Abstract
Free full text

Activation of NF-κB by the Human Herpesvirus 8 Chemokine Receptor ORF74: Evidence for a Paracrine Model of Kaposi's Sarcoma Pathogenesis
Abstract
Infection with human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS)-associated herpesvirus, is necessary for the development of KS. The HHV-8 lytic-phase gene ORF74 is related to G protein-coupled receptors, particularly interleukin-8 (IL-8) receptors. ORF74 activates the inositol phosphate/phospholipase C pathway and the downstream mitogen-activated protein kinases, JNK/SAPK and p38. We show here that ORF74 also activates NF-κB independent of ligand when expressed in KS-derived HHV-8-negative endothelial cells or primary vascular endothelial cells. NF-κB activation was enhanced by the chemokine GROα, but not by IL-8. Mutation of Val to Asp in the ORF74 second cytoplasmic loop did not affect ligand-independent signaling activity, but it greatly increased the response to GROα. ORF74 upregulated the expression of NF-κB-dependent inflammatory cytokines (RANTES, IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor) and adhesion molecules (VCAM-1, ICAM-1, and E-selectin). Supernatants from transfected KS cells activated NF-κB signaling in untransfected cells and elicited the chemotaxis of monocytoid and T-lymphoid cells. Expression of ORF74 conferred on primary endothelial cells a morphology that was strikingly similar to that of spindle cells present in KS lesions. Taken together, these data, demonstrating that ORF74 activates NF-κB and induces the expression of proangiogenic and proinflammatory factors, suggest that expression of ORF74 in a minority of cells in KS lesions could influence uninfected cells or latently infected cells via autocrine and paracrine mechanisms, thereby contributing to KS pathogenesis.
Kaposi's sarcoma (KS) is a neoplasm of mixed cellularity that until recently was rare and occurred in three forms: classical (in elderly males of Mediterranean descent), endemic (in parts of Africa), and iatrogenic (in transplant patients). KS lesions are highly vascularized and contain characteristic spindle-shaped cells believed to be of endothelial origin, angiogenic blood vessels, and infiltrates of immune cells. Inflammation and angioproliferation appear to play a key role in KS development.
A fourth form (AIDS-KS) appeared with the human immunodeficiency virus (HIV) epidemic. HIV type 1 (HIV-1) infection results in a 10,000- to 100,000-fold increase in the incidence of KS (6, 7, 55) but is not a primary cause of KS, since the other three forms of KS are negative for HIV-1. Thus, HIV-1, although not necessary for KS, is a very powerful cofactor. A new herpesvirus called KS-associated herpesvirus (KSHV) or, more formally, human herpesvirus 8 (HHV-8) was discovered in KS lesions (16) and was found to be present in virtually all cases of the four forms of KS, as well as in primary effusion lymphomas (14). An increase in HHV-8 antibody levels in serum and viremia precedes the onset of KS by 1 or 2 years (27, 41, 47, 57, 68). Taken together, these data indicate that HHV-8 infection is necessary for KS.
In spite of the strong epidemiological association of HHV-8 with KS, its pathogenic role(s) is not clear. The apparent prevalence of HHV-8 in normal populations ranges from a small percentage of the general population in the United States, the Caribbean, and countries in Southeast Asia (1, 61) to >50% in parts of Africa (3, 28, 44). This far exceeds the rate of KS in these regions, suggesting that although HHV-8 is necessary for KS, it is highly inefficient. Many genes of HHV-8 have been shown to transform cells, but infection with HHV-8 does not generally transform cells. Infection of endothelial cells in vitro greatly extends their life span, although only about 5% of the cells are infected at any given time (23). In early KS lesions, a minority of the spindle cells believed to constitute the abnormal cell population is infected (8). In later lesions, although most spindle cells are infected, the majority of infected cells are in viral latency, and lytic replication is occurring in only a few cells (8, 64). Taken together, these data suggest that HHV-8 infection could influence uninfected cells and contribute to KS pathogenesis by paracrine mechanisms involving soluble factors secreted by infected lytic-phase cells.
Lytic viral gene products or cellular gene products induced by them could act as soluble paracrine factors. HHV-8 codes for at least four biologically active secreted lytic-phase proteins: a viral interleukin-6 (vIL-6) that has functional similarities to its cellular homologue (46, 50, 51), and three homologues of cellular β chemokines (9, 38, 46, 51) that can elicit angiogenesis and chemotaxis. HHV-8 also has genes, including K1 (40), several v-IRFs (12, 26), v-FLIP (65), K15 (32, 54), and ORF74 (4, 33), that are related to cellular signal transduction genes and factors and that could induce the expression of soluble paracrine factors.
ORF74, a chemokine (IL-8) receptor homologue, is an early lytic-phase gene (37, 60) that is expressed in KS lesions at a very low level (33). ORF74 is an attractive candidate for a gene whose expression could alter the behavior of adjacent uninfected or latently infected cells. Indeed, it induces angiogenesis and vascular endothelial growth factor (VEGF) expression in vitro and in xenotransplants in mice (5). Transgenic mice expressing ORF74, regulated by a T-cell-specific promoter, develop angioproliferative lesions that exhibit many of the characteristics of KS but do not develop lymphoproliferative diseases, suggesting that paracrine mechanisms may cause their KS-like lesions (15, 69). ORF74 has been shown to signal constitutively through the inositol phosphate/phospholipase C pathways and to activate downstream mitogen-activated protein (MAP) kinases p38 and JNK/SAP (4, 62). Many cellular chemokines bind to ORF74, including IL-8 and Groα. Some enhance or inhibit, but most fail to change, its ability to signal through these pathways (29, 30, 31).
Inflammation is thought to play a major role in the developmental course of KS. Inflammatory cytokines, adhesion molecules, and endothelial cell activation are all centrally involved in this process (20), and the expression of many of these factors depends upon activation of the transcriptional regulatory factor NF-κB. We therefore sought to determine whether expression of ORF74 affected NF-κB activity and resulted in the secretion of inflammatory factors other than VEGF that could promote the development of KS by paracrine mechanisms. We present the results of these studies, which indicate that ORF74 indeed enhances NF-κB activation, resulting in the induction of expression of several NF-κB-dependent proinflammatory and pro-angiogenic cytokines, chemokines, and cell adhesion molecules.
MATERIALS AND METHODS
Cell culture and retroviral infections.
Human umbilical vein endothelial cells (HUVECs) and dermal microvascular endothelial cells (dMVECs) were obtained from Cell Systems Corp. (CS-C) (Kirkland, Wash.). Cells were maintained in CS-C complete medium in a 5% CO2 humidified incubator. HUVECs were expanded and used for experiments between passages 4 and 5. A. Albini (Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy) kindly provided KSIMM cells (2). KSIMM cells were maintained in RPMI (Life Technologies, Inc. [LTI], Gaithersburg, Md.) with 10% heat-inactivated fetal bovine serum (FBS; LTI) in a 5% CO2 humidified incubator. Penicillin G (100 U/ml) and 100 μg of streptomycin/ml were added to all culture media. For retroviral infections, HUVECs or dMVECs (passages 4 and 5) were treated with Polybrene (6 μg/ml) 12 h prior to infection. Supernatants containing retrovirus were prepared from the Moloney murine leukemia virus-based retroviral packaging cell line Phoenix(Ampho) by transfection with pMIGR1(ORF74) or pMIGR1 empty vector. W. Pear (University of Pennsylvania, Philadelphia) kindly provided the MIGR1 vector (53). Retrovirus preparations were added to the HUVECs or dMVECs, which were then gently rocked for 1 h. The virus titer was determined by expression of green fluorescent protein (GFP), translated from an independent ribosome entry site (IRES) in the retroviral vector from the same cistron containing the inserted gene. Cells were refed 24 h postinfection with fresh media.
Plasmids and transfections.
Cells were transfected with various plasmid constructs using Fugene 6 (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's instructions. Transfection efficiencies were estimated by cotransfection of a β-galactosidase (β-Gal) expression vector pCMV–β-Gal (Stratagene) or GFP (Green Lantern; Stratagene). Lysates were assayed for β-Gal activity, and cells were counted for reporter gene expression. The dominant-negative (DN) mutant ΙκBα32A/36A was a kind gift from J. DiDonato (University of California, San Diego, School of Medicine, La Jolla) (18). The DN constructs for ΙKK75α (K44A) and ΙKKβ (K44A) were generously provided by D. Goeddel (Tularik, South San Francisco, Calif.). The DN mutant of AKT (AKT-K179 M) was obtained from the lab of M. Greenberg (Harvard Medical School, Boston, Mass.) (19). The mutant construct of ORF74 (V142D) was generated as described below. LY294002, wortmannin, and the p38 inhibitor SB203580 were obtained from Sigma, St. Louis, Mo. They were added to cell culture media for 4 to 6 h on day 1 posttransfection at final concentrations of 40 μM, 20 nM, and 10 μM, respectively. Groα and IL-8 (R&D Systems, Minneapolis, Minn.) were added to the cell culture media at different concentrations (20 to 100 nM) 1 h prior to cell harvest for gel shift assays and 4 h prior to harvest for luciferase assays.
