Abstract
Free full text

Neisseria gonorrhoeae Coordinately Uses Pili and Opa To Activate HEC-1-B Cell Microvilli, Which Causes Engulfment of the Gonococci†
Abstract
This study was undertaken to examine concomitant roles of pili and colony opacity-associated proteins (Opa) in promoting Neisseria gonorrhoeae adherence to and invasion of human endometrial HEC-1-B cells. Adherence of N. gonorrhoeae to cultured HEC-1-B cells was saturable, even though organisms adhered to <50% of the cells. During 4 to 6 h of incubation, adherent mono- and diplococci formed microcolonies on the surfaces of the cells. Microvilli of the HEC-1-B cells adhered by their distal ends to individual cocci within the microcolonies. When the microcolonies grew from isogenic pilus-negative (P−) Opa−, P− Opa+, or P+ Opa− gonococci, microvilli did not elongate, and the colonies were not engulfed. In contrast, the microvilli markedly elongated during exposure to P+ Opa+ gonococci. The microvilli adhered to the organisms along their full lengths and appeared to actively participate in the engulfment of the microcolonies. Internalized microcolonies, with P+ Opa+ gonococci, contained dividing cocci and appeared to be surrounded by cell membrane but were not clearly within vacuoles. In contrast, degenerate individual organisms were within vacuoles. Low doses of chloramphenicol, which inhibits protein synthesis by both prokaryotes and eukaryotes, prevented the microvillar response to and internalization of the P+ Opa+ gonococci; higher doses caused internalization without microvillus activation. Cycloheximide and anisomycin, which inhibit only eukaryotic protein synthesis, caused dose-dependent enhancement of uptake. Cytochalasins reduced engulfment; colchicine had no effect. These results show that gonococci must express both pili and Opa to be engulfed efficiently by HEC-1-B cells.
Pili and colony opacity-associated proteins (Opa) are surface-exposed structures of Neisseria gonorrhoeae. Pili can extend several micrometers from the gonococcal cell surface, while Opa proteins are integral parts of the outer membrane. Pili and Opa have been studied individually and extensively for their relative roles in gonococcal adherence to and invasion of host epithelial cells.
Pili occur in small-colony variants of gonococci that can be selectively subcultured with a dissecting microscope (30, 32, 33, 68). Pili appear to mediate interactions between gonococci and host epithelial cells (42, 43, 53, 54, 63, 73). Pili are inhibitory to phagocytosis of gonococci by polymorphonuclear cells (18, 22, 48), but their effect on internalization by epithelial cells is unclear. Pili are composed of PilE, the major pilus subunit; PilC, an adherence-associated protein; and possibly other components (1, 54, 55). Gonococci undergo pilus phase variation, converting from pilus positive (P+) to P− and from P− to P+ in vitro and quite probably in vivo as well (67, 68). Transcellular passage through HEC-1-B cells in culture yields pilus phase variation from P+ to P− (26). In vitro, pili undergo rapid antigenic variation; such antigenic variation undoubtedly occurs in vivo also.
Opa proteins are heat-modifiable outer membrane proteins found in gonococcal colony color variants (64, 65). Opa proteins routinely are found in gonococci in opaque colonies and sometimes are found in gonococci in transparent colonies; however, Opa− gonococci form only transparent colonies, and these are generally similar to Opa+ transparent colonies when viewed under a dissecting microscope (17). Opa proteins confer upon the gonococci variation in surface appearance and size (17). It has been estimated that each strain of gonococcus has 9 to 12 opa genes (4, 14). Each Opa has hypervariable 1 (HV1) and HV2 regions (4, 14); the genetic codes for the HV1 and HV2 regions may appear more than once in a strain of gonococcus and in diverse strains as well (4, 8, 14). Shifts in reading the repetitive sequences within each opa locus result in frequent changes (≥1 in 103) in the expression of Opa proteins (40, 44, 61). Opa proteins have an important role in gonococcal adherence to host cells. Adherence of P− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate (11). Opa proteins confer on gonococci cell tropism, with some Opa proteins promoting adherence to polymorphonuclear cells and others promoting adherence to specific lines of epithelial or other cells in vitro (7, 12, 13, 34, 36). Opa proteins differentially recognize members of the carcinoembryonic antigen family (70), which may result in the tissue tropism (7, 12, 13).
Invasion requires the active participation of both the gonococcus and the host cell (10, 52, 58). During invasion, epithelial cell microvilli surround the bacteria as they are drawn into the eukaryotic cell (52, 58). Endocytosis is stopped by drugs that block actin filament rearrangement within the host cell (3, 10, 58). The gonococcal factors that signal the host cell to engulf them have not been defined completely but probably include Opa proteins (39, 54, 59, 74).
There is considerable indirect evidence that pili and Opa are concomitantly important in the infectious process. Gonococci have to be piliated to be infectious for human fallopian tube tissues in organ culture and for infection of male human volunteers (32, 33, 42, 69, 73). Piliated gonococci probably are present in vivo (28) and are present in primary clinical cultures from infected patients (30, 35, 60, 68). One to four Opa proteins are present in primary clinical isolates from the male urethra and rectum and from the cervix (27), whereas Opa− isolates either are not seen (27) or are uncommon (21, 29). Human male volunteers inoculated intraurethrally with Opa− gonococci almost always shed Opa+ gonococci on subsequent cultures (31, 56, 66). Both pili and Opa have been implicated in the signaling of epithelial cells to engulf gonococci (23, 25, 39, 54, 59, 74).
This study was undertaken to examine concomitant roles of pili and Opa in promoting adherence to and invasion of HEC-1-B cells. The data indicate that gonococci use pili and Opa coordinately in their interactions with the HEC-1-B cells.
MATERIALS AND METHODS
Gonococcal strains.
Three sets of N. gonorrhoeae strains were used in this study. The organisms were subcultured onto GC Agar Base supplemented with IsoVitaleX (BBL, Cockeysville, Md.) (GC agar). A dissecting microscope and standard criteria were used to select piliated (P+) and nonpiliated (P−) colony types with Opa proteins (Opa+) and without Opa proteins (Opa−).
The P− and P+ variants of strain MS11mk (4, 66) were kindly supplied by Herman Schneider (Walter Reed Army Institute of Research, Washington, D.C.). The nonrevertant P− MS11mk mutant lacked a complete pilE site and could not rearrange pilS loci; it was derived by exhaustive selection from the wild-type strain. A second, heavily piliated variant of MS11mk was also selected; it was designated P+. These MS11mk variants had transparent colony phenotypes and were Opa− (66); opaque colony variants that were Opa+ were selected from each of the P− and P+ variants.
Schneider et al. (56, 57) observed that the variant of MS11mk that expresses C lipooligosaccharides is virulent for the male urethra. This variant was obtained from Herman Schneider, and variants of it with piliated and Opa+ phenotypes were selected, as described above.
