Abstract
Free full text

Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats
Abstract
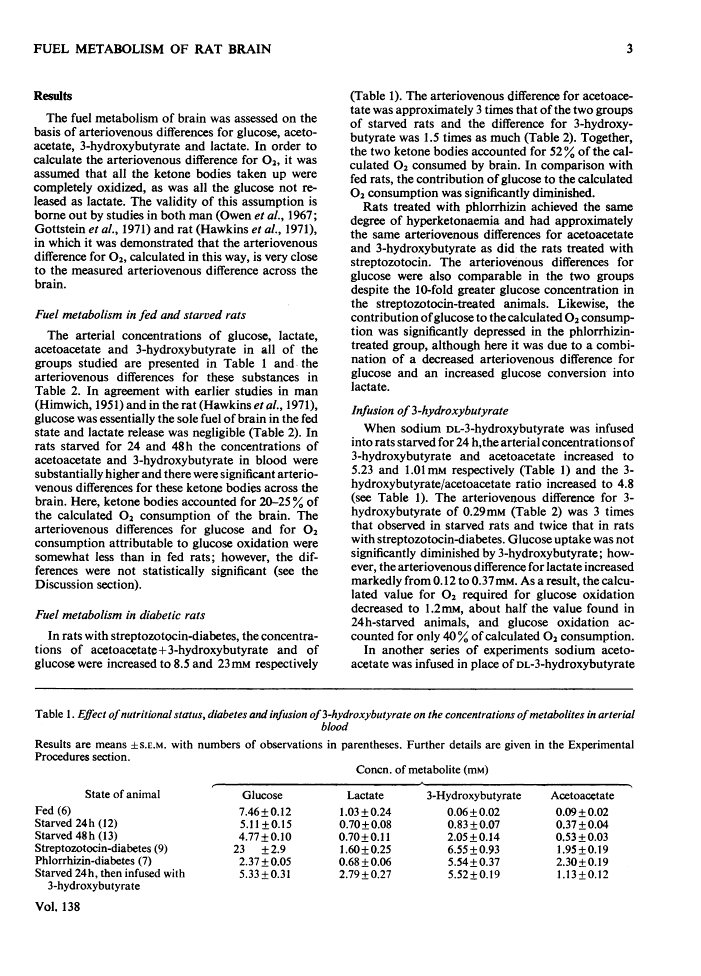
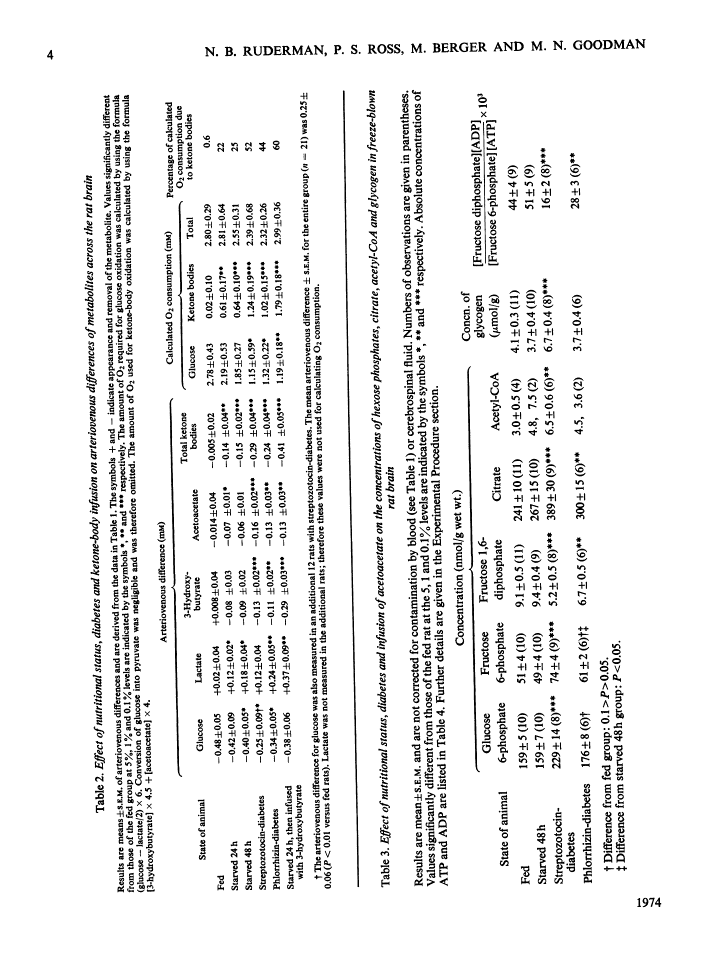
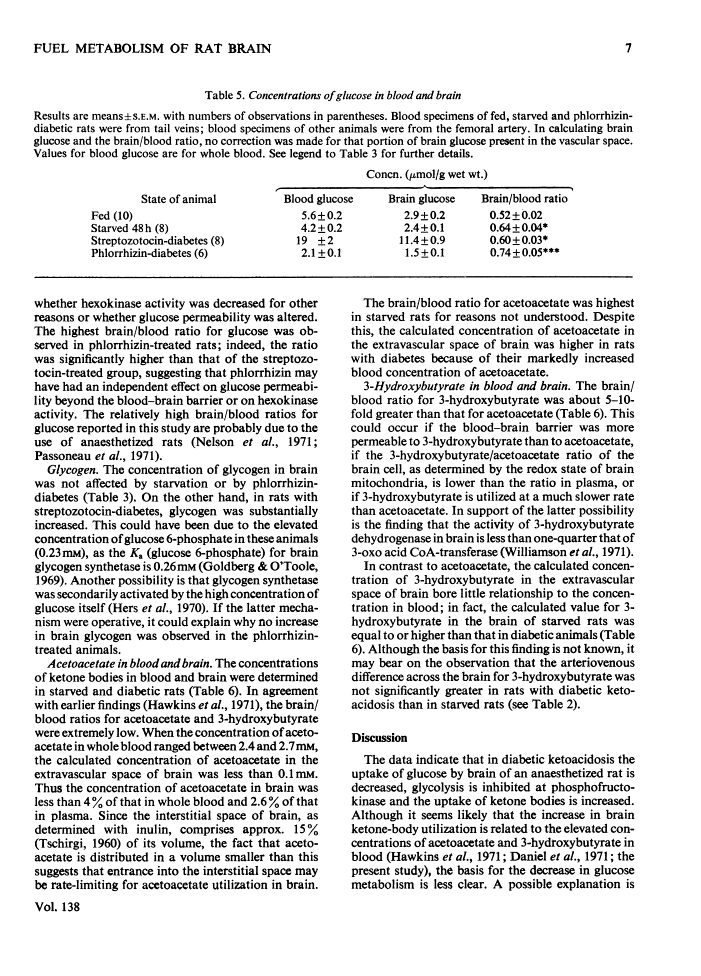
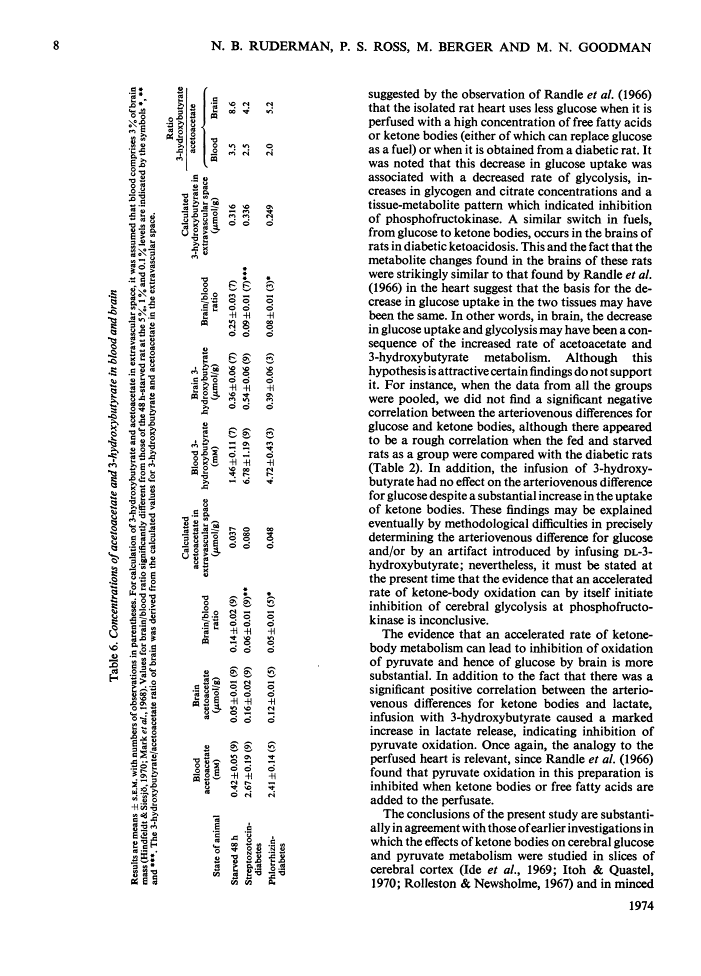
1. The effects of starvation and diabetes on brain fuel metabolism were examined by measuring arteriovenous differences for glucose, lactate, acetoacetate and 3-hydroxybutyrate across the brains of anaesthetized fed, starved and diabetic rats. 2. In fed animals glucose represented the sole oxidative fuel of the brain. 3. After 48h of starvation, ketone-body concentrations were about 2mm and ketone-body uptake accounted for 25% of the calculated O2 consumption: the arteriovenous difference for glucose was not diminished, but lactate release was increased, suggesting inhibition of pyruvate oxidation. 4. In severe diabetic ketosis, induced by either streptozotocin or phlorrhizin (total blood ketone bodies >7mm), the uptake of ketone bodies was further increased and accounted for 45% of the brain's oxidative metabolism, and the arteriovenous difference for glucose was decreased by one-third. The arteriovenous difference for lactate was increased significantly in the phlorrhizin-treated rats. 5. Infusion of 3-hydroxybutyrate into starved rats caused marked increases in the arteriovenous differences for lactate and both ketone bodies. 6. To study the mechanisms of these changes, steady-state concentrations of intermediates and co-factors of the glycolytic pathway were determined in freeze-blown brain. 7. Starved rats had increased concentrations of acetyl-CoA. 8. Rats with diabetic ketosis had increased concentrations of fructose 6-phosphate and decreased concentrations of fructose 1,6-diphosphate, indicating an inhibition of phosphofructokinase. 9. The concentrations of acetyl-CoA, glycogen and citrate, a potent inhibitor of phosphofructokinase, were increased in the streptozotocin-treated rats. 10. The data suggest that cerebral glucose uptake is decreased in diabetic ketoacidosis owing to inhibition of phosphofructokinase as a result of the increase in brain citrate. 11. The inhibition of brain pyruvate oxidation in starvation and diabetes can be related to the accelerated rate of ketone-body metabolism; however, we found no correlation between the decrease in glucose uptake in the diabetic state and the arteriovenous difference for ketone bodies. 