Abstract
Background
Human embryonic stem cells provide access to the earliest stages of human development and may serve as a source of specialized cells for regenerative medicine. Thus, it becomes crucial to develop protocols for the directed differentiation of embryonic stem cells into tissue-restricted precursors.Methods and findings
Here, we present culture conditions for the derivation of unlimited numbers of pure mesenchymal precursors from human embryonic stem cells and demonstrate multilineage differentiation into fat, cartilage, bone, and skeletal muscle cells.Conclusion
Our findings will help to elucidate the mechanism of mesoderm specification during embryonic stem cell differentiation and provide a platform to efficiently generate specialized human mesenchymal cell types for future clinical applications.Free full text

Derivation of Multipotent Mesenchymal Precursors from Human Embryonic Stem Cells
Associated Data
Abstract
Background
Human embryonic stem cells provide access to the earliest stages of human development and may serve as a source of specialized cells for regenerative medicine. Thus, it becomes crucial to develop protocols for the directed differentiation of embryonic stem cells into tissue-restricted precursors.
Methods and Findings
Here, we present culture conditions for the derivation of unlimited numbers of pure mesenchymal precursors from human embryonic stem cells and demonstrate multilineage differentiation into fat, cartilage, bone, and skeletal muscle cells.
Conclusion
Our findings will help to elucidate the mechanism of mesoderm specification during embryonic stem cell differentiation and provide a platform to efficiently generate specialized human mesenchymal cell types for future clinical applications.
Introduction
Embryonic stem (ES) cells are pluripotent cells derived from the inner cell mass of the blastocyst that can be maintained in culture for an extended period of time without losing differentiation potential. The successful isolation of human ES cells (hESCs) has raised the hope that these cells may provide a universal tissue source to treat many human diseases. However, directed differentiation of hESCs into specific tissue types poses a formidable challenge. Protocols are currently available for only a few cell types, mostly of neural identity [1–3], and differentiation into many of the cell types derived from the paraxial mesoderm has not been reported, with the exception of a recent study indicating osteoblastic differentiation [4]. Mesenchymal stem cells (MSCs) have been isolated from the adult bone marrow [5], adipose tissue [6], and dermis and other connective tissues [7]. Harvesting MSCs from any of these sources requires invasive procedures and the availability of a suitable donor. The number of MSCs that can be obtained from a single donor is limited, and the capacity of these cells for long-term proliferation is rather poor. In contrast, hESCs could provide an unlimited number of specialized cells. In this study, we present techniques for the generation and purification of mesenchymal precursors from hESCs and their directed differentiation in vitro into various mesenchymal derivatives, including skeletal myoblasts. Our isolation method for mesenchymal precursors is the first example, to our knowledge, of efficiently deriving structures of the paraxial mesoderm from ES cells, and further highlights the potential of hESCs for basic biology and regenerative medicine.
Methods
Cell Culture and FACS
Undifferentiated hESCs, H1 (WA-01, XY, passages 40–65) and H9 (WA-09, XX, passages 35–45), were cultured on mitotically inactivated mouse embryonic fibroblasts (Specialty Media, Phillipsburg, New Jersey, United States) and maintained under growth conditions and passaging techniques described previously [3]. OP9 cells were maintained in alpha MEM medium containing 20% fetal bovine serum (FBS) and 2 mM L-glutamine. Mesenchymal differentiation was induced by plating 10 × 103 to 25 × 103 cells/cm2 on a monolayer of OP9 cells in the presence of 20% heat-inactivated FBS in alpha MEM medium. Flow-activated cell sorting (FACS) (CD73-PE; PharMingen, San Diego, California, United States) was performed on a MoFlo (Cytomation, Fort Collins, Colorado, United States). All human ES cell–derived mesenchymal precursor cell (hESMPC) lines in this study are of polyclonal origin. Primary human bone marrow–derived MSCs and primary human foreskin fibroblasts (both from Poietics, Cambrex, East Rutherford, New Jersey, United States) were grown in alpha MEM medium containing 10% FBS and 2 mM L-glutamine.