Opa variants of strains F62-SF and FA1090 have been extensively described (5, 16). Janne Cannon (University of North Carolina, Chapel Hill) kindly provided the FA1090 Opa variants. Nonpiliated variants of FA1090 Opa− and piliated and nonpiliated variants of FA1090 OpaE were selected (17). Piliated and nonpiliated variants of F62-SF OpaA and Opa− also were selected.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
Pilus and Opa expression was monitored by solubilizing in sodium dodecyl sulfate whole-cell lysates of the gonococci used in each adherence assay and electrophoresing them through a 12.5% acrylamide separating gel (37). The proteins then were transferred to nitrocellulose and incubated with antibodies specific for pilin and Opa proteins (9, 38).
The Opa content was determined by comparing lysates prepared at 37 and 100°C and looking for the presence of a heat-modifiable protein. Transblots of FA1090 lysates were incubated with monoclonal antibodies that bind each of its Opa proteins (2) (kindly provided as hybridoma supernatants by Janne Cannon). Opa proteins of the other gonococcal strains were detected in immunoblots with use of monoclonal antibody B33, which reacts with all Opa proteins (38).
Rabbit antipilin antibody against the CNBr fragment 2 of strain R10 pili kindly was provided by Gary Schoolnik (Stanford University, Palo Alto, Calif.). This antibody was incubated with transblots of gonococcal lysates to confirm whether or not the organisms made pili.
Adherence assays.
HEC-1-B cells, a human endometrial adenocarcinoma cell line (ATCC HTB 113; American Type Culture Collection, Rockville, Md.), were maintained in Eagle minimal essential medium (MEM) containing Earle’s salts, nonessential amino acids, sodium pyruvate, and 10% fetal calf serum (FCS). To use them in adherence assays, the HEC-1-B cells were trypsinized from the surface of a tissue culture flask and suspended in tissue culture medium at 2 × 105 to 3 × 105 cells/ml, and 1 ml was plated onto each round Thermanox coverslip (Electron Microscopy Sciences, Fort Washington, Pa.) in 24-well tissue culture trays (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.). The cells were incubated for 20 to 24 h at 37°C in 5% CO2. Gonococcal inocula were suspended in MEM containing 0.05% HEPES buffer at pH 7.4.
For studies in which light microscopy was used to assess adherence and microcolony formation, P+ Opa+ MS11mkC and P+ FA1090 OpaE gonococci were inoculated onto HEC-1-B cells that had been plated, as described above, onto tissue culture chamber slides (four-well, Lab-Tek; Miles Scientific, Naperville, Ill.) at inocula of 1 × 104 to 2 × 104 to 2 × 107 CFU/ml. The inocula were removed at selected times of incubation, and the slides were rinsed with phosphate-buffered saline (PBS) to remove nonadherant bacteria and then Gram stained.
For studies in which electron microscopy (EM) was used to assess adherence, gonococci were inoculated onto the coverslips at approximately 2 × 107 CFU/ml. The inocula were removed after 30 min or 1 h of incubation, and the coverslips were rinsed to remove nonadherent bacteria. The incubation was continued after the addition of fresh medium. The coverslips were rinsed again at selected times and fixed at 4°C in 2.5% glutaraldehyde in 0.1 M cacodylate buffer of pH 7.38 for 4 h. The coverslips were held in the cacodylate buffer until they were prepared for scanning EM (SEM).
When culture was used to assess adherence, 2 × 105 HEC-1-B cells were plated directly into each well of the 24-well tissue culture trays. The gonococcal strains were used at inocula of 1 × 108 to 2 × 108 CFU/ml. The HEC-1-B cells were incubated with the bacteria for 2 to 3 h and then rinsed four times with PBS to remove nonadherant bacteria. The HEC-1-B cells were dislodged from the wells with trypsin and lysed in 1% saponin with vortexing. The lysates were mixed by vortex action and then diluted in PBS. Aliquots of appropriate dilutions were plated onto GC agar, and the colonies were counted after overnight incubation. The assays were done in duplicate or triplicate.
SEM.
For SEM, the coverslips were prepared as previously described (16). The cells were postfixed in 2% (vol/vol) OsO4 in 0.1 M cacodylate buffer and placed sequentially in 1% (vol/vol) aqueous tannic acid and 2% (wt/vol) uranyl acetate, with rinse steps between each solution. The cells were dehydrated by placing them in a series of graded ethanol solutions (50 to 100%). The coverslips were rinsed in hexamethyldisilazane (Electron Microscopy Sciences), mounted on SEM stubs, and sputter coated with 25 nm of platinum. A scanning electron microscope (DS130; International Scientific Instruments, Milpitas, Calif.) was used to examine the specimens.
Invasion assays.
Assays for invasion differed from assays for adherence in that the HEC-1-B cells were incubated with gonococcal variants for 10 h before being processed for transmission EM (TEM) and for 6 h before being processed for culture. The coverslips were rinsed and fixed in glutaraldehyde-cacodylate prior to preparation for TEM. When culture was used to assess invasion, gentamicin (250 μg/ml), which kills extracellular but not intracellular gonococci, was added to the medium after 4 h of incubation, and the incubation was continued for an additional 2 h, after which the HEC-1-B cells were processed and quantitative cultures of intracellular organisms were done, as for the adherence assays (see above).
TEM.
For TEM, the cell layers on the coverslips were fixed in glutaraldehyde and OsO4 as described for SEM and then transferred to cacodylate buffer in glass microbeakers. The cell layers were dehydrated in a graded acetone series and infiltrated with resin (Araldite/Medcast; Ted Pella, Redding, Calif.). After 3 h of infiltration in whole resin, the coverslips containing the cell layers were placed on a glass surface, and several rectangles were cut out of the centers of the coverslips. The rectangles were placed cell side up in flat embedding molds with fresh resin and polymerized overnight at 60°C. Cross sections of the cells were obtained by sectioning through the embedded coverslips. Sections were placed on bare 150-mesh copper grids, stained with uranyl acetate and lead citrate, and viewed with an electron microscope (100CX; JEOL USA, Medford, Mass.).
Inhibitors.
Chloramphenicol, cycloheximide, and anisomycin were used to inhibit protein synthesis, cytochalasin B and D were used to block actin polymerization, and colchicine was used to block tubulin polymerization. All except chloramphenicol, which was obtained from the hospital pharmacy, were from Sigma (St. Louis, Mo.).
Inhibitors were dissolved in either dimethyl sulfoxide or MEM (chloramphenicol, cycloheximide, and anisomycin) as stock solutions, aliquoted, and stored at −20°C. Prior to use reactants were diluted in MEM supplemented with 10% (vol/vol) FCS and added to the cultured HEC-1-B cells. The highest concentration of dimethyl sulfoxide that resulted from the dilutions was prepared without inhibitor in MEM with FCS and included as a control. The highest concentration of each reactant used was incubated with the organisms for the same period in a well without HEC-1-B cells to find out whether it affected bacterial survival or growth.