12. The data also suggest that the rates of acetoacetate and 3-hydroxybutyrate utilization by brain are governed by their concentrations in plasma. 13. The finding of very low concentrations of acetoacetate and 3-hydroxybutyrate in brain compared with plasma suggests that diffusion across the blood–brain barrier may be the rate-limiting step in their metabolism.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill GF, Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. [Europe PMC free article] [Abstract] [Google Scholar]
- Daniel PM, Love ER, Moorehouse SR, Pratt OE, Wilson P. Factors influencing utilisation of ketone-bodies by brain in normal rats and rats with ketoacidosis. Lancet. 1971 Sep 18;2(7725):637–638. [Abstract] [Google Scholar]
- Goldberg ND, O'Toole AG. The properties of glycogen synthetase and regulation of glycogen biosynthesis in rat brain. J Biol Chem. 1969 Jun 10;244(11):3053–3061. [Abstract] [Google Scholar]
- Gottstein U, Müller W, Berghoff W, Gärtner H, Held K. Zur Utilisation von nicht-veresterten Fettsäuren und Ketonkörpern im Gehirn des Menschen. Klin Wochenschr. 1971 Apr 1;49(7):406–411. [Abstract] [Google Scholar]
- Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. [Europe PMC free article] [Abstract] [Google Scholar]
- Hers HG, De Wulf H, Stalmans W. The control of glycogen metabolism in the liver. FEBS Lett. 1970 Dec 28;12(2):73–82. [Abstract] [Google Scholar]
- Hindfelt B, Siesjö BK. The effect of ammonia on the energy metabolism of the rat brain. Life Sci II. 1970 Sep 22;9(18):1021–1028. [Abstract] [Google Scholar]
- HUGGETT AS, NIXON DA. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. [Abstract] [Google Scholar]
- Ide T, Steinke J, Cahill GF., Jr Metabolic interactions of glucose, lactate, and beta-hydroxybutyrate in rat brain slices. Am J Physiol. 1969 Sep;217(3):784–792. [Abstract] [Google Scholar]
- Ito T, Quastel JH. Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem J. 1970 Feb;116(4):641–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Krebs HA, Eggleston LV. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [Europe PMC free article] [Abstract] [Google Scholar]
- Krebs HA, Dierks C, Gascoyne T. Carbohydrate synthesis from lactate in pigeon-liver homogenate. Biochem J. 1964 Oct;93(1):112–121. [Europe PMC free article] [Abstract] [Google Scholar]