Adipocytic Differentiation
hESMPCs are grown to confluence followed by exposure to 1 mM dexamethasone, 10 μg/ml insulin, and 0.5 mM isobutylxanthine (all from Sigma, St. Louis, Missouri, United States) in alpha MEM medium containing 10% FBS for 2–4 wk. Data were confirmed in hESMPC-H1.1, -H1.2, -H1.3, and -H9.1 (hESMPC-H1.4 was not tested).
Chondrocytic Differentiation
Differentiation of hESMPCs was induced in pellet culture [5] by exposure to 10 ng/ml TGF-β3 (R & D Systems, Minneapolis, Minnesota, United States) and 200 μM ascorbic acid (Sigma) in alpha MEM medium containing 10% FBS for 3–4 wk. Data were confirmed in hESMPC-H1.1, -H1.3, and -H9.1 (hESMPC-H1.2 and -H1.4 were not tested).
Osteogenic Differentiation
hESMPCs were plated at low density (1 × 103 to 2.5 × 103 cells/cm2) on tissue-culture-treated dishes in the presence of 10 mM β-glycerol phosphate (Sigma), 0.1 μM dexamethasone, and 200 μM ascorbic acid in alpha MEM medium containing 10% FBS for 3–4 wk. Data were confirmed in hESMPC-H1.1, -H1.3, and -H9.1 (hESMPC-H1.2 and -H1.4 were not tested).
Myogenic Differentiation
Confluent hESMPCs were maintained for 2–3 wk in alpha MEM medium with 20% heat-inactivated FBS. More rapid induction was observed in the presence of medium conditioned for 24 h by differentiated C2C12 cells. Coculture of hESMPCs and C2C12 cells was carried out in alpha MEM with 3% horse serum and 1% FBS [8]. Data were confirmed in hESMPC-H1.3, -H1.4, and -H9.1 (hESMPC-H1.1 and -H1.2 were not tested).
Cytochemistry
Immunocytochemistry for all surface markers was performed on live cells. Monoclonal antibodies VCAM, STRO-1, ICAM-1(CD54), CD105, CD29, and MF20 were from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, Iowa, United States); CD73, CD44, and ALCAM(CD166) were from BD Biosciences Pharmingen (San Diego, California, United States). All other immunocytochemical analyses were performed after fixation in 4% paraformaldehyde and 0.15% picric acid, followed by permeabilization in 0.3% Triton X100. Polyclonal antibodies used were MyoD (Santa Cruz Biotechnology, Santa Cruz, California, United States) and nestin (gift from R. McKay); monoclonal antibodies were vimentin, alpha smooth muscle actin, fast-switch myosin, pan-cytokeratin (all from Sigma), and human nuclear antigen (Chemicon, Temecula, California, United States).
Alkaline phosphatase reaction was performed using a commercially available kit (Kit-86; Sigma) and the mineral was stained with silver nitrate according to the von Kossa method. Fat granules were visualized by Oil Red O staining solution (Sigma). Alcian Blue (Sigma) was used to detect extracellular matrix proteoglycans in chondrogenic cultures.
Gene-Expression Analyses
RT-PCR analysis
Total RNA was extracted by using the RNeasy kit and DNase I treatment (Qiagen, Valencia, California, United States). Total RNA (2 μg each) was reverse transcribed (SuperScript; Invitrogen, Carlsbad, California, United States). PCR conditions were optimized and linear amplification range was determined for each primer by varying annealing temperature and cycle number. PCR products were identified by size, and identity was confirmed by DNA sequencing. Primer sequences, cycle numbers, and annealing temperatures are provided in Table S1.