Antagonist assay.
HEC-1-B cells on coverslips were incubated at 37°C for 30 min in chloramphenicol at concentrations of 0 to 50 μg/ml. The spent medium was aspirated, and the cells were inoculated with gonococci diluted in medium containing chloramphenicol and incubated for an additional hour. After the nonadherant gonococci had been removed, fresh medium with chloramphenicol was added. Coverslips were removed at specified times and fixed for EM.
Culture was used to assess the inhibitory effects of cycloheximide, cytochalasins, and colchicine; 100 ml of the dilutions of each inhibitor was added to wells that contained HEC-1-B cells so as to achieve a range of concentrations in final volumes of 1 ml. The cells were incubated with the reactants for 1 h, after which the bacteria were added and the incubation was continued for 3 to 4 h more. The trays then were processed as for the invasion assays (see above).
RESULTS
Gonococcal adherence to HEC-1-B cells is selective and saturable.
Adherence of P+ Opa+ N. gonorrhoeae to HEC-1-B cells was saturable. Approximately 104 gonococci, as represented by CFU, were adherent to 2 × 105 to 3 × 105 HEC-1-B cells 4 h after inoculation, even when inocula of 106 or 107 were used (Table (Table1).1). As seen by light microscopy of Gram-stained tissue cultures, gonococci adhered to <50% of the individual HEC-1-B cells, even when inocula of >106 were used (Fig. (Fig.1A).1A). Uninfected HEC-1-B cells were not visually different from infected ones.
TABLE 1
Adherence to and invasion of HEC-1-B cells by P+ Opa+ variants of strains MS11mkC and FA1090 OpaE after 4 h of incubation
incubation
| Inoculum | MS11mkC
| FA1090 OpaE
| ||||
|---|---|---|---|---|---|---|
| Adherence (CFU, 104)a | Invasion
| Adherence (CFU, 104) | Invasion
| |||
| CFU (102)b | %c | CFU (102) | % | |||
| 1 × 107–2 × 107 | 3.6 | 2.2 | 0.6 | 1–2 | 2–3.7 | 2.0 |
| 1 × 106–2 × 106 | 3.6 | 0.6 | 0.2 | 1–2 | 0.5–1 | 0.5 |
| 1 × 105–2 × 105 | 1.6 | 0.4 | 0.25 | 0.5–1 | 0.1–0.4 | 0.3 |
| 1 × 104–2 × 104 | 0.3 | 0.1 | 0.33 | 0.1–0.2 | 0.1–0.2 | 1.0 |
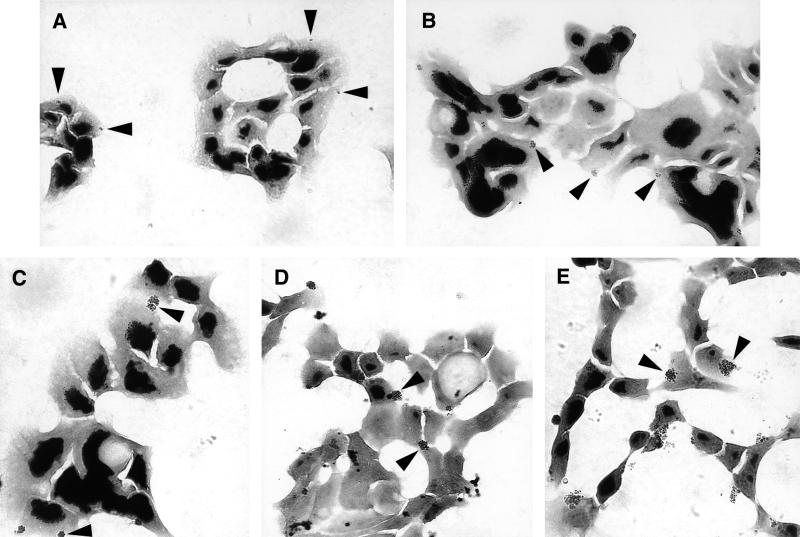
Gonococci form microcolonies on or in HEC-1-B cells. Representative micrographs of Gram-stained cell monolayers after 30 min (A), 60 min (B), 90 min (C), 2 h (D), and 4 h (E) of incubation are shown. Fewer than half of the cells appear to be colonized by gonococci. The gonococcal strain is FA1090 OpaE. Representative colonies are indicated by arrowheads; other colonies are also visible. At 30 min (A) adherent organisms appear as mono- and diplococci. By 60 min (B) tetracoccal and larger colonies are visible. Microcolonies are clearly visible along with new diplococcal and tetracoccal colonies. As colonies continue to grow (C), new microcolonies continue to be established (D). After 4 h of incubation, quite large colonies are visible adherent to or within the HEC-1-B cells (E).
Adherent gonococci form microcolonies on HEC-1-B cells.
P+ Opa+ gonococci adhered to HEC-1-B cells as mono- and diplococci, as seen by light microscopy 30 min after inoculation (Fig. (Fig.1A).1A). Cells that were infected usually had only one or two adherent mono- or diplococci, and these adherent organisms were not clustered (Fig. (Fig.1A).1A). Adherent gonococci gradually formed microcolonies on the surfaces of the HEC-1-B cells during subsequent incubation (Fig. (Fig.1B1B to E). After 1 h of incubation, colonies with four, six, and eight gonococci predominated, but colonies with higher numbers of organisms also were visible (Fig. (Fig.1B).1B). By 90 min most colonies were too large for enumeration of individual cocci, but as the colonies continued to grow, new colonies with two and four gonococci could be seen (Fig. (Fig.1C1C and D). After 4 h of incubation, quite large colonies that had formed from the P+ Opa+ inoculum could be seen adherent to or within HEC-1-B cells (Fig. (Fig.11E).
The CFU of adherent P+ Opa− organisms also increased between 30 min and 2 h of incubation. In contrast, there was no apparent increase in the CFU of adherent P− Opa− or P− Opa+ gonococci over incubation times of between 30 min and 7 h.
Adherent gonococci invade HEC-1-B cells by 4 h.
Invasion of HEC-1-B cells paralleled adherence but was not saturable (Table (Table1).1). No more than 2% of the CFU that were associated with HEC-1-B cells after 4 h of incubation were within the cells, as judged by gonococcal survival in the presence of gentamicin. The proportion of associated CFU that was intracellular by 4 h was roughly constant for inocula of from 104 to 106 CFU/ml but was four times greater for the 107 inoculum than for the 106 inoculum. The number of intracellular FA1090 OpaE CFU was the same at 4 h as the number of MS11mkC CFU, even though 2 to 3 times fewer FA1090 OpaE variants than MS11mkC variants adhered.
Effects of piliation and Opa expression on gonococcal microcolony and HEC-1-B cell microvillus morphologies.