Luciferase reporter gene assays.
The NF-κB reporter construct expresses the firefly luciferase gene under regulation by a synthetic promoter containing five tandem binding sites for NF-κB (Stratagene, La Jolla, Calif.). AP1, CRE, and p53 luciferase reporter gene constructs were also obtained from Stratagene. KSIMM cells were transfected using Fugene 6 with 0.5 μg of the NF-κB luciferase reporter-gene construct, ORF74-pSG5, or pSG5 alone, various DN constructs, and pCMV–β-Gal (Stratagene). The amount of total DNA transfected was equalized with the appropriate amounts of control vectors. At 20 h posttransfection, cells were harvested and lysed in cell lysis buffer (a proprietary formulation) (Promega, Madison, Wis.). Protein concentration was normalized by bicinchoninic acid (BCA) assay (Pierce Biochemicals, Rockford, Ill.). Luciferase activity was determined using Luciferase Assay Reagent (Promega) and a luminometer (Turner Designs, Sunnyvale, Calif.). Transfection efficiencies were normalized by assaying β-Gal activity from the cotransfected pCMV–β-Gal.
Western blot analysis.
KSIMM cells were transfected with pSG5 control vector or ORF74-pSG5 using Fugene 6 (Boehringer Mannheim). Cells were harvested and lysed in lysis buffer (5% Triton X-100 in phosphate-buffered saline [PBS], pH 7.4) with protease inhibitors (complete minileupeptin, aprotinin, and Pefabloc) (Boehringer Mannheim). Protein quantification was carried out by BCA assay. Protein was loaded in each well and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 4 to 12% gels. Transfer to nitrocellulose membranes was performed in transfer buffer (12 mM Tris base; 96 mM glycine, pH 8.3; 15% methanol). Membranes were probed with primary antibodies to phospho-Akt (ser473), Akt (New England Biolabs, Beverly, Mass.), β-actin (Sigma), or ORF74 (rabbit polyclonal—gift of Gary Hayward, Johns Hopkins University). Secondary antibody conjugated to horseradish peroxidase (HRP) was used. Protein was detected using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's protocol.
Cytokine quantitation by ELISA.
KSIMM cells were transfected with pSG5-ORF74 or pSG5 alone using Fugene 6. Cell counts were taken to ensure equality of cell numbers in different wells. Supernatants from equal numbers of cells were collected 20 h posttransfection and centrifuged (12,000 rpm, 15 min) to remove cell debris. Supernatants were also collected from HUVECs 3 days after infection with the MIGR1-ORF74 or the control MIGR1 retrovirus. Cytokines in supernatants were quantified using commercial enzyme-linked immunosorbent assay (ELISA) plates precoated with the appropriate antibodies (R&D Systems). Samples were added to plates, which were incubated at room temperature for 1 h. Plates were washed four times with PBS–0.5% Tween 20, and biotinylated anticytokine antibodies were added to the plates, which were incubated at room temperature for 1 h. After a washing step, streptavidin-HRP was added to the plates for 30 min, and the plates were washed again and then incubated with TMB (3,3′,5,5′-tetramethylbenzidine) substrate for 20 min. Reactions were stopped with 2 N H2SO4, and the absorbance at 450 to 570 nm was determined. All readings were done in duplicate.
Flow cytometric analysis.
KSIMM cells were transfected with pSG5-ORF74 or pSG5 alone. Cells were detached 20 h posttransfection with 1 mM EDTA and counted. Aliquots (0.5 × 106) of transfected cells were washed twice in ice-cold PBS with 2% FBS. Cells treated with tumor necrosis factor alpha (TNF-α; 5 ng/ml) were included as a positive control for induction of adhesion marker expression. Appropriate antibodies were aliquoted into each tube and incubated on ice in the dark for 60 min. Cells were washed twice in PBS–2% FBS and fixed in 0.5 ml of 2% paraformaldehyde. Samples were analyzed using a Becton-Dickinson FACSCalibur flow cytometer. Phycoerythrin (PE)-conjugated antibodies for VCAM-1, ICAM-1, and E-selectin were obtained from PharMingen (San Diego, Calif.). Appropriate isotype controls were used for each antibody as a control for nonspecific antibody binding.
Generation of ORF74 expression constructs.
By using DNA from a lambda clone of HHV-8 (λ6-1) (33) containing ORFs 72 to 75 (GenBank accession number U82242) as a template, a fragment containing ORF74 was generated by PCR amplification using Bio-X-Act polymerase (Bioline, Reno, Nev.) and primers ORF74sense (5′-CGCAT GAATTCCTTGTTATTGTAGCATGGCGG-3′) and ORF74antisense (5′-CGATG AGATCTGGGCTACGTGGTGGCG-3′). The fragment was separated on an agarose gel, extracted with a Concert Rapid Gel Extraction kit (LTI), and digested with EcoRI and BglII (underlined). The purified fragment was ligated into the EcoRI/BglII sites of the expression plasmid vector pSG5 (Stratagene) with T4 ligase and transfected into STBL2 bacterial cells (LTI). Transformants were screened for the presence of the ORF74 insert by restriction digest analysis of DNA minipreps using BglII and EcoRI. Two clones were identified and sequenced at the University of Maryland, Baltimore, Biopolymer Core Facility with an ABI Prism model 373, version 3.0, DNA sequencer to verify the presence of the correct open reading frame.
A retroviral construct expressing ORF74 was prepared using pMIGR1 (53), which expresses GFP using an encephalomyocarditis virus (EMCV)-derived IRES and the same promoter used to drive ORF74 expression. To clone ORF74 into pMIGR1, λ6-1 DNA was used as a template for PCR amplification with Bio-X-Act polymerase and the primers 495B (5′-CTCTAGATCTTTGTTATTGTTGGCCATGGC-3′) and 494 (5′-CAACGAATTCCTACGTGGTGGCGCCGGAC-3′). The PCR product was purified from an agarose gel with the Concert Rapid PCR Purification Kit (LTI), digested with BglII and EcoRI, repurified as described above, ligated into the BglII/EcoRI sites of pMIGR1 using T4 DNA ligase, and transfected into STBL2 cells. Transformants were screened for the presence of the ORF74 insert by restriction digest analysis of DNA minipreps using BglII and EcoRI. Endotoxin-free plasmid DNA was prepared from two positive clones using the EndoFree Plasmid Maxi Kit (Qiagen, Valencia, Calif.). Both clones were analyzed by DNA sequencing and found to contain the correct ORF.
Generation of mutant ORF74 (V142D).
The V142D mutant of ORF74 was generated by PCR in two steps. (i) ORF74 was PCR amplified from pMIGR1(ORF74) in two segments that overlapped in the region of Val142. Val was changed to Asp in both fragments using a partly mismatched primer. The primers for the 5′ portion of ORF74 were 495B and MP3 (5′-TGCCACCAGGAGGTACCTATCTTGACTGACGCACAC-3′) and for the 3′ portion were 494 and MP1 (5′-GTGCGTCAGTCTAGATAGGTACCTCCTGGTGGCATA-3′). The mismatched bases are in boldface italics. The PCR products were gel purified as described above. (ii) The two fragments were joined by PCR overlap extension. An equimolar mixture of the two purified mutant fragments was amplified by PCR using the outer primers 495B and 494. The resultant PCR product was gel purified as before, digested with BglII and EcoRI, repurified, ligated into the cognate sites of MIGR1, and transfected into STBL2 cells. Transformants were grown and analyzed by digestion with XbaI, which is unique to the mutant ORF74 sequence, and EcoRI plus BglII. Endotoxin-free plasmid DNA was prepared using EndoFree Maxi Plasmid Kit (Qiagen) from two positive clones. The mutant ORF74 sequence was confirmed by sequence analysis of both clones.
Chemotaxis assays.
Chemotaxis assays were performed with the U937 monocytoid and Jurkat CD4+ T-lymphoid cell lines (American Type Culture Collection, Manassas, Va.) as described previously (11). Cells were harvested and resuspended at a concentration of 1.6 × 106 cells/ml in assay medium (RPMI 1640 containing 25 mM HEPES without phenol red or sodium bicarbonate). Cells then were labeled with 0.1 μM Calcein AM (Molecular Probes, Eugene, Oreg.) and an equal volume of Pluronic F-127 (Molecular Probes) at 37°C for 30 min. After being loaded, the cells were washed twice in assay medium and resuspended at a concentration of 4 × 106 cells/ml. Chemotaxis was measured in 96-well ChemoTx disposable chambers with 5-μm-diameter pores (Neuroprobe, Cabin John, Md.). Supernatants from ORF74- or control vector-transfected cells (total volume, 29 μl) were placed in the lower chamber and covered with a filter. A 25-μl drop containing 105 cells was then placed on the filter top. The chamber was placed in a 37°C incubator for 6 h. The fluorescence emission in the lower chamber was measured at 517 nm by using a Victor fluorescence plate reader (Wallac, Gaithersburg, Md.). The fluorescence values were converted to cell number based on a standard curve generated by staining serial concentrations of cells with 0.1 μM Calcein AM as described above.