Affymetrix analysis
Total RNA (5 μg) from primary MSCs, from hESMPC-H9.1, hESMPC-H1.2, and three samples of undifferentiated hESCs (H1; passages 42–46), were processed by the Memorial Sloan-Kettering Cancer Center Genomics Core Facility and hybridized on Affymetrix (Santa Clara, California, United States) U133A human oligonucleotide arrays. Data were analyzed using MAS5.0 (Affymetrix) software. Transcripts selectively expressed in each of the mesenchymal cell populations (MSC, hESMPC-H9.1, and hESMPC-H1.2) were defined as those called “increased” by the MAS5.0 algorithm in each of three comparisons with independent samples of undifferentiated hESCs. A Venn diagram was generated to visualize overlap in gene expression. Further statistical analyses were performed as described below.
Results
Mesenchymal differentiation of hESCs (lines H1 [WA-01] and H9 [WA-09]) [9] was induced by plating undifferentiated hESCs on a monolayer of murine OP9 stromal cells [10], in the presence of 20% heat-inactivated FBS in alpha MEM medium. OP9 cells have been previously shown to induce blood cell differentiation from mouse ES cells [11]. After 40 d of coculture, cells were harvested and sorted by FACS for CD73, a surface marker expressed in adult MSCs [5] (Figure 1A). An average of 5% CD73+ cells was obtained from the mixed culture of OP9 and differentiated hESC progeny. CD73+ cells were replated in the absence of stromal feeders on tissue culture plates and expanded in alpha MEM medium with 20% FBS for 7–14 d. We next established the membrane antigen profile of the resulting population of flat spindle-like cells. The H1- and H9-derived CD73+ cells expressed a comprehensive set of markers that are considered to define adult MSCs, including CD105(SH2), STRO-1, VCAM (CD106), CD29(integrin β1), CD44, ICAM -1(CD54), ALCAM(CD166), vimentin, and alpha smooth muscle actin (Figure 1B and and1C).1C). The cells were negative for hematopoietic markers such as CD34, CD45, and CD14. They were also negative for neuroectodermal, epithelial, and muscle cell markers including nestin, pancytokeratin, and desmin (data not shown). The human identity of these presumed mesenchymal cells (termed hESMPC-H1.1, -H1.2, -H1.3, -H1.4, and -H9.1) was confirmed for all experiments by immunocytochemistry for human nuclear antigen to rule out the possibility of contamination with OP9 cells (Figure S1).
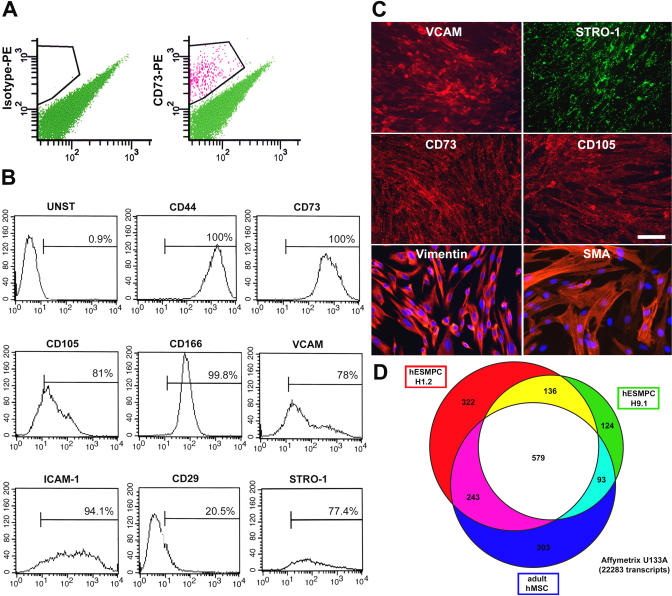
(A) FACS (MoFlo, Cytomation) for the isolation of CD73+ precursors (right) and isotype control (left).
(B) Flow cytometry analysis of the CD73+ hESMPC population for various markers characteristic of MSCs, including CD44, CD73, CD105, CD166, VCAM, ICAM-1, CD29, and STRO-1.