- LOWRY OH, PASSONNEAU JV. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [Abstract] [Google Scholar]
- Mansour TE. Phosphofructokinase. Curr Top Cell Regul. 1972;5:1–46. [Abstract] [Google Scholar]
- Mark J, Godin Y, Mandel P. Glucose and lactic acid content of the rat brain. J Neurochem. 1968 Feb;15(2):141–143. [Abstract] [Google Scholar]
- Openshaw H, Bortz WM. Oxidation of glucose, acetoacetate, and palmitate in brain mince of normal and ketotic rats. Diabetes. 1968 Feb;17(2):90–95. [Abstract] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. [Europe PMC free article] [Abstract] [Google Scholar]
- Passonneau JV, Brunner EA, Molstad C, Passonneau R. The effects of altered endocrine states and of ether anaesthesia on mouse brain. J Neurochem. 1971 Dec;18(12):2317–2328. [Abstract] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA, Denton RM, Pogson CI. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. [Abstract] [Google Scholar]
- Rolleston FS, Newsholme EA. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruderman NB, Goodman MN. Regulation of ketone body metabolism in skeletal muscle. Am J Physiol. 1973 Jun;224(6):1391–1397. [Abstract] [Google Scholar]
- Ruderman NB, Houghton CR, Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. [Europe PMC free article] [Abstract] [Google Scholar]
- Siess E, Wittmann J, Wieland O. Interconversion and kinetic properties of pyruvate dehydrogenase from brain. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):447–452. [Abstract] [Google Scholar]
- Siesjö BK, Folbergrová J, MacMillan V. The effect of hypercapnia upon intracellular pH in the brain, evaluated by the bicarbonate-carbonic acid method and from the creatine phosphokinase equilibrium. J Neurochem. 1972 Nov;19(11):2483–2495. [Abstract] [Google Scholar]
- Start C, Newsholme EA. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. [Europe PMC free article] [Abstract] [Google Scholar]
- Tildon JT, Sevdalian DA. CoA transferase in the brain and other mammalian tissues. Arch Biochem Biophys. 1972 Feb;148(2):382–390. [Abstract] [Google Scholar]
- Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [Abstract] [Google Scholar]
- Veech RL, Harris RL, Veloso D, Veech EH. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973 Jan;20(1):183–188. [Abstract] [Google Scholar]