Gel shift assays.
Cells were transfected with pSG5-ORF74 or pSG5 alone. Day 1 posttransfection cells were harvested, and nuclear extracts were prepared. Cells were centrifuged at 4°C and 1,200 rpm for 10 min, washed in ice-cold PBS, suspended in 100 μl of sucrose buffer (0.32 M sucrose; 3 mM CaCl2; 2 mM magnesium acetate; 0.1 mM EDTA, pH 8; 10 mM Tris-HCl, pH 7.9; 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF]; 0.5% NP-40; 1 mM NaF; 1 mM Na3VO4; 0.01 mg of aprotinin/ml; 0.01 mg of leupeptin/ml; 0.01 mg of pepstatin A/ml) per 107 cells and incubated on ice. After centrifugation at 600 × g for 5 min at 4°C, the supernatants were removed and the nuclear pellets were washed in sucrose buffer lacking NP-40. Nuclear pellets were lysed in 50 μl of Dignam buffer (20 mM Tris-Cl, pH 7.9; 1.5 mM MgCl2; 420 mM NaCl; 2 mM EDTA, pH 8; 1 mM PMSF; 1 mM NaF; 1 mM Na3VO4; 0.01 mg of aprotinin/ml; 0.01 mg of leupeptin/ml; 0.01 mg of pepstatin A/ml; 50 mM β-glycerol phosphate). Lysed pellets were incubated on ice for 20 min and centrifuged at 9,000 × g. Supernatants were collected and stored at –80°C. Oligonucleotide probes containing NF-κB binding sites were labeled with [α-32P]dCTP. Protein concentrations of each sample were determined by BCA assay. A total of 4 to 5 μg of nuclear protein was incubated on ice in DNA binding buffer (10 mM HEPES, pH 7.9; 50 mM KCl; 0.2 mM EDTA; 2.5 mM DTT; 10% glycerol; 0.05% NP-40), 2 to 3 μg of poly(dI-dC), and labeled NF-κB probe (4 × 104 cpm). Samples were electrophoresed on a 4% nondenaturing acrylamide gel, and the gel was dried and autoradiographed.
ELISA-based NF-κB DNA binding assay.
KSIMM cells were transfected with 100 or 500 ng of pSG5-ORF74 or pSG5 in 25-cm2 plates. Cells were harvested at 16 h posttransfection. Alternatively, cell lysates were prepared from dMVECs infected with retrovirus MIGR1-ORF74 or MIGR1. Whole-cell lysates were assayed for activated NF-κB DNA binding ability by an ELISA-based assay kit (Active Motif, Carlsbad, Calif.). This assay, which has been reported to be more sensitive than standard gel shift assays (56), uses 96-well plates coated with oligonucleotides containing the NF-κB consensus site (5′-GGGACTTTCC-3′). Cells were lysed in 100 μl of lysis buffer (20 mM HEPES, 0.35 M NaCl, 20% glycerol, 1% NP-40, 1 mM MgCl2 · 6H2O, 0.5 mM EDTA, 0.1 mM EGTA) containing a protease inhibitor cocktail provided in the kit. Various amounts of lysate were incubated in binding buffer [4 mM HEPES, 100 mM KCl, 8% glycerol, 5 mM DTT, 0.2% bovine serum albumin, 0.016% poly(dI-dC)] for 1 h. Excess (20 pmol) mutant probe (5′-AGTTGAGGCCATTTCCCAGGC-3′) and wild-type probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were added to reactions in the competition experiments. Plates were washed three times in wash buffer (PBS, 0.1% Tween 20), incubated with rabbit anti-p65 antibody for an hour, rewashed three times, incubated with an HRP-conjugated anti-rabbit immunoglobulin G (IgG) antibody for 1 h, and rewashed four times. Wells were incubated with 100 μl of TMB for 5 min, and 100 μl of stop solution was added as per the kit directions. Plates were analyzed with an ELISA plate reader at 450 nm with a reference wavelength of 655 nm.
RESULTS
ORF74 activates NF-κB.
Transient expression of ORF74 in KSIMM cells, an HHV-8-negative KS-derived cell line, resulted in the activation of an NF-κB-sensitive reporter gene. As shown in Fig. Fig.1A,1A, NF-κB activation was seen upon transfection using as little as 20 ng of ORF74-pSG5 per well. There was a progressive increase in NF-κB activity with increasing amounts of transfected ORF74 DNA. In samples transfected with 500 ng of ORF74 plasmid DNA, NF-κB was activated about eightfold above the baseline. Transfection with an empty control vector failed to appreciably induce NF-κB. Figure Figure1B1B shows that the levels of ORF74 protein, as judged by Western blotting, roughly correlated both with the increasing amounts of transfected ORF74 plasmid DNA and with the corresponding increase in NF-κB activity seen in Fig. Fig.1A.1A. The fuzzy bands representing ORF74 in the Western blots have an apparent molecular mass of about 45 kDa, somewhat larger than the calculated mass of 38 kDa. This is likely due to glycosylation of the multiple consensus glycosylation sites in ORF74 or to other posttranslational modifications.

Dose response of ORF74-induced activation of NF-κB in KSIMM cells. (A) Dose response to transfected ORF74. KSIMM cells (105) were transfected with a NF-κB luciferase reporter construct (100 ng/sample) and increasing amounts (20 to 500 ng) of an expression vector encoding ORF74 (ORF74-pSG5). Total input DNA was balanced with the empty expression vector (pSG5). A CMV–β-Gal vector (200 ng) was cotransfected to serve as an internal control for the efficiency of transfection. Transfection efficiencies were ca. 20%. Cells were harvested and assayed for luciferase and β-Gal activity 24 h after transfection. Luciferase values are expressed as relative light units (RLU) and were normalized to the constant β-Gal activity. The values shown are averages of three independent samples, with standard deviations represented by the error bars. (B) ORF74 protein expression. KSIMM cells were transfected with increasing amounts of ORF74-pSG5 DNA (lanes 2 to 5) as described for panel A. Cell lysates were analyzed for ORF74 protein by SDS-PAGE and Western blotting. Lane 1 contains protein lysate from KSIMM cells transfected with pSG5 alone. The band in lane 1 is a nonspecific band given by the ORF74 rabbit polyclonal antibody. Blots were stripped and reprobed with an antibody to β-actin to ensure that equal amounts of protein were loaded in each lane.
Figure Figure2a2a shows the kinetics of NF-κB activation by ORF74. For samples transiently transfected with 100 ng of ORF74 plasmid DNA, activation was rapid, reaching a maximum within 24 h of transfection and then diminishing completely by 72 h. For samples transfected with 500 ng of DNA NF-κB activity reached a maximum by 24 h and diminished slightly thereafter but was still evident at 72 h posttransfection. We wanted to determine how NF-κB activation kinetics at the two input levels of plasmid DNA (100 ng and 500 ng) correlated with ORF74 protein levels. As shown in Fig. Fig.2B,2B, the NF-κB activity over time correlates well with ORF74 protein levels. In samples transfected with 100 ng of ORF 74, protein expression is maximal at 24 h and diminishes to undetectable levels by 72 h posttransfection. In samples transfected with 500 ng of ORF74 DNA, protein expression is also maximal at 24 h and diminishes slightly thereafter, but it is still present at 72 h posttransfection.
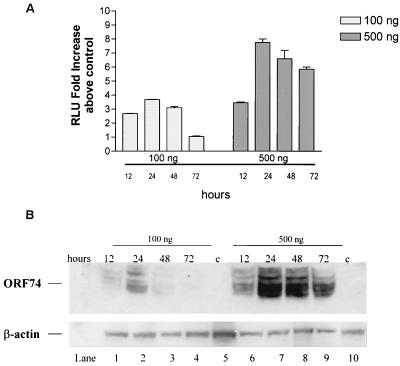
Time course of ORF74-induced activation of NF-κB in KSIMM cells. (A) Time course of NF-κB signaling. KSIMM cells (105) were transfected with an NF-κB luciferase reporter construct (100 ng) and 100 or 500 ng of pSG5-ORF74. Transfection efficiencies were ca. 20%. Controls were transfected with pSG5 alone. Cells were harvested at the indicated time points and assayed for luciferase activity. Readings are expressed as the fold increase of luciferase above the control vector-transfected cells. Values shown are averages of three independent samples, with standard deviations represented by the error bars. (B) ORF74 protein expression. KSIMM cells were transfected with 100 ng (lanes 1 to 4) or 500 ng (lanes 6 to 9) of ORF74 DNA as described for panel A. The cells for lanes 5 and 10 (designated “c” for control) were transfected with pSG5 alone and harvested at 24 h posttransfection. Cell lysates were analyzed for ORF74 protein by SDS-PAGE and Western blotting. Blots were stripped and reprobed with an antibody to β-actin to ensure that equal amounts of protein were loaded into each lane.