(C) Immunocytochemistry of hESMPCs for MSC markers (VCAM, STRO-1, CD73, and CD105). The cells also express vimentin and alpha smooth muscle actin. Scale bar = 50 μm.
(D) Venn diagram presenting the overlap among transcripts selectively expressed in hESMPC-H1.2, hESMPC-H9.1, and primary adult human MSCs.
To further characterize hESMPCs, we performed genome-wide expression analysis using oligonucleotide arrays (Affymetrix U133A). The expression profiles of hESMPC-H1.2 and hESMPC-H9.1 were compared with that of human primary adult MSCs. Housekeeping genes for each of the mesenchymal cell populations were eliminated by subtracting those transcripts also expressed in at least one of three independent samples of undifferentiated hESCs. Based on this analysis, 1,280 transcripts were selectively expressed in hESMPC-H1.2, 932 transcripts in hESMPC-H9.1, and 1,218 transcripts in primary adult MSCs. A remarkable overlap of 579 transcripts shared among the three mesenchymal populations was observed (Figure 1D). Using the genes that were selected in the initial filter, we performed a statistical analysis on the expression levels to determine whether the genes were expressed significantly differently in the two cell types. We used a Bayesian extension to the standard t-test [12] to assess this difference. Of the 579 genes, 412 of them were significantly different, at a false discovery rate cutoff of 0.05. The relative fold changes were also extremely large in many of the cases. We also looked at the variance of the expression levels within the cell types. For the MSCs, 94% had a coefficient of variation less than 20% for the expression (log transformed); for the ES-derived cells, 72% had a coefficient of variation less than 20%. Numerous known MSC markers were included in the list of 412 genes, such as the mesenchymal stem cell protein DSC54 (13.9-fold increase, p < 0.001), neuropilin 1 (30.4-fold increase, p < 0.001), hepatocyte growth factor (48.1-fold increase, p < 0.001), forkhead box D1 (14.8-fold increase, p < 0.001), and notch homolog 2 (2.9-fold increase, p < 0.001) . Table S2 lists the p-values from the test, the mean and standard deviation of the expression levels, and the relative fold change of all 412 genes between the two types.
Known markers of MSCs, such as mesenchymal stem cell protein DSC54, were all included within the 579 shared transcripts. These findings support the immunocytochemical data and suggest that hESMPCs and primary MSCs are highly related.
MSCs are characterized functionally by their ability to differentiate into mesenchymal tissues, such as fat, cartilage, and bone. Therefore, we tested whether hESMPCs have the same potential (Figure 2).
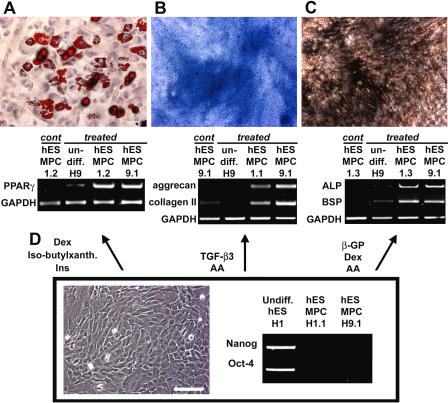
(A) Adipocytic differentiation in the presence of dexamethasone, insulin, and isobutylxanthine. Adipocytic characterization by Oil Red O staining and RT-PCR analysis for PPARγ.
(B) Chondrocytic differentiation in the presence of TGF-β3 and ascorbic acid. Chondrocytic characterization by Alcian Blue staining and RT-PCR for aggrecan and collagen II.
(C) Osteogenic differentiation in the presence of β-glycerolphosphate, dexamethasone, and ascorbic acid. Osteocytic characterization by von Kossa staining and RT-PCR for bone-specific alkaline phosphatase (ALP) and bone sialoprotein (BSP).
(D) Phase-contrast image of hESMPCs and RT-PCR for the ES cell markers Nanog and Oct-4 in hESMPC-H1.1 and -H9.1 compared with undifferentiated H1 hESCs.
