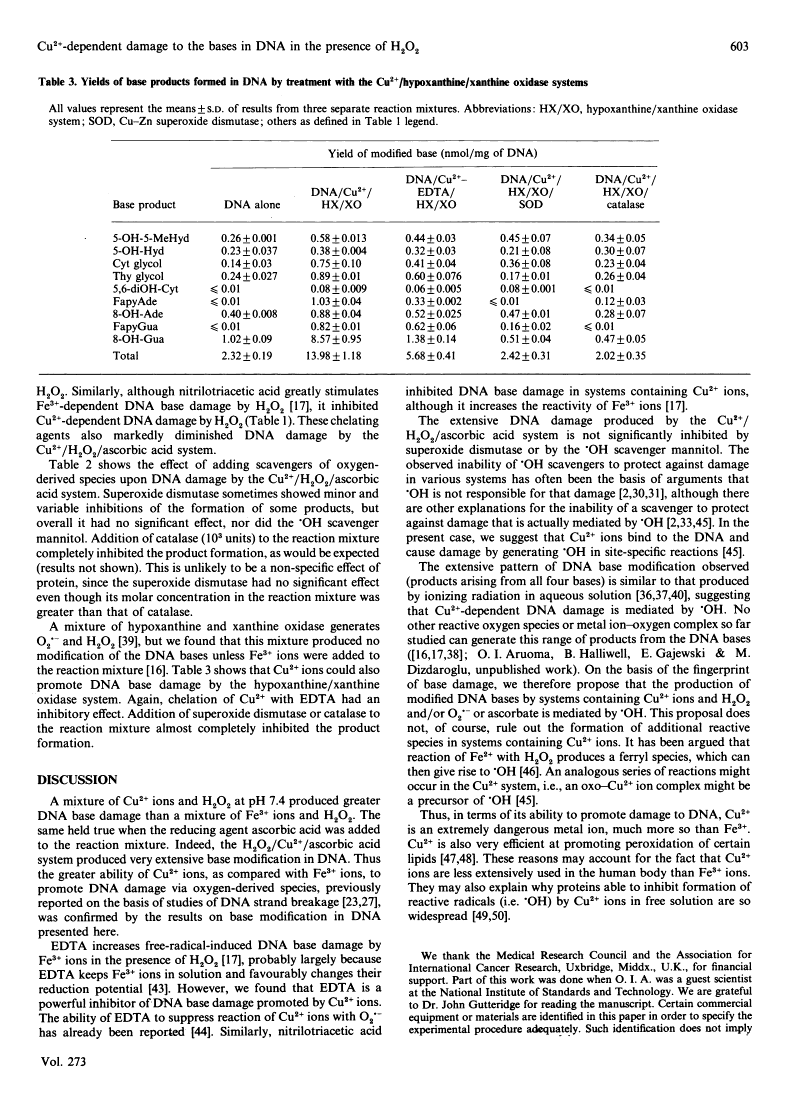
- Yamamoto K, Kawanishi S. Hydroxyl free radical is not the main active species in site-specific DNA damage induced by copper (II) ion and hydrogen peroxide. J Biol Chem. 1989 Sep 15;264(26):15435–15440. [Abstract] [Google Scholar]
- Halliwell B, Grootveld M, Gutteridge JM. Methods for the measurement of hydroxyl radicals in biomedical systems: deoxyribose degradation and aromatic hydroxylation. Methods Biochem Anal. 1988;33:59–90. [Abstract] [Google Scholar]
- Teoule R, Cadet J. Radiation-induced degradation of the base component in DNA and related substances--final products. Mol Biol Biochem Biophys. 1978;27:171–203. [Abstract] [Google Scholar]
- Dizdaroglu M, Aruoma OI, Halliwell B. Modification of bases in DNA by copper ion-1,10-phenanthroline complexes. Biochemistry. 1990 Sep 11;29(36):8447–8451. [Abstract] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [Abstract] [Google Scholar]
- Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985 Feb 1;144(2):593–603. [Abstract] [Google Scholar]
- Fuciarelli AF, Wegher BJ, Gajewski E, Dizdaroglu M, Blakely WF. Quantitative measurement of radiation-induced base products in DNA using gas chromatography-mass spectrometry. Radiat Res. 1989 Aug;119(2):219–231. [Abstract] [Google Scholar]
- Dizdaroglu M, Gajewski E. Selected-ion mass spectrometry: assays of oxidative DNA damage. Methods Enzymol. 1990;186:530–544. [Abstract] [Google Scholar]
- Grootveld M, Halliwell B. An aromatic hydroxylation assay for hydroxyl radicals utilizing high-performance liquid chromatography (HPLC). Use to investigate the effect of EDTA on the Fenton reaction. Free Radic Res Commun. 1986;1(4):243–250. [Abstract] [Google Scholar]
- Halliwell B. The superoxide dismutase activity of iron complexes. FEBS Lett. 1975 Aug 1;56(1):34–38. [Abstract] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. [Abstract] [Google Scholar]
- Gutteridge JM. Age pigments: role of iron and copper salts in the formation of fluorescent lipid complexes. Mech Ageing Dev. 1984 Apr-May;25(1-2):205–214. [Abstract] [Google Scholar]
- Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. [Abstract] [Google Scholar]
- Gutteridge JM, Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. [Abstract] [Google Scholar]
- Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988 Feb 15;37(4):569–571. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2730601
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1149805?pdf=render
Citations & impact
Impact metrics
Article citations
Phaeolschidin F, a new symmetrical bis(styrylpyrone) derivative with redox-catalyzing activity from the mushroom Gymnopilus aeruginosus (order Agaricales).
J Antibiot (Tokyo), 76(4):236-238, 02 Feb 2023
Cited by: 0 articles | PMID: 36732638
Iron Availability and Homeostasis in Plants: A Review of Responses, Adaptive Mechanisms, and Signaling.
Methods Mol Biol, 2642:49-81, 01 Jan 2023
Cited by: 1 article | PMID: 36944872
Review
Ascorbate Is a Primary Antioxidant in Mammals.
Molecules, 27(19):6187, 21 Sep 2022
Cited by: 10 articles | PMID: 36234722 | PMCID: PMC9572970
Review Free full text in Europe PMC
Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review.
Front Surg, 9:905892, 05 Aug 2022
Cited by: 22 articles | PMID: 35990090 | PMCID: PMC9388913
Review Free full text in Europe PMC
Development of a next-generation chikungunya virus vaccine based on the HydroVax platform.
PLoS Pathog, 18(7):e1010695, 05 Jul 2022
Cited by: 7 articles | PMID: 35788221 | PMCID: PMC9286250
Go to all (233) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions.
Arch Biochem Biophys, 285(2):317-324, 01 Mar 1991
Cited by: 139 articles | PMID: 1654771
Modification of bases in DNA by copper ion-1,10-phenanthroline complexes.
Biochemistry, 29(36):8447-8451, 01 Sep 1990
Cited by: 51 articles | PMID: 2123717
Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase.
J Biol Chem, 264(22):13024-13028, 01 Aug 1989
Cited by: 178 articles | PMID: 2546943
Hydrogen peroxide-induced base damage in deoxyribonucleic acid.
Radiat Res, 121(3):338-343, 01 Mar 1990
Cited by: 66 articles | PMID: 2315450