Electrophoretic mobility gel shift assays (EMSAs) were performed to determine whether ORF74 activation of NF-κB correlated with induction of protein binding to NF-κB sites. Figure Figure3A3A shows that this is indeed the case. Retardation of migration of an oligonucleotide containing a consensus NF-κB site was evident after incubation with nuclear extracts prepared from ORF74-transfected cells. Supershift assays were performed using antibodies to members of the NF-κB family. As shown in Fig. Fig.3A,3A, the bound complexes were shifted by antibodies to p65(RelA) and p50, suggesting that NF-κB binding was at least in part by p65-p50 heterodimers. To further characterize NF-κB binding, an ELISA-based DNA-binding assay was performed for p65 (see Materials and Methods) (56). In this assay, the NF-κB complex bound to an immobilized oligonucleotide containing an NF-κB consensus DNA binding site is detected with an antibody to p65. A secondary antibody conjugated to HRP provides a colorimetric readout. Figure Figure3B3B shows that cell lysates from KSIMM transfected with either 100 or 500 ng of pSG5-ORF74 show enhanced NF-κB binding to the oligonucleotide containing the consensus site. This increase binding is inhibited by addition of excess soluble wild-type oligonucleotide but not excess oligonucleotide containing a mutant consensus site.
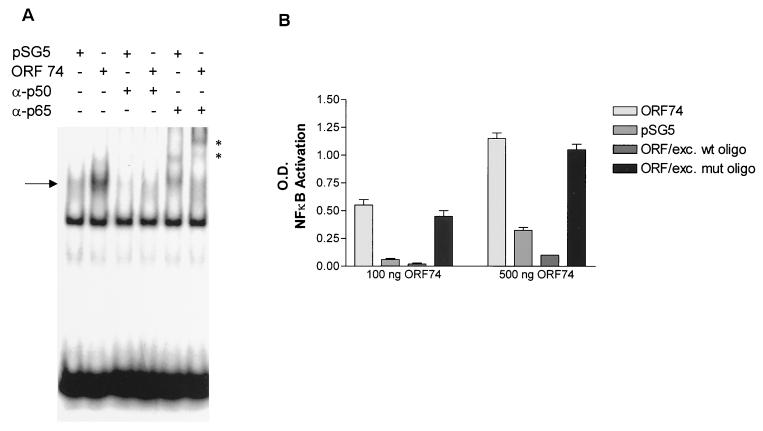
Induction of NF-κB binding activity by ORF74. (A) NF-κB binding activity is induced by ORF74. KSIMM cells were transfected with ORF74-pSG5 or pSG5 control vector; the transfection efficiency was 28%. Cells were harvested and nuclear extracts were prepared and used in an electrophoretic mobility shift assay as described in Materials and Methods. Supershift assays were performed with the indicated antibodies. Complexes supershifted with p65 antibody are marked with an asterisk. (B) ELISA-based NF-κB DNA binding assay. KSIMM cells were transfected with 100 or 500 ng of ORF74-pSG5 or pSG5. Cells were harvested at 16 h posttransfection. Cell lysates were assayed for NF-κB DNA binding using an ELISA-based assay kit (Active Motif) in which 96-well plates are coated with oligonucleotides containing the NF-κB consensus site. Excess (20 pmol) mutant (ORF/exc. mut oligo) or wild-type (ORF/exc. wt oligo) probes were added to the indicated samples as competitors. Plates were incubated with rabbit anti-p65 antibody for 1 h, followed by an HRP-conjugated anti-rabbit IgG, and developed with TMB (see Materials and Methods). The binding was quantified spectrophotometrically.
Mutant ORF74(V142D) constitutively activates NF-κB.
One of the striking characteristics of ORF74-mediated signaling is its activity in the absence of ligand. Recent work by Burger et al. (10) has shown that mutation of a DRY motif present in the second transmembrane loop of the IL-8 receptor CXCR2, which is common to most cellular chemokine receptors, results in ligand-independent signaling by CXCR2. We were interested in whether the opposite change in ORF74, from VRY to DRY, would eliminate the ligand-independent activation of NF-κB. Figure Figure44 shows that the mutant ORF74(V142D) retained the ability to activate NF-κB in the absence of added ligand and did so at a slightly enhanced level compared to the wild-type ORF74. Interestingly, however, signaling by ORF74(V142D) was greatly enhanced in the presence of Groα, an ORF74 ligand. Groα at 20 nM did not enhance activation of NF-κB by wild-type ORF74 compared with >8-fold activation of ORF74(V142/D) above that for the control. Wild-type ORF74 could only be stimulated at the increased dose of 100 nM Groα, with the mutant showing a much more pronounced NFκB activation at 100 nM Groα. Cells transfected with an empty control vector were not affected by addition of Groα at either concentration. IL-8 did not enhance the activation of NF-κB by either mutant or wild-type ORF74 constructs (data not shown).
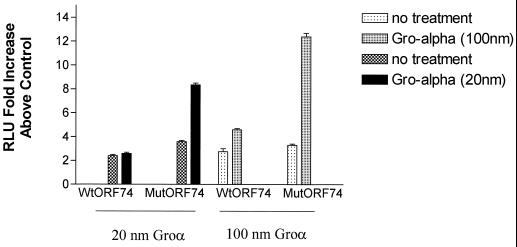
Inducible NF-κB signaling by mutant ORF74. KSIMM cells (105) were transfected with an NF-κB luciferase reporter plasmid and wild-type ORF74 (ORF74-MIGR1), mutant ORF74 (V142D) in the same vector, or the control vector (MIGR1). At 16 h posttransfection, cells were treated with Groα for 4 h. Cells were harvested, lysed, and assayed for luciferase activity as described in Materials and Methods. Readings are expressed as the fold increase of luciferase above that of the control vector-transfected cells. Transfection efficiencies were ca. 25%.
ORF74 activates NF-κB in primary endothelial cells.
To determine whether ORF74 could activate NF-κB in primary endothelial cells, dMVECs were infected with a retrovirus expressing ORF74 from a Moloney murine leukemia virus long terminal repeat (LTR) (see Materials and Methods). This retrovirus (MIGR1) also expresses GFP using an EMCV-derived IRES and the same promoter used to drive ORF74, thereby allowing visualization of infected cells expressing ORF74. Lysates from these cells were assayed for activation of NF-κB DNA binding by the ELISA-based NF-κB DNA binding assay described above. Figure Figure5A5A shows that cell lysates from dMVECs infected with a retrovirus expressing ORF74 show enhanced NF-κB DNA binding relative to lysates from control retrovirus-infected cells. This increase in NF-κB DNA binding is inhibited by addition of excess wild-type oligonucleotide but not excess oligonucleotide containing a mutant consensus site. Western blots (Fig. (Fig.5B)5B) show that ORF74 protein is expressed but at somewhat lower levels than in KSIMM cells transfected with 100 ng of ORF74 DNA and analyzed 24 h posttransfection.
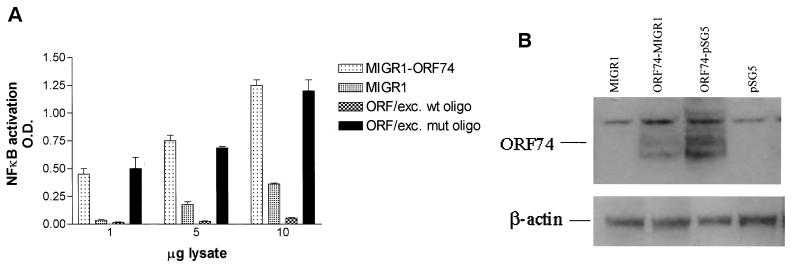
ORF74 activates NF-κB in primary endothelial cells. (A) Activation of NF-κB DNA binding in endothelial cells expressing ORF74. dMVECs were infected with a retrovirus expressing ORF74 from the Moloney murine leukemia virus LTR (ORF74-MIGR1) (see Materials and Methods) or control virus alone (MIGR1). Lysates from these cells were assayed for activation of NF-κB DNA binding by ELISA-based NF-κB DNA-binding assays (see Materials and Methods). Excess (20 pmol) mutant probe (ORF/exc. mut oligo) or probes (ORF/exc. wt oligo) wild-type were added to reactions as competitors. (B) Expression of ORF74 protein in primary endothelial cells. Cell lysates were harvested 10 days postinfection, and 25 μg of protein lysate was analyzed by SDS-PAGE and Western blotting for ORF74. Twenty-five micrograms of protein lysate from KSIMM cells transfected with 100 ng of ORF74-pSG5 or empty vector (pSG5) was included for comparison of the protein expression levels. Lysates from transfected cells were harvested at 24 h posttransfection. The transfection efficiency was 25%. Blots were stripped and reprobed with an antibody to β-actin to ensure that equal amounts of protein were loaded in each lane.