Scale bar = 50 μm for all panels.
Adipocytic differentiation of hESMPCs was induced under conditions described previously for primary adult MSCs [5]. Appearance of cells harboring fat granules was observed after 10–14 d in culture. After 3 wk of induction, more than 70% of the cells displayed Oil Red O+ fat granules, and PPARγ, a marker of adipocytic differentiation, was detected by RT-PCR. (Figure 2A).
Chondrocytic differentiation was achieved using the pellet culture system [5]. After 28 d in culture, more than 50% of all cells exhibited robust staining for Alcian Blue, a marker specific for extracellular matrix proteoglycans. Chondrocytic differentiation was confirmed by the gene expression of collagen II and aggrecan, two components of extracellular matrix selectively expressed by chondrocytes, using RT-PCR (Figure 2B).
Osteogenic differentiation was induced in the presence of β-glycerolphosphate [5]. Osteogenesis was demonstrated by specific staining for calcium deposition in the matrix (von Kossa, Figure 2C; or Alizarin Red, Figure S2A) and increased expression of bone-specific alkaline phosphatase and bone sialoprotein at day 28 of treatment (Figures 2C and S2B). At day 28, Alizarin Red staining was detected in approximately 70% of all cells. Throughout these studies, human adult MSCs and foreskin fibroblasts were used as positive and negative controls, respectively.
In addition to adipocytic, chondrocytic, and osteogenic differentiation, reports suggested that adult MSCs can form skeletal muscle [13]. Although generation of skeletal muscle cells from adult MSCs remains controversial, we tested whether hESMPCs exhibit this potential. Under the conditions previously described [13], hESMPC-H1.1 and -H9.1 did not yield significant numbers of MyoD+ cells after 15–20 d in culture. However, when confluent cells were maintained in culture in the presence or absence of 5-AzaC without passage for more than 21 d, expression of specific skeletal muscle markers such as MyoD and fast-switch myosin was observed (Figure 3A). More rapid myogenic differentiation was obtained in the presence of 24-h-conditioned medium from the murine myoblastic cell line C2C12 previously induced to form myotubes [14]. Direct coculture of hESMPCs with C2C12 cells led to the formation of hESMPC-derived myotubes, as visualized by expression of human nuclear antigen (Figure 3B), similar to those formed by host C2C12 cells. After 1 wk of coculture, myotubes composed of human nuclei accounted for more than 10% of the total number of human cells present, and each human myotube was composed of up to ten human nuclei. Human cell contribution to myotubes in coculture was confirmed by expression of human muscle-specific transcripts such as MyoD, myosin heavy chain IIa, and myogenin (data not shown). These data demonstrate that hESMPCs can give rise to mesenchymal derivatives typically obtained from primary adult MSCs, as well as to cells expressing markers of skeletal muscle.
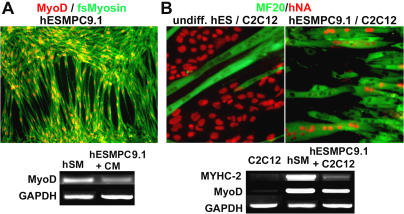
(A) Immunocytochemistry for MyoD (red) and fast-switch myosin (green). RT-PCR for MyoD in human skeletal muscle as a positive control (hSM), and in hESMPC-H9.1 cells differentiated for 10 d in the presence of C2C12-conditioned medium (hESMPC).
(B) Myotube formation induced at high cell densities in the presence of C2C12 cells. Myotube characterization by immunocytochemistry for MF20 against sarcomeric myosin (green) and human nuclear antigen (hNA, red). Left panel: Control undifferentiated hESCs (H9) do not fuse with C2C12. Right panel: Under identical culture conditions, hESMPCs (line 9.1) efficiently fuse with C2C12 cells, forming myotubes containing human nuclei. RT-PCR for human specific muscle transcripts myosin heavy chain IIa (MYHC-2) and MyoD in C2C12 cells, in human skeletal muscle as positive control (huSM), and in hESMPC-H9.1 cells cocultured with C2C12 cells.
