Abstract
Free full text

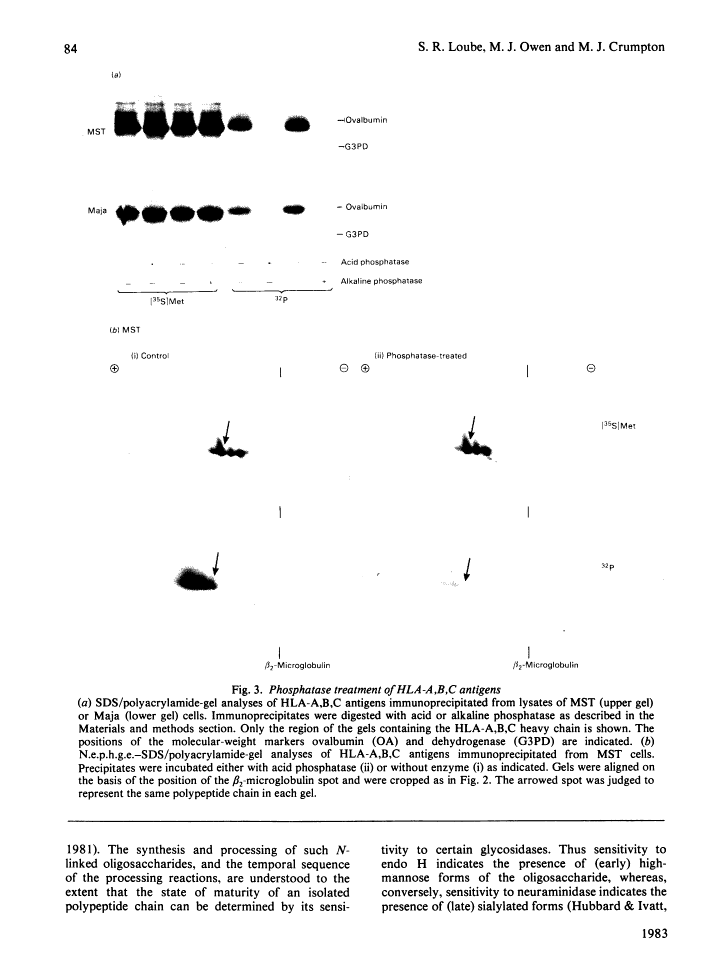
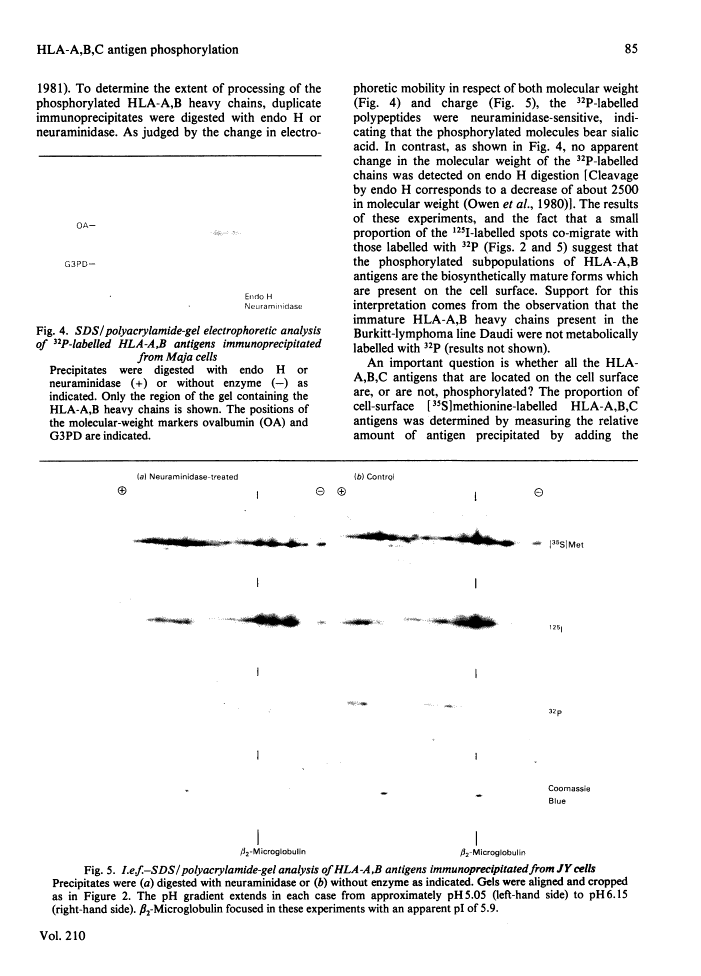
Human class I histocompatibility antigens (HLA-A,B,C). A small proportion only is phosphorylated.