Expression of ORF74 in endothelial cells results in spindle-shaped cells.
Interestingly, transduction of ORF74 into primary endothelial cells (both HUVECs and dMVECs) using a retroviral vector resulted in profound changes in their morphology. As shown in Fig. Fig.6B6B and D, cells infected with the ORF74 retrovirus assume a spindle shape compared to control virus-infected cells (Fig. (Fig.6A6A and C), an appearance strikingly similar to that of the spindle cells present in KS lesions. Infection efficiencies for both control and ORF74-expressing viruses were ca. 80%, as determined by counting GFP-positive cells.
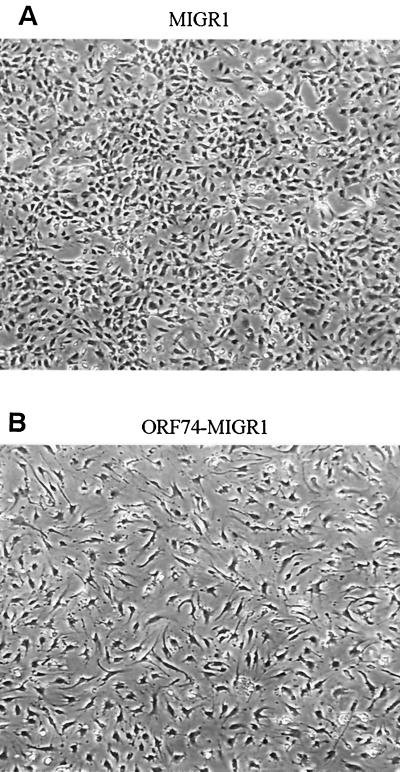
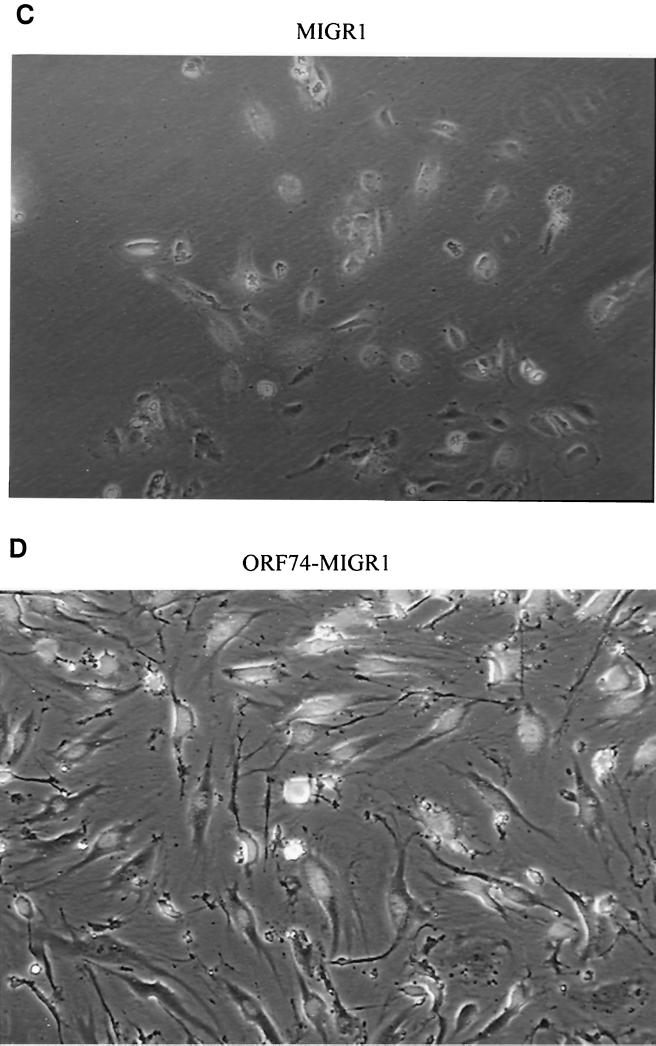
Induction of spindle morphology in primary endothelial cells by ORF74. dMVECs (passages 4 and 5) were infected with the retroviral expression construct MIGR1-ORF74 (B and D) or the control vector MIGR (A and C). A reporter gene for GFP is expressed on the same mRNA as ORF74 and translated from an IRES in the retroviral vector (see Materials and Methods). Fluorescence was detected using a 395-nm excitation light source for illumination as seen in panels C and D. Photographs were taken by phase-contrast microscopy at 20× magnification at 10 days postinfection. The whitish hue on cells in panels C and D represents GFP expression. Panels A and B (4× magnification) show the morphologic changes in the culture population as a whole (infection efficiency was ca. 80%).
DN mutants of IKKα, IKKβ, and IκBα inhibit activation of NF-κB by ORF74.
To further characterize NF-κB activation by ORF74, we tested whether DN mutants of proteins involved in NF-κB signaling could inhibit the observed activation. In unstimulated cells, NF-κB is sequestered in an inactive form in the cytoplasm bound to Ικβ proteins. Appropriate stimulation of cells, by viral genes or exogenous stimuli such as TNF-α, leads to a rapid phosphorylation by the IκB kinase (IKK) complex. IKK then phosphorylates IκB, which is ubiquitinated and degraded, freeing NF-κB to translocate to the nucleus and activate transcription of its target genes. Figure Figure77 shows that DN IKKβ and DN IκBα completely inhibited NF-κB activation by ORF74 in KSIMM cells. Partial inhibition was obtained under the same conditions with DNIKKα, another kinase in the NF-κB pathway.
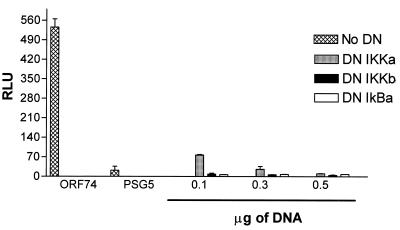
Inhibition of NF-κB activation by ORF74 by DN mutants. KSIMM cells (3 × 105 cells) were transfected with pSG5-ORF74, the NF-κB luciferase reporter plasmid, and increasing amounts of the indicated DN mutant expression constructs. Total DNA inputs were equalized with control vector. Cells were lysed and luciferase expression was quantified at 18 h posttransfection. KSIMM cells were transfected with ORF74 and increasing amounts of DN constructs of IKKα, IKKβ, and IκB. All samples were analyzed in triplicate, and the values were averaged. The transfection efficiencies were ca. 30%.
ORF74 is known to activate the AP1 and CRE transcription factor pathways, which are downstream of the MAP kinase pathways. To understand how the levels of activation of NF-κB by ORF74 compare with those of AP1 and CRE, KSIMM cells were transfected with luciferase reporter gene constructs and ORF74 (200 ng) or pSG5 (200 ng). p53 luciferase was included as a negative control to ensure that ORF74-mediated activation of these reporter constructs was specific. The fold activations of NF-κB, AP1, CRE, and p53 luciferase by ORF74 were 5.5, 5.6, 4.7, and 1, respectively (data not shown). These results indicate that the activation of NF-κB by ORF74 in reporter gene assays is similar to that of other factors known to be activated by ORF74 and has specificity.
PI 3-kinase inhibitors and DN Akt, but not a p38 inhibitor, block activation of NF-κB by ORF74.
To further elucidate the mechanism of NF-κB activation by ORF74, KSIMM cells transfected with pSG5-ORF74 were treated with either LY294002 and wortmannin, two specific inhibitors of phosphatidylinositol 3-kinase (PI 3-kinase), or SB203580, a specific inhibitor of p38 MAP kinase. Alternatively, samples were cotransfected with an expression construct of a DN mutant of Akt(K179M) (19). PI 3-kinase and its downstream kinase Akt activate NF-κB in response to stimuli such as TNF-α and platelet-derived growth factor (PDGF) (52, 58). The appropriate concentrations of LY294002 and DN Akt were determined by their ability to fully abrogate NF-κB activation in KSIMM cells induced by TNF-α (data not shown). NF-κB activation by ORF74 was almost completely inhibited by 40 μM LY294002 and 20 nM wortmannin (Fig. (Fig.8A).8A). Consistent with the possibility that activation by ORF74 involves signaling through PI 3-kinase and Akt, cotransfection with 0.2 μg of DN Akt inhibited NF-κB activation, although inhibition was incomplete. The p38 inhibitor SB203580 (10 μM) neither inhibited nor enhanced NF-κB activation by ORF74. Furthermore, Western blots of Akt (Fig. (Fig.8B)8B) showed enhanced phosphorylation of Akt in cells expressing ORF74. Quantification of the bands using a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) showed that the ratio of the intensity of phosphorylated Akt to the intensity of total Akt was 0.4 when pSG5 was transfected, compared to 0.83 when ORF74-pSG5 was cotransfected and 0.23 with the LY294002 control.