One concern for the clinical application of hESC-derived progeny in regenerative medicine is the risk of teratoma formation due to the presence of residual undifferentiated ES cells among the differentiated progeny. We did not detect markers of undifferentiated hESCs, such as Nanog [15] or Oct-4 [16], in any of the hESMPCs by RT-PCR (see Figure 2D) and immunocytochemistry (data not shown), suggesting the lack of any undifferentiated ES cells in hESMPC cultures. However, future in vivo studies are required to rule out the potential of these cells for teratoma formation.
Discussion
Previous studies have demonstrated the derivation of neural cells [1–3], hematopoietic [17] and endothelial lineages [18], and cardiomyocytes [19] from hESCs. This study presents the induction of paraxial mesoderm with the generation of multipotent mesenchymal precursors. We calculate that under these conditions a single undifferentiated hESC yields an average of one CD73+ cell at day 40 of differentiation, suggesting a balance between cell proliferation and cell selection. There were no obvious differences in marker and gene-expression profile or in differentiation behavior among the five hESMPC lines generated. However, some of the lines (e.g., hESMPC9.1) exhibited a tendency of spontaneous osteogenic differentiation after long-term propagation. Directed differentiation of hESCs into somatic stem-cell-like precursors represents a substantial advancement in harnessing the developmental potential of hESCs. The high purity, unlimited availability, and multipotentiality of hESMPCs will provide the basis for future therapeutic efforts using these cells in preclinical animal models of disease. Such in vivo studies will also be required to properly assess the safety profile of these cells. Furthermore, our system also offers a novel platform to study basic mechanisms of mesodermal induction and differentiation during early human development.
Supporting Information
Figure S1
Human Identity of CD73+ Cells after FACS:All cells as visualized by DAPI+ nuclei express human nuclear antigen (hNA) confirming the absence of any contaminating OP9 cells. Scale bar = 50 μm.
(148 KB PDF).
Figure S2
Additional Markers of Bone Differentiation:(A) Alizarin Red staining for calcium deposition in the matrix in hESMPCs untreated (left panel) or treated in the presence of β-glycerolphosphate (right panel; compare to Figure 2C).
(B) Increasing alkaline phosphatase reactivity during osteogenic differentiation of hESMPC-H1.1. Scale bar = 250 μm for main panels, 50 μm for insets.
(278 KB PDF).
Table S2
List of Shared Genes:List of 421 genes that are shared between primary and hESC-derived mesenchymal precursors but significantly different from undifferentiated hESCs (see main text for details).
(107 KB XLS).
Accession Numbers
The Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo) accession number for all raw microarray data used in this study is GSE2248.
The Unigene (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=unigene) accession numbers for the gene products discussed in this paper are aggrecan (Hs.2159 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=2159; bone sialoprotein (Hs.518726 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=518726; bone-specific alkaline phosphatase (Hs.75431 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=75431; collagen II (Hs.408182 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=408182; forkhead box D1 (Hs.519385 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=519385; hepatocyte growth factor (Hs.396530 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=396530; mesenchymal stem cell protein (DSC54, Hs.157461 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=157461; MyoD (Hs.520119 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=520119; myogenin (Hs.2830 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=2830; myosin heavy chain IIa (Hs.513941 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=513941; Nanog (Hs.329296 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=329296]) [15]; neuropilin 1 (Hs.131704 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=131704; notch homolog 2 (Hs.549056 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=549056; Oct-4 (Hs.504658 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=504658; and PPARγ (Hs.162646 [http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=162646]).
Acknowledgments
We thank R. McKay for nestin antibody; P. Song and the Sloan-Kettering Genomics and Flow Cytometry Core Facilities for technical assistance; and R. Stan, V. Tabar, M. Tomishima, Y. Elkabetz, and S. Desbordes for critical review of the manuscript. This work was supported in part by the Kinetics Foundation. The funder had no role in the study design, data analysis, decision to publish, or manuscript preparation and content.