Microcolonies formed by piliated gonococci appeared under SEM to be quite different from those formed by nonpiliated organisms. Figure Figure2A2A shows SEM of a typical P− Opa− microcolony after 6 h of incubation on an HEC-1-B cell. P− Opa− colonies were loose, with moderate coccus-to-coccus adhesion. Microvilli of the HEC-1-B cells appeared to adhere only at their distal ends to individual cocci within the microcolony, and these microvilli were not distorted except by simple contiguity. Expression of Opa by nonpiliated gonococci (Fig. (Fig.2B)2B) did not change the appearance of their microcolonies appreciably and did not result in changes in the morphology of HEC-1-B cell microvilli or their interactions with the organisms.
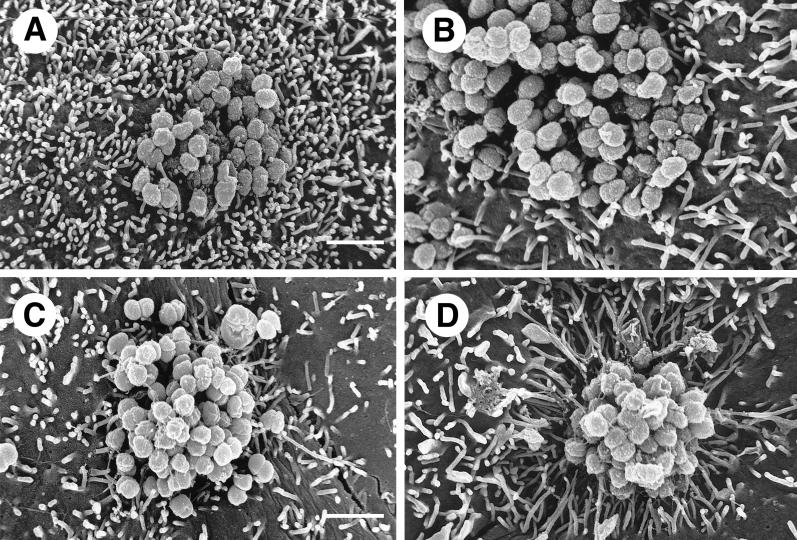
Gonococci must express both Opa and pili (P) in order to signal HEC-1-B cells to engulf them. (A and B) SEM photomicrographs of typical microcolonies of MS11mk P− organisms after 6 hours of growth on HEC-1-B cells. The bacteria in panel B also make Opa. Pili are not seen. (C and D) SEM photomicrographs of typical microcolonies of MS11mk P+ organisms. The bacteria in panel D also make Opa. Strands of pili are visible. (A) The P− Opa− microcolony is loose, with moderate bacterium-bacterium adhesion. Microvilli can be seen protruding from between individual gonococci, but there is little, if any, distortion of microvillus architecture other than that by simple contiguity. Some microvillus distal ends appear to have adhered to individual cocci. (B) The interaction between microvilli and the P− Opa+ microcolony is not different from that seen with P− Opa− organisms (panel A). Opa expression appears to have little influence on the reaction of the epithelial cells to the organisms. (C) Pili appear to “lash” individual P+ Opa− gonococci together to form a more compact colony. Some pili have bound microvilli together or pulled them toward the microcolony, modestly distorting microvillus architecture. Other strands of pili are visible radiating some distance from the microcolony along the surface of the epithelial cell. (D) The microvillus architecture has been altered dramatically as microvilli extend toward the microcolony of P+ Opa+ organisms. The microcolony consists of tightly adherent organisms that are bound together. More peripheral microvilli have extended themselves toward the microcolony to extraordinary lengths. As highly elongated peripheral microvilli adhere to the colony, the microvilli appear to pull the HEC-1-B cell around the colony and lift the HEC-1-B cell from the coverslip. Magnification, ×8,000; bar, 2 μm.
Microcolonies formed by piliated gonococci were quite different. The cocci appeared to be bound together by their pili into compact colonies (Fig. (Fig.2C2C and D). Expression of Opa by piliated organisms resulted in even more compact microcolonies in which organisms were bound together by extensive coccus-coccus adhesions and webs of pili (Fig. (Fig.2D).2D). Some pili of Opa− variants adhered to microvilli and occasionally seemed to be “pulling” them toward the microcolony, thereby modestly distorting the microvilli (Fig. (Fig.2C).2C). The effect of Opa expression on the HEC-1-B cells, and in particular on the morphology of their microvilli, was most dramatic. The microvilli elongated toward the P+ Opa+ microcolonies and appeared to be pulling the HEC-1-B cell surface up around the gonococci (Fig. (Fig.2D).2D). Some microvilli extended themselves to extraordinary lengths in order to surround P+ Opa+ cocci at the apex of the microcolony. This very active microvillus engagement of cocci within microcolonies was absent when the organisms made Opa but not pili (Fig. (Fig.2B)2B) or pili but not Opa (Fig. (Fig.22C).
The extent to which piliated Opa+ organisms activated HEC-1-B cell microvilli was clearer at higher magnifications (Fig. (Fig.3).3). Microvilli adhered to nonpiliated Opa+ gonococci primarily at their distal ends (Fig. (Fig.3A),3A), whereas they bound to piliated Opa+ gonococci along their full lengths (Fig. (Fig.3B).3B). This caused elongated microvilli to wrap tightly around a considerable circumference of individual cocci. As microvilli elongated to engage the apex of the colony, they drew the cell surface up around it (Fig. (Fig.3B),3B), and the colony appeared to sink into the underlying cell cytoplasm (Fig. (Fig.4).4).
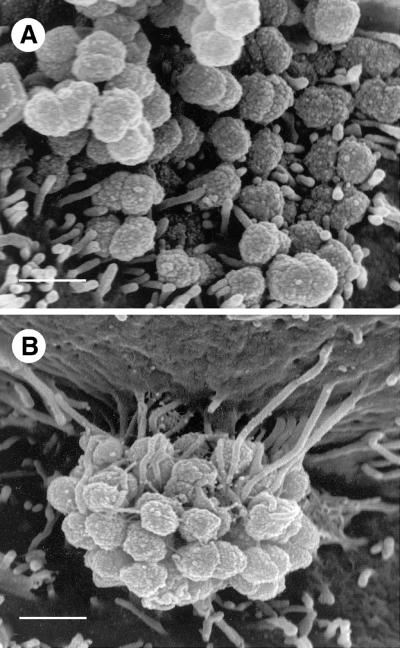
HEC-1-B cell microvilli adhere to P+ Opa+ gonococci along the full lengths of the microvilli. (A) At this SEM magnification it is clear that after 6 h of incubation only the distal ends of microvilli adhere to MS11mk P− Opa+ organisms. Microvilli have not been elongated. Both true diplococci and adherent individual cocci are visible. (B) In this SEM photomicrograph microvilli can be seen to have wrapped themselves tightly around MS11mk P+ Opa+ cocci in the microcolony after 6 h of incubation on HEC-1-B cells. Microvilli adhere tightly to individual cocci up to the full lengths of the microvilli. Microvillus adherence is clearly separate from bacterium-pilus interactions. Microvilli at the top of the micrograph appear to have pulled the cell surface up along the side of the colony, creating the appearance of a microcolony hanging from a pedestal of microvilli. (The electron beam is focused vertically, not horizontally.) The extent of microvillus elongation is remarkable, and the contrast between microvillar responses to piliated and nonpiliated Opa+ bacteria is striking. Magnification, ×15,500; bar, 1 μm.