- WILLIAMSON DH, MELLANBY J, KREBS HA. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. [Europe PMC free article] [Abstract] [Google Scholar]
- Williamson DH, Bates MW, Page MA, Krebs HA. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj1380001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1166169?pdf=render
Citations & impact
Impact metrics
Article citations
β-hydroxybutyrate reduces blastocyst viability via trophectoderm-mediated metabolic aberrations in mice.
Hum Reprod, 37(9):1994-2011, 01 Aug 2022
Cited by: 1 article | PMID: 35856159 | PMCID: PMC9433850
Differential ketogenic diet-induced shift in CSF lipid/carbohydrate metabolome of pediatric epilepsy patients with optimal vs. no anticonvulsant response: a pilot study.
Nutr Metab (Lond), 18(1):23, 01 Mar 2021
Cited by: 6 articles | PMID: 33648550 | PMCID: PMC7923458
Emerging Trends in the Use of Therapeutic Hypothermia as a Method for Neuroprotection in Brain Damage (Review).
Sovrem Tekhnologii Med, 12(5):94-104, 28 Oct 2020
Cited by: 1 article | PMID: 34796010 | PMCID: PMC8596265
Review Free full text in Europe PMC
Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks.
J Biol Chem, 295(42):14379-14390, 12 Aug 2020
Cited by: 39 articles | PMID: 32796035 | PMCID: PMC7573269
Review Free full text in Europe PMC
Whole-Body Metabolism, Carbohydrate Utilization, and Caloric Energy Balance After Sport Concussion: A Pilot Study.
Sports Health, 12(4):382-389, 10 Jun 2020
Cited by: 4 articles | PMID: 32520660 | PMCID: PMC7787565
Go to all (139) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The rate of cerebral utilization of glucose, ketone bodies, and oxygen: a comparative in vivo study of infant and adult rats.
Pediatr Res, 10(11):910-917, 01 Nov 1976
Cited by: 34 articles | PMID: 980550
Ketone-body utilization by adult and suckling rat brain in vivo.
Biochem J, 122(1):13-18, 01 Mar 1971
Cited by: 368 articles | PMID: 5124783 | PMCID: PMC1176682
Cerebral utilization of glucose, ketone bodies and oxygen in starving infant rats and the effect of intrauterine growth retardation.
Acta Physiol Scand, 98(2):237-247, 01 Oct 1976
Cited by: 10 articles | PMID: 983734
Preferential utilization of ketone bodies in the brain and lung of newborn rats.
Fed Proc, 44(7):2352-2358, 01 Apr 1985
Cited by: 22 articles | PMID: 3884391
Review