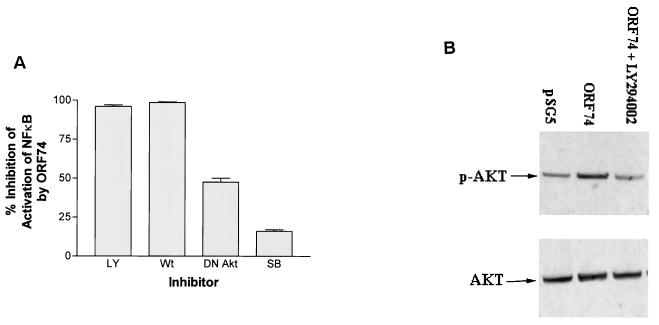
Involvement of PI 3-kinase and Akt but not p38 in NF-κB activation by ORF74. (A) Inhibition of NF-κB activation. KSIMM cells (106) were transfected with 0.3 μg of pSG5-ORF74 and 0.5 μg of the NF-κB luciferase reporter plasmid. Cells were treated with the PI 3-kinase inhibitors LY294002 (LY) (40 μM) and wortmannin (Wt) (20 nM) or by the p38 inhibitor SB203580 (SB) (10 μM) in dimethyl sulfoxide (DMSO) for 12 h and then assayed for luciferase activity as described in Materials and Methods. Controls were treated with DMSO alone. Alternatively, cells were cotransfected with ORF74-pSG5 and 0.2 μg of DN Akt mutant expression construct or a control plasmid and then assayed for luciferase activity as described above. All samples were analyzed in triplicate, and the values were averaged. Data are presented as the percent inhibition of NF-κB activity induced by ORF74 as assayed by measuring luciferase activity. (B) Akt phosphorylation. Cell lysates from KSIMM cells transfected with pSG5 or ORF74-pSG5 were subjected to SDS-PAGE and Western blot analysis using an antibody specific for phosphorylated Akt (p-AKT). Lysates from cells transfected with ORF74 and treated with the PI 3-kinase inhibitor LY294002 (40 μM) were also analyzed. Blots were stripped and reprobed with an antibody to total Akt (AKT) to determine the relative phosphorylation levels. The transfection efficiencies were ca. 25%.
CM from ORF74-expressing cells activates NF-κB in ORF74-negative cells and also induces T-cell and monocyte chemotaxis.
We next investigated whether ORF74 expression in cells could result in the release of soluble factors that could activate NF-κB in ORF74-negative cells in trans. We transfected KSIMM cells with an NF-κB luciferase reporter construct and subsequently treated these target cells with conditioned medium (CM) from cells transfected with pSG5-ORF74 or pSG5 alone at 24 h posttransfection. Figure Figure99 shows that activation of NF-κB in target cells by ORF74 CM was nearly threefold greater than that obtained with CM medim from cells transfected with the control vector. To further analyze the effects of CM from KSIMM cells expressing ORF74 on neighboring cells, we tested its ability to elicit chemotaxis of U937 monocytoid cells and Jurkat CD4+ T cells. Figure Figure1010 shows that CM from ORF74-transfected KSIMM cells indeed elicits chemotaxis of U937 cells and, to a lesser extent, of Jurkat cells.
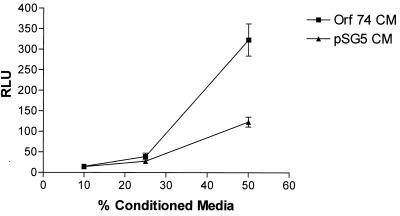
Paracrine stimulation of NF-κB by CM from cells transfected with ORF74. KSIMM cells (106) were transfected with ORF74-pSG5 or pSG5. CM from these cells was collected at 24 h posttransfection. Equal numbers of KSIMM target cells transfected with an NF-κB reporter plasmid were suspended in dilutions of 10, 25, and 50% CM in fresh medium, cultured for 6 h, and harvested. Luciferase activity was assayed as described in Materials and Methods. Datum points are the average of triplicate results from three wells.
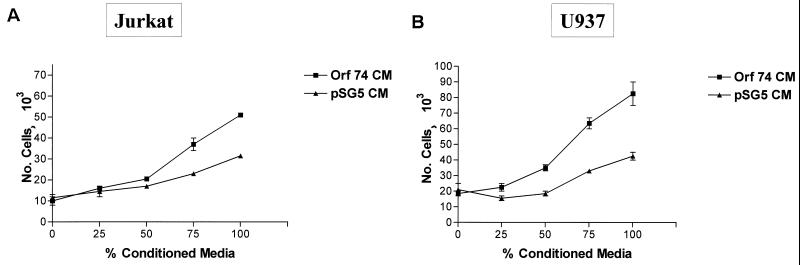
Chemotaxis of U937 and Jurkat cells to CM from KSIMM cells transfected with ORF74. CM from KSIMM cells transfected with ORF74-pSG5 or pSG5 was collected at 24 h posttransfection. Chemotaxis assays with the Jurkat CD4+ T-lymphoid cell lines (A) and U937 monocytoid cells (B) were performed as described in Materials and Methods using the indicated concentrations of CM. The number of cells was calculated from fluorescence of calcein-labeled cells in the lower chamber by comparison with a standard curve.
ORF74 induces the expression of NF-κB-dependent chemokines and cytokines: IL-6, IL-8, GM-CSF, and RANTES.
Inflammation is thought to play a key role in the development of KS, and inflammatory growth factors and cytokines such as gamma interferon are present in KS lesions (22). NF-κB activation is critical for the transcription of genes involved in inflammatory responses, including those encoding TNF-α, RANTES, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, GM-CSF, granulocyte colony-stimulating factor (G-CSF) cell adhesion molecules, acute-phase proteins, and transcription factors such as p53 and c-myc. To determine whether ORF74 enhances the expression of inflammatory cytokines and chemokines regulated by NF-κB, we tested the cytokine levels in supernatants from transfected cells. Table Table11 shows that the levels of IL-6, IL-8, and RANTES are all increased in supernatants of HUVECs and KSIMM cells expressing ORF74. GM-CSF expression was increased by ORF74 expression in KSIMM cells but not in HUVECs. To verify that ORF74 caused the upregulation of cytokines through an NF-κB-dependent mechanism, we cotransfected ORF74-pSG5 and the DN IκBα mutant into KSIMM cells. The increases in IL-6, IL-8, and RANTES were reduced 60, 30, and 40%, respectively, although GM-CSF expression was not significantly inhibited (not shown). TNF-α, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, and G-CSF were not changed upon expression of ORF74 in HUVECs or KSIMM (data not shown).
TABLE 1
Cytokine and growth factor expression in KSIMM and HUVECsa
| Cytokine | Cell type | Mean ELISA reading (pg/ml) ± SD
| Fold increase | |
|---|---|---|---|---|
| ORF 74 | Control | |||
| IL-6 | HUVECs | 223 ± ± 16 16 | 149 ± ± 6.55 6.55 | 1.5 |
| KSIMM | 1,187 ± ± 17.6 17.6 | 415 ± ± 45.9 45.9 | 2.86 | |
| IL-8 | HUVECs | 198 ± ± 47 47 | 121 ± ± 17 17 | 1.6 |
| KSIMM | 86,700 ± ± 1,110 1,110 | 54,400 ± ± 298 298 | 1.59 | |
| RANTES | HUVECs | 784 ± ± 14 14 | 56 ± ± 14 14 | 14 |
| KSIMM | 401 ± ± 59 59 | 46 ± ± 9.9 9.9 | 8.7 | |
| GM-CSF | KSIMM | 199 ± ± 22.7 22.7 | 117 ± ± 2.516 2.516 | 1.7 |
| VEGF | HUVECs | 350 ± ± 30 30 | 170 ± ± 14 14 | 2.05 |
| KSIMM | 728 ± ± 50 50 | 596 ± ± 30 30 | 1.22 | |
ORF74 induces the expression of NF-κB-dependent adhesion molecules VCAM-1, ICAM-1, and E-selectin.
We sought to determine by flow cytometry whether ORF74 enhances the expression of NF-κB-dependent adhesion molecules. Cell surface levels of VCAM-1, ICAM-1, and, to a lesser degree, E-selectin were enhanced on KSIMM cells expressing ORF74 (Fig. (Fig.11A,11A, B, and C). To ensure that ORF74 expression did not affect all cell surface markers, transfected cells were also stained with antibody against αVβ3, the vitronectin receptor. Figure Figure11D11D demonstrates no appreciable change in the surface expression of αVβ3 in cells expressing ORF74.