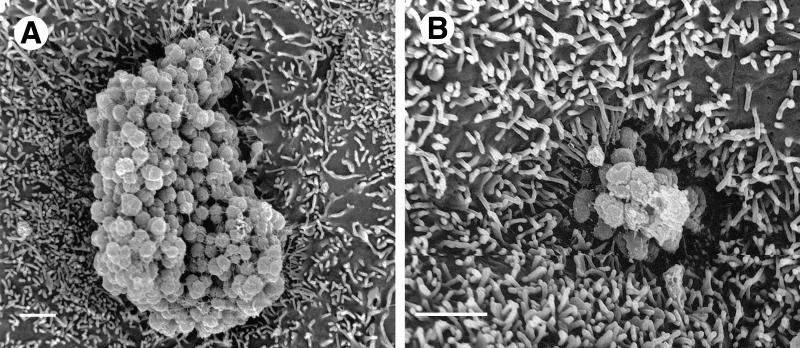
HEC-1-B cells engulf microcolonies with P+ Opa+ gonococci. After 6 h of incubation, the MS11mk P+ Opa+ microcolonies appear to sink into the HEC-1-B cell in these SEM photomicrographs as activated microvilli pull the underlying cell surfaces up around the colony. Magnification, ×8,000; bar, 2 μm.
The requirement for gonococci to express both pili and Opa in order to activate microvilli was not unique to the MS11mk strain. HEC-1-B cellular responses to the pilus and Opa variants of strains F62-SF and FA1090 were the same as those to the MS11mk variants. Only piliated Opa+ variants of F62-SF and FA1090 activated microvillus-directed engulfment.
Microvillus elongation did not begin before 2 h of incubation with P+ Opa+ organisms, with the exception of the piliated FA1090 Opa variant E (OpaE), and most of the P+ Opa+ variants needed 4 to 6 h of incubation before microvilli elongated. The piliated FA1090 OpaE variant was unique in that it stimulated microvillus elongation within 1 h.
TEM was used to confirm that microvilli contributed to the internalization of piliated Opa+ organisms. Elongated microvilli adhered full length to P+ Opa+ organisms (Fig. (Fig.5A5A and B), whereas microvilli were not elongated in the presence of P− Opa+ gonococci, and the very few microvilli that engaged the bacteria did so only at their distal ends (Fig. (Fig.5C5C and D). Some nonpiliated Opa+ organisms lay on the cell surface without perturbing it.
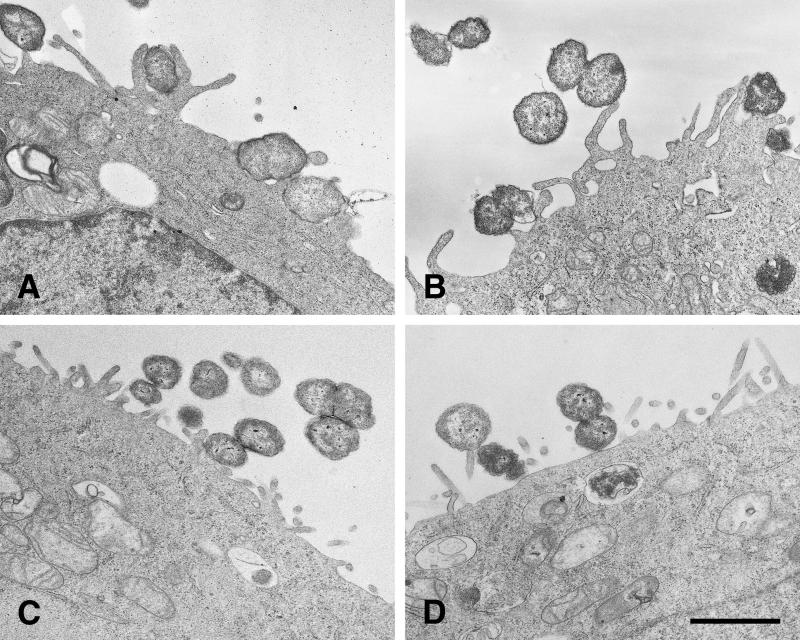
TEM of P+ Opa+ organisms with activated microvilli adherent along their full lengths. (A and B) FA1090 P+ OpaE after 10 h of incubation on HEC-1-B cells. Microvilli have elongated and are adhering along their full lengths to gonococci, causing a perturbation of the HEC-1-B cell surface. Internalized cocci that are visible in both panels A and B appear to be free in the cell cytoplasm; vacuole membranes are not clearly visible (A). A bundle of pili is visible extending from the surface of a nonadherent coccus in panel B. (C and D) FA1090 P− OpaE after 10 h of incubation on HEC-1-B cells. Microvilli have not elongated, and the HEC-1-B cell surface is not perturbed. Some of these nonpiliated organisms lie in contiguity with the cell surface, but they are not being internalized. Some vacuoles within the cell cytoplasm appear to contain degenerating organisms. Bacterium-bacterium adhesion is apparent in both panels; tip adherence by microvilli also can be seen in both panels. Bar, 1 μm.
After 10 h of incubation, the HEC-1-B cells that had engulfed P+ Opa+ gonococci were filled with organisms that were not within vacuoles (Fig. (Fig.6A).6A). At higher magnifications (Fig. (Fig.7),7), cell membranes could be seen to be surrounding engulfed P+ Opa+ organisms, but the membrane fragments were closely apposed, or adherent, to the bacterial membranes, without any vacuolar space between them. Many of the internalized P+ Opa+ gonococci maintained the close bacterium-bacterium adherence, and dividing cocci could be seen within the cell (Fig. (Fig.6A).6A). Other HEC-1-B cells, often in sufficiently close contiguity to have formed tight junctions with infected HEC-1-B cells, contained no organisms (Fig. (Fig.6A),6A), reflecting the very selective nature of invasion. Only an occasional P− Opa+ gonococcus was found within a cell, and they were clearly within vacuoles (Fig. (Fig.6B).6B). Some vacuoles contained degenerated bacterial forms (Fig. (Fig.5D).5D).