Abbreviations
| ES | embryonic stem |
| FACS | flow-activated cell sorting |
| FBS | fetal bovine serum |
| hESC | human embryonic stem cell |
| hESMPC | human embryonic stem cell–derived mesenchymal precursor cell |
| MSC | mesenchymal stem cell |
Footnotes
Citation: Barberi T, Willis LM, Socci ND, Studer L (2005) Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2(6): e161.
References
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. [Europe PMC free article] [Abstract] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. [Abstract] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. [Abstract] [Google Scholar]
- Sottile V, Thomson A, McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5:149–155. [Abstract] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. [Abstract] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. [Abstract] [Google Scholar]
- Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. [Abstract] [Google Scholar]
- Shi D, Reinecke H, Murry CE, Torok-Storb B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood. 2004;104:290–294. [Abstract] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. [Abstract] [Google Scholar]
- Kodama H, Nose M, Niida S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [Abstract] [Google Scholar]
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. [Abstract] [Google Scholar]
- Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: Regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. [Abstract] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. [Abstract] [Google Scholar]
- Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7:159–166. [Abstract] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. [Abstract] [Google Scholar]
- Scholer HR, Ciesiolka T, Gruss P. A nexus between Oct-4 and E1A: Implications for gene regulation in embryonic stem cells. Cell. 1991;66:291–304. [Abstract] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. [Europe PMC free article] [Abstract] [Google Scholar]
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. [Europe PMC free article] [Abstract] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. [Abstract] [Google Scholar]
Articles from PLOS Medicine are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pmed.0020161
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosmedicine/article/file?id=10.1371/journal.pmed.0020161&type=printable
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
INDUCED PLURIPOTENT STEM CELL-DERIVED MESENCHYMAL STEM CELLS-DERIVED EXTRACELLULAR VESICLES ATTENUATE LPS-INDUCED LUNG INJURY AND ENDOTOXEMIA IN MICE.
Shock, 62(2):294-303, 23 May 2024
Cited by: 0 articles | PMID: 38813932
Hyaluronan in mesenchymal stromal cell lineage differentiation from human pluripotent stem cells: application in serum free culture.
Stem Cell Res Ther, 15(1):130, 03 May 2024
Cited by: 0 articles | PMID: 38702837
Multifaceted Characterization of Human Embryonic Stem Cell-Derived Mesenchymal Stem/Stromal Cells Revealed Amelioration of Acute Liver Injury in NOD-SCID Mice.
Cell Transplant, 33:9636897231218383, 01 Jan 2024
Cited by: 0 articles | PMID: 38173232 | PMCID: PMC10768578
Therapeutic potential of mesenchymal stem cells from human iPSC-derived teratomas for osteochondral defect regeneration.
Bioeng Transl Med, 9(2):e10629, 29 Nov 2023
Cited by: 3 articles | PMID: 38435815 | PMCID: PMC10905541
The Current Proceedings of PSC-Based Liver Fibrosis Therapy.
Stem Cell Rev Rep, 19(7):2155-2165, 25 Jul 2023
Cited by: 1 article | PMID: 37490204
Review
Go to all (284) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
GEO - Gene Expression Omnibus
- (1 citation) GEO - GSE2248
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542.
J Bone Miner Res, 25(6):1216-1233, 01 Jun 2010
Cited by: 81 articles | PMID: 20200949
In utero transplantation of human bone marrow-derived multipotent mesenchymal stem cells in mice.
J Orthop Res, 24(3):301-312, 01 Mar 2006
Cited by: 19 articles | PMID: 16482576
Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2.
Calcif Tissue Int, 84(1):56-64, 04 Dec 2008
Cited by: 42 articles | PMID: 19052794
From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera?
Int J Dev Biol, 52(8):1023-1032, 01 Jan 2008
Cited by: 64 articles | PMID: 18956335
Review