Abstract
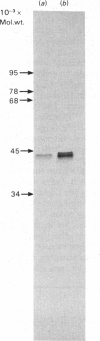
Human Class I HLA antigens (HLA-A,B,C) were isolated by immune precipitation from cells labelled with 32P, [35S]methionine or 125I (by lactoperoxidase-catalysed cell-surface iodination) and were analysed using both one- and two-dimensional electrophoretic systems. In several B-lymphoblastoid cell lines and in human peripheral blood lymphocytes the electrophoretic mobility of the 32P-labelled HLA-A,B,C heavy chains consistently differed from that of molecules labelled by other means. Thus the 32P-labelled heavy chains appeared to be larger and to possess a more acidic pI than did heavy chains labelled with [35S]methionine or 125I, or identified by Coomassie Blue staining. Phosphatase treatment of immunoprecipitates, under conditions where 32P-labelled antigens were shown to be dephosphorylated, did not affect the mobilities of the [35S]methionine-labelled heavy chains. On glycosidase treatment, the positions of the 32P-labelled heavy chains were affected by neuraminidase but not by endo-beta-N-acetylglucosaminidase H. These results imply that phosphorylated HLA-A,B,C antigens comprise only a small proportion (relative to the total cellular HLA-A,B,C antigens) of the biosynthetically mature molecules. The possible significance of such heterogeneity is discussed.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z, Huang KP. Dephosphorylation of rabbit skeletal muscle glycogen synthase (phosphorylated by cyclic AMP-independent synthase kinase 1) by phosphatases. J Biol Chem. 1981 Jan 25;256(2):757–760. [Abstract] [Google Scholar]
- Alt FW, Bothwell AL, Knapp M, Siden E, Mather E, Koshland M, Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. [Abstract] [Google Scholar]
- Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. [Abstract] [Google Scholar]
- Brodsky FM, Parham P, Barnstable CJ, Crumpton MJ, Bodmer WF. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. [Abstract] [Google Scholar]
- Chaplin DD, Wedner HJ, Parker CW. Protein phosphorylation in human peripheral blood lymphocytes: mitogen-induced increases in protein phosphorylation in intact lymphocytes. J Immunol. 1980 May;124(5):2390–2398. [Abstract] [Google Scholar]
- Early P, Rogers J, Davis M, Calame K, Bond M, Wall R, Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. [Abstract] [Google Scholar]
- Johnstone AP, DuBois JH, Crumpton MJ. Phosphorylated lymphocyte plasma-membrane proteins. Biochem J. 1981 Jan 15;194(1):309–318. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Farrell PZ, Goodman HM, O'Farrell PH. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. [Abstract] [Google Scholar]
- Owen MJ, Kissonerghis AM. Immunoglobulin G biosynthesis in a human lymphoblastoid cell line. Differences between membrane-bound and secretory forms of gamma chains. Eur J Biochem. 1982 May;124(1):79–87. [Abstract] [Google Scholar]
- Owen MJ, Kissonerghis AM, Lodish HF. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [Abstract] [Google Scholar]
- Parham P, Humphreys RE, Turner MJ, Strominger JL. Heterogeneity of HL-A antigen preparations is due to variable sialic acid content. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3998–4001. [Europe PMC free article] [Abstract] [Google Scholar]
- Ploegh HL, Cannon LE, Strominger JL. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. [Europe PMC free article] [Abstract] [Google Scholar]
- Ploegh HL, Orr HT, Strominger JL. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. [Abstract] [Google Scholar]