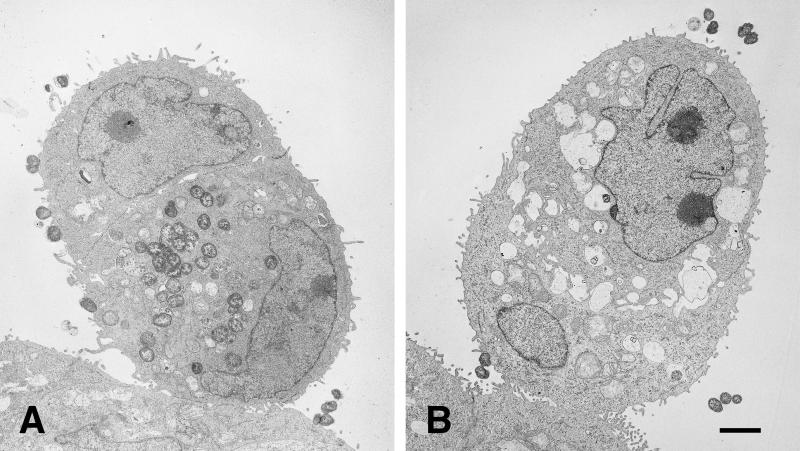
Only P+ Opa+ organisms are found within HEC-1-B cells. TEM photomicrographs of HEC-1-B cells after 10 h of incubation with FA1090 P+ OpaE (A) and FA1090 P− OpaE (B) organisms are shown. (A) Two cells are closely apposed, appearing as a single, ovoid cell. (Note the two nuclei, the tight junctions at the periphery, and microvilli in the space below the upper nucleus.) The lower (and larger) of the two cells contains what appears to be an internalized microcolony in which the individual cocci remain tightly adherent to each other; cocci may be in the process of being internalized into the upper cell. Vacuole membranes are not apparent around the ingested organisms. (B) Two cells are closely apposed, appearing as a single, ovoid cell. (Note the two nuceli, the tight junctions at the periphery, and the microvilli in the space between the nuclei.) The lower (and smaller) of the two cells contains a single internalized coccus, visible at the lower right; it appears to be within a vacuole. Other vacuoles within the cytoplasm are empty. (The “vacuoles” between the nuclei are intercellular spaces.) A second coccus is in close contact with the cell membrane. Bar, 1 μm.
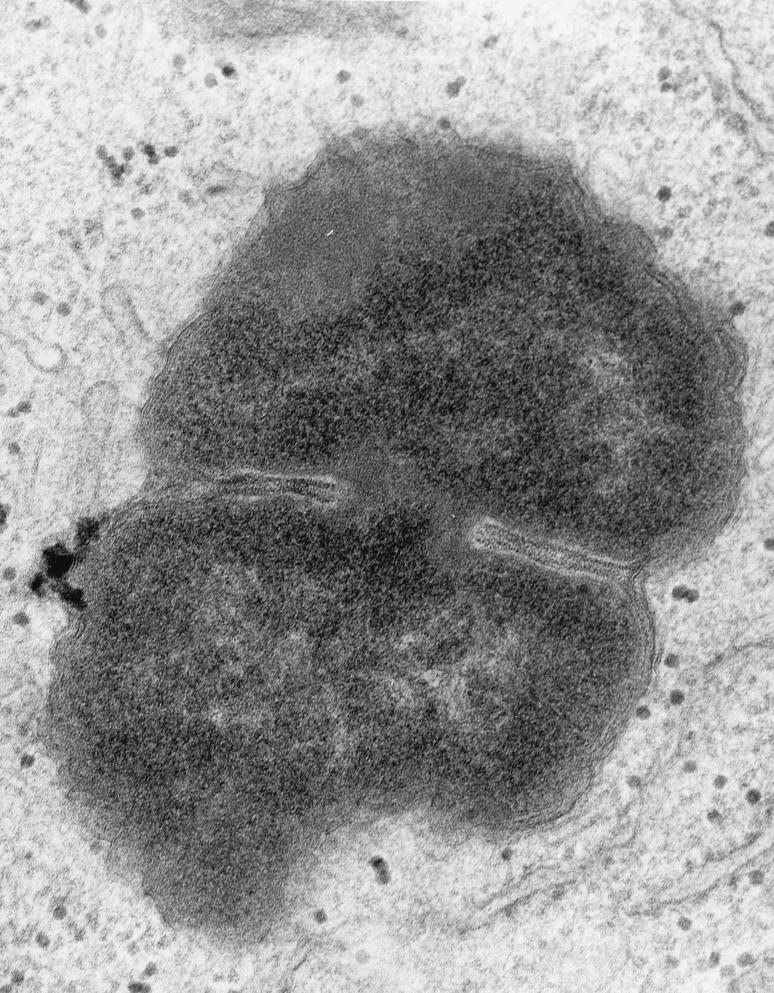
High magnification (×50,000) of an intracellular FA1090 P+ OpaE organism that is in the process of dividing. Fragments of host cell membrane are visible in very close apposition to the bacterial outer membrane. This is most visible at the “waist” of the dividing coccus, but redundant fragments can be seen surrounding most of the organism. Note the absence of a clear vacuolar space between the host cell and bacterial cell membranes.
Effects of protein synthesis inhibitors.
Treatment with 1 μg of chloramphenicol per ml, which would inhibit protein synthesis by the bacteria but not that by the eukaryotic cells, caused a diminution in microvillus elongation at 6 h and stopped engulfment of P+ Opa+ organisms (Fig. (Fig.8).8). In the presence of chloramphenicol concentrations of ≥5 μg/ml (Fig. (Fig.8),8), Opa+ organisms no longer made abundant pili (Fig. (Fig.8),8), and microvillus elongation was abolished altogether. At chloramphenicol concentrations that would inhibit protein synthesis by the eukaryotic cells (≥25 μg/ml), the poorly piliated and loose bacterial colonies again appeared to sink into the underlying HEC-1-B cells, but this was not accompanied by the elongation of microvilli (Fig. (Fig.8).8).