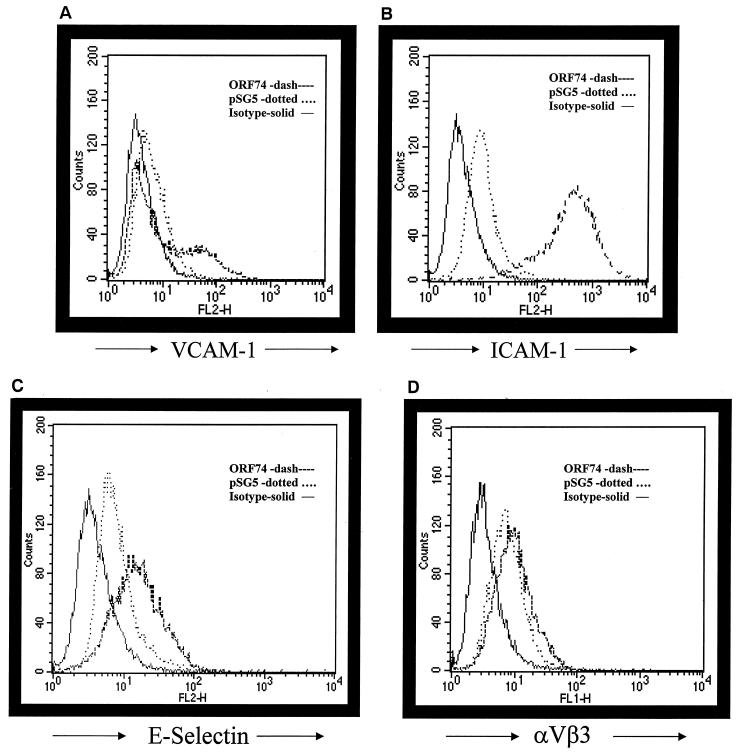
Induction of adhesion molecule expression by ORF74. KSIMM cells were transfected with pSG5-ORF74 or pSG5 alone. Cells were detached at 20 h posttransfection with 1 mM EDTA and counted. Aliquots (0.5 × 106) of transfected cells were stained with the indicated antibodies. Cells treated with TNF-α (5 ng/ml) were included as a positive control for the induction of adhesion marker expression (data not shown). Samples were analyzed using a Becton Dickinson FACSCalibur flow cytometer. PE-conjugated antibodies for VCAM-1, ICAM-1, E-selectin, and αVβ3 integrin were obtained from PharMingen. Appropriate isotype-matched controls were used for each antibody as a control for nonspecific antibody binding. Cell Quest Analysis (Becton Dickinson version 3.1f) software was used to analyze the raw fluorescence-activated cell-sorting data and obtain quantitative values. (A) Analysis of expression of cell surface ICAM-1. (B) Analysis of expression of cell surface VCAM-1. (C) Analysis of expression of cell surface E-selectin. (D) Analysis of expression of cell surface αVβ3. The solid line represents the isotype control, the dotted line represents pSG5-transfected cells, and the dashed line represents ORF74-transfected cells. The transfection efficiency was ca. 35%.
DISCUSSION
Infection with HHV-8 is required for the development of KS. Previous studies have indicated that inflammatory cytokines and chemokines contribute to the development of KS (20), at least in part by stimulating the angioproliferation and immune cell infiltration characteristic of KS lesions. Since expression of many of these inflammatory factors is dependent on the transcription factor NF-κB, we sought to determine whether the HHV-8 chemokine receptor gene ORF74 could activate NF-κB and thereby induce elevated expression levels of factors that could contribute to the development of KS by paracrine mechanisms. We wanted to carry out these studies in a relevant cell type, so we used KSIMM (an HHV-8-negative KS endothelial cell line) (2) and primary human endothelial cells (HUVECs and dMVECs).
Our studies indicate that ORF74 indeed activates NF-κB in both KS and primary endothelial cells, in a ligand-independent fashion. Expression of ORF74 in KSIMM cells transactivated an NF-κB-dependent promoter and induced DNA binding to NF-κB sites, as judged by luciferase reporter gene activity, gel shift assays, and an ELISA-based DNA binding assay. Activation of NF-κB by other G protein-coupled receptors (GPCR) has recently been demonstrated, although the exact mechanisms have not fully been elucidated (45). It is clear, however, that there is a significant amount of cross talk between signaling pathways, and activation of NF-κB by chemokine receptors may be more common than is currently appreciated.
Upon transfection of lower amounts of plasmid DNA, NF-κB activation occurred rapidly, reached a maximum by 24 h, and then diminished and disappeared by 72 h. This activation correlated directly with expression of ORF74 protein. Transfection of higher levels of ORF74 plasmid DNA resulted in maintenance of NF-κB activation at up to 72 h posttransfection, although it was slightly diminished from its maximum at 24 h, and protein expression was also maintained. We do not know why the expression of ORF74 diminishes so rapidly. There are a number of possibilities, one being that expression is toxic, although we did not see an increase in cell death in ORF74-transfected KSIMM cells. Toxicity of ORF74 would not be disadvantageous to virus replication, since cells normally die during lytic phase. In the retrovirus-infected endothelial cells, ORF74 expression is present and does not seem to be toxic to the cells. This may be because ORF74 is expressed at lower levels by the Moloney murine leukemia virus promoter than by the simian virus 40 promoter. It also may be possible that even though we did not note an increase in cell death in the samples transfected with ORF74, untransfected cells have a growth advantage over the transfected cells, resulting in an overall decrease in ORF74 levels. The decrease seems too fast, however, for this to be a reasonable explanation.
The IL-8 receptor CXCR2 is the closest cellular homologue to ORF74, suggesting that ORF74 may be a captured CXCR1 or CXCR2 gene. Among chemokine receptors, a DRY motif in the second intracellular loop is highly conserved. Burger et al. (10) exchanged Val for Asp to change the DRY of CXCR2 to the VRY found in ORF74 and showed that this resulted in a ligand-independent active receptor with transforming potential. We performed the reverse mutagenesis, changing the VRY motif in ORF74 to DRY, to see if it would abrogate ligand-independent activation of NF-κB. This same mutation has recently been reported elsewhere to not alter ligand binding or basal inducible signaling through phospholipase C (59). The V142D mutation did not abrogate constitutive activation of NF-κB; it slightly enhanced it. There was an increased induction of NF-κB signaling, however, after binding of Groα to the mutant ORF74 compared to the wild type. Recent work (34) confirms that the V142D mutation does not inhibit ligand- independent signaling by ORF74 but rather enhances it. These findings suggest that other differences between cellular chemokine receptors and ORF74 influence ligand-independent signaling and that different domains of ORF74 are involved in Groα stimulation of NF-κB and phospholipase C activation.
The multisubunit IκB kinase (IKK) is responsible for inducible phosphorylation of IκB and appears to be the point of convergence for most stimuli that activate NF-κB. This apparently includes ORF74 signaling. IKKβ and IKKα are the two catalytic subunits of the IKK complex, and both phosphorylate IκB. Gene knockout studies of these kinases indicate that IKKβ is primarily responsible for the activation of NF-κB in response to proinflammatory stimuli, whereas IKKα may not be as critical (42, 43). DN mutants of IKKβ and IκBα completely inhibited NF-κB activation by ORF74 in KSIMM cells, whereas DN IKKα only partially inhibited activation under the same conditions. This pattern is similar to what would be seen in the case of NF-κB activation by proinflammatory stimuli such as TNF-α and suggests that ORF74 activates related pathways.
Two specific inhibitors of PI 3-kinase, LY294002 and wortmannin, almost completely inhibited activation of NF-κB by ORF74. PI 3-kinase has been implicated in a number of cellular functions, including cell adhesion, vesicular trafficking, protein synthesis, and cell survival. GPCRs, including the IL-8 receptor, have been shown to activate PI 3-kinase and its downstream target kinase Akt through G-protein β-γ dimers (49, 66). The serine-threonine kinase Akt itself has been shown to control cell survival, glycogen metabolism, and cellular transformation (24). Recent work by other groups has demonstrated NF-κB to be a target of Akt activation by stimulators such as TNF-α and PDGF (58). Our findings suggest that ORF74 indeed activates the PI 3-kinase/Akt/NF-κB pathway. It appears from our results with LY294002 and wortmannin that this pathway is the major contributory pathway leading to activation of NF-κB by ORF74. This would also be consistent with what has been reported for other GPCRs and activation of NF-κB. We cannot completely rule out the possibility of other contributory pathways feeding into the activation of NF-κB, such as the MAP kinase pathways, since there is a large degree of cross talk between pathways. The PI 3-kinase/Akt pathway may contribute significantly to the enhancement of angiogenesis and VEGF production induced by ORF74 in KS, since recent findings have shown that PI 3-kinase/Akt signaling mediates angiogenesis and the expression of VEGF in endothelial cells (35). We find that treatment of KSIMM cells with the PI 3-kinase inhibitor LY294002 suppresses the increase in VEGF production by ORF74 (data not shown), suggesting that ORF74 utilizes pathways in addition to the p38 MAP kinase pathway to upregulate VEGF (62).