- Pober JS, Guild BC, Strominger JL. Phosphorylation in vivo and in vitro of human histocompatibility antigens (HLA-A and HLA-B) in the carboxy-terminal intracellular domain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6002–6006. [Europe PMC free article] [Abstract] [Google Scholar]
- Racevskis J, Sarkar NH. Phosphorylation of murine mammary tumor virus precursor polypeptides. J Virol. 1979 Apr;30(1):241–247. [Europe PMC free article] [Abstract] [Google Scholar]
- Rangel-Aldao R, Kupiec JW, Rosen OM. Resolution of the phosphorylated and dephosphorylated cAMP-binding proteins of bovine cardiac muscle by affinity labeling and two-dimensional electrophoresis. J Biol Chem. 1979 Apr 10;254(7):2499–2508. [Abstract] [Google Scholar]
- Robb RJ, Terhorst C, Strominger JL. Sequence of the COOH-terminal hydrophilic region of histocompatibility antigens HLA-A2 and HLA-B7. J Biol Chem. 1978 Aug 10;253(15):5319–5324. [Abstract] [Google Scholar]
- Rogers J, Choi E, Souza L, Carter C, Word C, Kuehl M, Eisenberg D, Wall R. Gene segments encoding transmembrane carboxyl termini of immunoglobulin gamma chains. Cell. 1981 Oct;26(1 Pt 1):19–27. [Abstract] [Google Scholar]
- Rothbard JB, Hopp TP, Edelman GM, Cunningham BA. Structure of the heavy chain of the H-2Kk histocompatibility antigen. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4239–4243. [Europe PMC free article] [Abstract] [Google Scholar]
- Steinmetz M, Moore KW, Frelinger JG, Sher BT, Shen FW, Boyse EA, Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. [Abstract] [Google Scholar]
- Walsh FS, Crumpton MJ. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2100079
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1154192?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Immortalized B Cells Transfected with mRNA of Antigen Fused to MITD (IBMAM): An Effective Tool for Antigen-Specific T-Cell Expansion and TCR Validation.
Biomedicines, 11(3):796, 06 Mar 2023
Cited by: 4 articles | PMID: 36979775 | PMCID: PMC10045729
KSHV-K5 inhibits phosphorylation of the major histocompatibility complex class I cytoplasmic tail.
Virology, 288(2):369-378, 01 Sep 2001
Cited by: 18 articles | PMID: 11601908
BoLA class I charge heterogeneity reflects the expression of more than two loci.
Anim Genet, 25(3):165-172, 01 Jun 1994
Cited by: 6 articles | PMID: 7943950
CD8 and beta 2-microglobulin-free MHC class I molecules in T cell immunoregulation.
Int J Clin Lab Res, 23(2):61-69, 01 Jan 1993
Cited by: 20 articles | PMID: 8518416
Review
The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells.
Cell Immunol, 142(1):103-113, 01 Jun 1992
Cited by: 43 articles | PMID: 1586951
Go to all (15) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phosphorylation of class I histocompatibility antigens in human B lymphocytes. Regulation by phorbol esters and insulin.
Biochem J, 256(3):763-768, 01 Dec 1988
Cited by: 6 articles | PMID: 3066355 | PMCID: PMC1135481
A biochemical analysis of the secregation of HLA-DR heavy and light chains in a family studied by two-dimensional gel electrophoresis.
Ann Immunol (Paris), 134C(1):55-81, 01 Jan 1983
Cited by: 0 articles | PMID: 6407385
HLA-DR antigens of autologous melanoma and B lymphoblastoid cell lines: differences in glycosylation but not protein structure.
J Immunol, 133(1):315-320, 01 Jul 1984
Cited by: 13 articles | PMID: 6609984
The synthesis and expression of HLA-A and -B antigens in Xenopus laevis oocytes.
Mol Immunol, 23(5):525-532, 01 May 1986
Cited by: 2 articles | PMID: 3092030