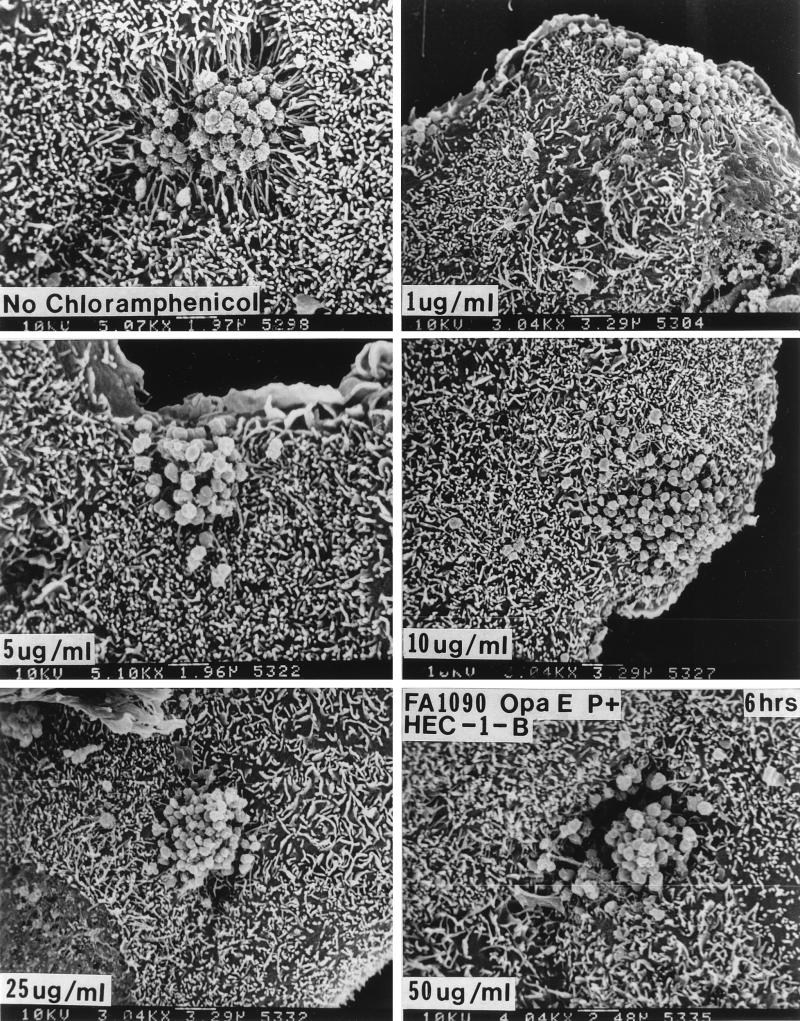
Chloramphenicol abolishes microvillus elongation and bacterial internalization at low doses but causes microvillus-independent HEC-1-B cell engulfment at higher doses. For these experiments FA1090 P+ OpaE variants were used, as these organisms stimulate microvillus elongation within 1 h. The HEC-1-B cells were incubated for 30 min with 0 to 50 μg of chloramphenicol per ml, the spent medium was aspirated, the HEC-1-B cells were inoculated with gonococci that had been diluted in medium containing the appropriate concentration of chloramphenicol, and the HEC-1-B cells were incubated for various times. As little as 5 μg of chloramphenicol per ml abolished microvillus elongation at 6 h; 10 μg of chloramphenicol per ml was needed to abolish microvillus elongation within the first hour. (A) FA1090 P+ OpaE after 6 h of incubation with HEC-1-B cells in the absence of chloramphenicol. (B to D) FA1090 P+ OpaE after 6 h of incubation with HEC-1-B cells in the presence of 1, 5, and 10 μg of chloramphenicol per ml. Note that microvillus-dependent engulfment appears to be lost at these concentrations of chloramphenicol. (E and F) FA1090 P+ OpaE after 6 h of incubation with HEC-1-B cells in the presence of 25 and 50 μg of chloramphenicol per ml. Note that the microcolonies are sinking into the HEC-1-B cells at these concentrations of chloramphenicol but that microvilli are not participating in their internalization. The magnification is given at the bottom of each panel.
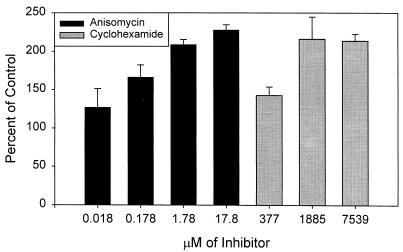
These results suggested that the higher chloramphenicol concentrations caused the HEC-1-B cells to lose their ability to resist gonococcal invasion, whereas the lower concentrations primarily affected the bacteria. If so, then drugs that would affect only eukaryotic protein synthesis should enhance gonococcal invasion. We tested this hypothesis by repeating the experiments in the presence of increasing concentrations of anisomycin, an antibiotic that blocks peptidyl transferase activity on eukaryotic ribosomes, and cycloheximide, which blocks translocation on eukaryotic ribosomes. We used culture to quantitate the effects of the two drugs, as neither affected N. gonorrhoeae viability. Increasing the concentrations of both drugs increased gonococcal uptake by the human cells; anisomycin was the more potent on a molar basis (Fig. (Fig.9).9).
Effects of cytoskeleton inhibitors.
Treatment of HEC-1-B cells with cytochalasins B and D, which block actin polymerization and paralyze cell movement, stopped gonococcal uptake in a dose-dependent way (data not shown). Cytochalasin D was 10-fold more active (50% inhibitory doses of 50 versus 500 ng). Treatment with colchicine, which binds tubulin and prevents its polymerization, did not affect gonococcal uptake, even at concentrations of 50 μM (data not shown). None of these drugs affected the viability of gonococci or their ability to adhere to the human cells.
DISCUSSION
The data presented in this paper strongly support the conclusion that pili and Opa function coordinately in N. gonorrhoeae adherence to and invasion of HEC-1-B cells. The gonococci that lacked pili or Opa or both did not demonstrate the same active interaction with the HEC-1-B cells as the Opa+ P+ gonococci.
Initial adherence of the Opa+ P+ gonococci was to the distal ends of microvilli of only a few HEC-1-B cells. Adherence was saturable and was to only a subpopulation of cells, as initially no gonococci were visibly attached to a majority of the cells, also suggesting that the ligand that mediates adherence was not present on many cells. Opa− and Opa+ gonococci both adhered to the distal ends of the microvilli. The adherence of the Opa− gonococci suggests that adhesins other than Opa mediate the initial close attachment of the gonococci to the microvilli (4, 14, 36, 39, 59, 74). Several studies have identified lectin-like adhesins other than pili and Opa that bind human glycosphingolipid structures (15, 47, 50, 51, 62). Gonococcal adherence to the distal ends of microvilli in the fallopian tube organ culture model was noted previously (20).
Initially the adherent Opa+ P+ gonococci were single cocci or diplococci. Over the next 30 min to few hours, the single cocci and diplococci became microcolonies. These appeared to be similar to the clusters of gonococci described by Novotny and coworkers after light microscopic and EM examination of pus from infected men and women; these clusters were termed infectious units (45, 46). The microcolonies of gonococci might be more representative of naturally occurring gonococcal infection than the single coccus or diplococcus.
The pili adhered to epithelial cell surfaces and microvilli, as seen by SEM, and also formed webs that bound the gonococci into compact colonies. The overall effect seemed to be to anchor these compact microcolonies onto the cell surface; similar observations have been made previously (16, 17).
As the microcolonies of Opa+ P+ gonococci developed, the HEC-1-B cell microvilli elongated and bound to the gonococci along much of their full lengths. Microvillus elongation and attachment to gonococci along the length of the microvilli in the fallopian tube organ culture model was noted previously (19). The microvilli appeared to be actively engaging the gonococci. The interaction of the microvilli with the gonococci that lacked pili or Opa or both was much different. Although pili were required for HEC-1-B cell microvillus activation and elongation, the pili were not sufficient; Opa also was required.