In spite of the strong epidemiologic association of HHV-8 with KS, it is not clear how the virus initiates or facilitates the disease. As mentioned previously, in early KS lesions, only a minority of the spindle cells believed to constitute the abnormal cell population are infected (8). In later lesions, although most spindle cells are infected, the majority of infected cells are in viral latency, and only a few cells are undergoing lytic replication (8, 64). Infection of a minority of primary endothelial cells has been reported to transform the culture as a whole (23). Recently, transgenic mice expressing ORF74 in hematopoietic cells have been shown to develop angioproliferative lesions in the skin that display many characteristics of KS, suggesting a paracrine pathogenic mechanism of ORF74 (69) that the authors of that study attribute primarily to the induction of VEGF expression.
We found that CM from KSIMM cells transfected with ORF74 is capable of activating NF-κB in cells not expressing ORF74, suggesting that a minority of cells in KS lesions expressing ORF74 could affect the proliferation and activation of neighboring cells through the release of soluble factors. The finding that CM from cells transfected with ORF74 can itself activate NF-κB suggests that part of the NF-κB activity that we see in ORF74-transfected KSIMM cells could be by a paracrine mechanism. It is difficult to discriminate between a direct effect and a paracrine effect, although the rapidity of induction of luciferase activity and NF-κB binding to oligonucleotides argues that in this system much of the activation by ORF74 is direct. The same CM was also able to elicit enhanced chemotaxis of immune cells (U937 cells and, to a lesser extent, Jurkat cells), suggesting that ORF74 may contribute to the characteristic immune cell infiltration of KS lesions. Analysis of this CM showed elevated levels of NF-κB-dependent chemokines and cytokines, including IL-6, IL-8, GM-CSF, and RANTES. Increases were similar with both KSIMM CM and HUVEC CM, except for GM-CSF, which was increased only in KSIMM CM. The contribution of NF-κB to the increases in these cytokines and chemokines by ORF74 was evident from the partial inhibition of these increases by cotransfection of a DN mutant of IκBα. There was also an induction in VEGF expression by ORF74, as reported by other groups (5, 62). The most striking ORF74-induced increase was in RANTES, which increased 14- and 9-fold in HUVECs and KSIMM, respectively. RANTES could contribute to enhanced chemotaxis toward KS cells expressing ORF74. It is possible, however, that many of these factors cooperate in producing the angioproliferation and inflammation seen in KS lesions. IL-8 is an α chemokine that is chemotactic for lymphocytes and neutrophils and has been shown to be proangiogenic through its ability to stimulate the proliferation, migration, and chemotaxis of endothelial cells (39). IL-6 has also been implicated in angiogenesis and has been shown to induce expression of VEGF in endothelial cells (17, 48). GM-CSF can also enhance the migration and proliferation of human endothelial cells (13). These findings suggest that ORF74 helps to upregulate the production of a number of proangiogenic, inflammatory factors, in addition to VEGF, which all may contribute significantly to the development of KS by both paracrine and autocrine mechanisms.
As mentioned previously, KS lesions are composed of a complex mixture of different cell types, including not only the characteristic spindle cells but also fibroblasts, microvascular endothelial cells, dendritic cells, and a prominent infiltrate of extravasated erythrocytes and lymphocytes. Recently, expression on KS cells of adhesion molecules known to be involved in endothelial cell-leukocyte interactions has been reported to contribute to the extravasation of leukocytes into the lesions (25). During tissue inflammation, normal endothelial cells can be induced to become adhesive for circulating blood cells and to support their transmigration into inflamed tissue. Much of this process is mediated by adhesion molecules expressed on either lymphocytes or endothelium under regulation by NF-κB. Galea et al. (25) have shown that the LFA1–ICAM-1 interaction is the primary one involved in the adhesion of peripheral blood lymphocytes to KSY1 cells (another KS cell line). We find that ORF74 upregulates the expression of VCAM-1, ICAM-1, and E-selectin on KSIMM cells, with the most significant increase in ICAM-1. It is worth noting that the fraction of cells expressing ICAM-1 (100%) exceeds the fraction of cells that we estimate are transfected (30%). Expression of ICAM-1 by cells not expressing ORF74 could occur by induction of expression of soluble factors by ORF74 or could be due to physical contact with cells expressing ORF74.
Although the cell of origin of KS is a source of controversy, phenotypic characterization and electron microscopic analyses of the long-term-cultured cells such as KSIMM indicate an endothelial cell origin (67). Several in vitro studies have shown that normal endothelial cells can be induced to acquire a typical spindle morphology by cytokines or the HIV-1 Tat protein (21). In our studies, HUVECs and dMVECs transduced with a retroviral vector expressing ORF74 adopted a spindle-shaped morphology strikingly similar to that of the spindle-shaped cells found in KS lesions.
NF-κB regulates the transcription of an exceptionally large number of genes, particularly many that participate in immune and inflammatory responses. Inappropriate regulation of NF-κB contributes to a wide range of disorders, including cancer and numerous other inflammatory conditions. Recently, Keller et al. have demonstrated that the inhibition of NF-κB induces apoptosis in HHV-8-infected primary effusion lymphoma cells, suggesting that NF-κB is necessary for their survival (36). NF-κB activation also functions in the antiviral response through regulation of interferon expression (63). However, many viruses, including HIV-1 and human T-cell leukemia virus type 1, exploit NF-κB to activate their own genes and to stimulate the survival and proliferation of the cells in which they replicate. HHV-8, via genes such as ORF74, appears to have also exploited these pathways to promote its own survival and propagation.
We have shown that expression of a single gene of HHV-8, ORF74, is capable of affecting neighboring cells through induction of expression of a variety of soluble proinflammatory factors. These may contribute substantially to the development of KS via paracrine mechanisms. The induction appears to be mediated at least in part by the ligand-independent activation of NF-κB signaling pathways by ORF74. These findings suggest a way in which the minority of cells in lesions in which HHV-8 lytic-phase replication is occurring may be able to influence the biology of the lesions as a whole.
ACKNOWLEDGMENTS
This work was supported by NCI grants P01 CA78817 to M.R. and RO1 CA55293 to R.A.F. S. Pati was supported by a predoctoral training grant from the National Cancer Center.
We thank Gary Hayward from Johns Hopkins University for the generous gift of antibody against ORF74. We also gratefully acknowledge the editorial assistance of Paula Dean.
REFERENCES
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.75.18.8660-8673.2001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc115111?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.75.18.8660-8673.2001
Article citations
Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses.
Annu Rev Virol, 9(1):329-351, 07 Jun 2022
Cited by: 9 articles | PMID: 35671566 | PMCID: PMC9584139
Review Free full text in Europe PMC
Cancers associated with human gammaherpesviruses.
FEBS J, 289(24):7631-7669, 02 Oct 2021
Cited by: 28 articles | PMID: 34536980 | PMCID: PMC9019786
Review Free full text in Europe PMC
In Vivo Models of Oncoproteins Encoded by Kaposi's Sarcoma-Associated Herpesvirus.
J Virol, 93(11):e01053-18, 15 May 2019
Cited by: 12 articles | PMID: 30867309 | PMCID: PMC6532075
Review Free full text in Europe PMC
Molecular Biology of KSHV in Relation to HIV/AIDS-Associated Oncogenesis.
Cancer Treat Res, 177:23-62, 01 Jan 2019
Cited by: 14 articles | PMID: 30523620 | PMCID: PMC7119181
Kaposi's Sarcoma-Associated Herpesvirus-Encoded Viral IL-6 (vIL-6) Enhances Immunoglobulin Class-Switch Recombination.
Front Microbiol, 9:3119, 18 Dec 2018
Cited by: 9 articles | PMID: 30619193 | PMCID: PMC6305588
Go to all (99) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences
- (1 citation) ENA - U82242
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat.
J Virol, 77(10):5759-5773, 01 May 2003
Cited by: 39 articles | PMID: 12719569 | PMCID: PMC154031
Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways.
J Virol, 76(4):1744-1752, 01 Feb 2002
Cited by: 54 articles | PMID: 11799169 | PMCID: PMC135879
Differential activation of murine herpesvirus 68- and Kaposi's sarcoma-associated herpesvirus-encoded ORF74 G protein-coupled receptors by human and murine chemokines.
J Virol, 78(7):3343-3351, 01 Apr 2004
Cited by: 26 articles | PMID: 15016856 | PMCID: PMC371069
Insights into the viral G protein-coupled receptor encoded by human herpesvirus type 8 (HHV-8).
Biol Cell, 96(5):349-354, 01 Jun 2004
Cited by: 12 articles | PMID: 15207903
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: R01 CA55293
Grant ID: P01 CA78817
Grant ID: R01 CA055293