There have been previous reports that Opa expression by gonococci was required for invasion of various epithelial cell lines in vitro (39, 59, 74). Kupsch et al. found that Opa expression alone was not sufficient to support gonococcal invasion of HEC-1-B cells (36). The data in the present paper show that Opa expression was required for invasion, but, as with pili, it was not sufficient to direct engulfment by HEC-1-B cells; pili also were required.
The studies with experimental gonococcal infections of human volunteers have demonstrated that pili were essential for infection to occur (32, 33, 69). When the volunteers were inoculated with Opa− gonococci, there was a lag period during which the subjects had no symptoms (31, 56, 66). The gonococci cultured from the urine sediment shortly after inoculation tended to be the Opa− variants that were in the initial inoculum. Subsequently, the subjects developed symptoms and the gonococci present in the urinary sediments were uniformly Opa+. It is likely that the Opa− gonococci in the inoculum either were shed and excreted in the urine or shifted to Opa+ and proceeded to invade the urethral mucosa and cause symptomatic disease. The presence of Opa in the gonococci from the symptomatic volunteers strongly suggested that Opa, along with pili, was required for the gonococci to cause disease. This in vivo demonstration of the coordinate use of pili and Opa in initiating gonococcal infection was fully consistent with the observations in the present in vitro study, where pili and Opa coordinately promoted epithelial cell invasion.
Microscopy of naturally occurring infections shows viable, dividing gonococci, which do not appear to be within vacuoles, inside epithelial cells and polymorphonuclear leukocytes (24, 46, 71, 72). Internalized P+ Opa+ organisms looked like those seen during natural infection (24, 46, 71, 72). In contrast, the few P− Opa+ bacteria that were ingested appeared to be degenerate and within clearly visible parasitophorous vacuoles.
Chen et al. reported that preincubation of gonococcal strain F62 with glutaraldehyde-fixed HEC-1-B cells enabled the bacteria then to invade viable cells without the normal 6- to 8-h lag (10). Preincubation with the fixed cells did not make the organisms more adherent, but for invasion to be enhanced, the gonococci had to have contact with the fixed membranes of the HEC-1-B cells (10). These observations indicate that HEC-1-B cells have on their surfaces a molecule(s) that can signal adherent gonococci to begin synthesizing molecules that then promote active endocytosis by the HEC-1-B cells.
The OpaE variant of strain FA1090 differed from the other Opa+ strains in that it caused microvillus elongation in only 1 h. It therefore must have expressed constitutively the necessary ligand(s) that was induced in the other Opa variants and strains of gonococci.
Treatment with doses of chloramphenicol that interfered with bacterial but not eukaryotic protein synthesis abolished both intimate attachment to microvilli and gonococcal invasion. This is consistent with the notion that protein synthesis by the gonococcus yielded a ligand that engaged proximal microvillus receptors. Chen et al. also reported that chloramphenicol treatment during preincubation abolished enhancement of invasion (10).
Drugs that blocked eukaryotic protein synthesis caused enhanced gonococcal invasion. At doses of chloramphenicol that interrupted eukaryotic protein synthesis, gonococcal microcolonies entered HEC-1-B cells without engaging microvilli. This same passive process may have accounted for the enhanced invasion that accompanied treatment of the eukaryotic cells with anisomycin and cyclohexamide. The HEC-1-B cells may naturally resist invasion. Activation of microvillus-mediated engulfment, then, can be seen as a strategy for circumventing natural resistance to invasion (6).
The finding that cytochalasins inhibited gonococcal invasion of various epithelial cell lines has been reported previously (3, 10, 58). Richardson and Sadoff found that colchicine also inhibited gonococcal invasion (52), but this drug did not inhibit uptake of FA1090 OpaE organisms by HEC-1-B cells in the present study. The discrepancy can explained by Richardson and Sadoff’s use of hamster kidney cells, rather than human epithelial cells. As N. gonorrhoeae is an obligate pathogen of human epithelial cells and polymorphonuclear leukocytes, its entry into rodent kidney cells could be expected to involve mechanisms different from those used to invade human cells. In addition, Richardson and Sadoff used different gonococcal strains.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI 21620 (to J.M.G.) and PO1 AI 21912 (to G.F.B.) and by the Research Service of the Department of Veterans Affairs (to J.M.G.).
May Fong helped administer the grants and prepared the manuscript, for which we thank her.
Footnotes
†Report no. 79 from the Centre for Immunochemistry, University of California, San Francisco.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.67.7.3469-3480.1999
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/67/7/3469.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/67/7/3469
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/content/full/67/7/3469
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/67/7/3469
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/168145644
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.67.7.3469-3480.1999
Article citations
Neisseria gonorrhoeae drives Chlamydia trachomatis into a persistence-like state during in vitro co-infection.
Infect Immun, 92(1):e0017923, 28 Nov 2023
Cited by: 0 articles | PMID: 38014981 | PMCID: PMC10790821
Three-dimensional models of the cervicovaginal epithelia to study host-microbiome interactions and sexually transmitted infections.
Pathog Dis, 80(1):ftac026, 01 Aug 2022
Cited by: 7 articles | PMID: 35927516 | PMCID: PMC9419571
Gonococcal invasion into epithelial cells depends on both cell polarity and ezrin.
PLoS Pathog, 17(12):e1009592, 01 Dec 2021
Cited by: 0 articles | PMID: 34852011 | PMCID: PMC8668114
Distinct Patterns of Host Adherence by Neisseria gonorrhoeae Isolated from Experimental Gonorrhea.
Can J Infect Dis Med Microbiol, 2021:7865405, 13 May 2021
Cited by: 1 article | PMID: 34093925 | PMCID: PMC8140856
Pathogenesis of Neisseria gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube.
Front Immunol, 9:2710, 21 Nov 2018
Cited by: 31 articles | PMID: 30524442 | PMCID: PMC6258741
Review Free full text in Europe PMC
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells.
Cell Microbiol, 3(10):681-691, 01 Oct 2001
Cited by: 22 articles | PMID: 11580753
Effect of attachment factors (pili plus Opa) on Neisseria gonorrhoeae invasion of human fallopian tube tissue in vitro: quantitation by computerized image analysis.
Microb Pathog, 13(2):93-108, 01 Aug 1992
Cited by: 7 articles | PMID: 1360614
Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton.
Infect Immun, 64(5):1621-1630, 01 May 1996
Cited by: 75 articles | PMID: 8613370 | PMCID: PMC173971
Adhesion of Neisseria gonorrhoeae and disease.
Ciba Found Symp, 80:188-201, 01 Jan 1981
Cited by: 4 articles | PMID: 6114820
Review
Funding
Funders who supported this work.
NIAID NIH HHS (3)
Grant ID: AI 21620
Grant ID: P01 AI 21912
Grant ID: R01 AI021620