Abstract
Free full text

Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review.
Abstract
Two distinct types of thalamic nucleus are proposed on the basis of the afferent fibres that they receive from ascending pathways and from the cerebral cortex. 'First order nuclei' receive primary afferent fibres, definable on the basis of their origin and their intrathalamic synaptic relationships, from ascending pathways. These nuclei receive corticothalamic afferents from pyramidal cells in cortical layer 6, which also send branches to the thalamic reticular nucleus and appear to have a modulatory function. 'Higher order nuclei' receive most or all of their 'primary afferents' from pyramidal cells in cortical layer 5. These resemble the ascending primary afferents in the first order nuclei in terms of fine structure, synaptic relationships and in lacking a branch to the thalamic reticular nucleus. The higher order nuclei also receive modulatory afferents from layer 6. It is proposed that the higher-order nuclei are largely concerned with transmitting information about the output of one cortical area to another cortical area, and that they are likely to play a key role in corticocortical communication and higher cortical functions.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson BP, Chalupa LM. The laminar distribution of cortical connections with the tecto- and cortico-recipient zones in the cat's lateral posterior nucleus. Neuroscience. 1985 May;15(1):81–95. [Abstract] [Google Scholar]
- Asanuma C, Porter LL. Light and electron microscopic evidence for a GABAergic projection from the caudal basal forebrain to the thalamic reticular nucleus in rats. J Comp Neurol. 1990 Dec 1;302(1):159–172. [Abstract] [Google Scholar]
- Aumann TD, Rawson JA, Finkelstein DI, Horne MK. Projections from the lateral and interposed cerebellar nuclei to the thalamus of the rat: a light and electron microscopic study using single and double anterograde labelling. J Comp Neurol. 1994 Nov 8;349(2):165–181. [Abstract] [Google Scholar]
- Bender DB. Visual activation of neurons in the primate pulvinar depends on cortex but not colliculus. Brain Res. 1983 Nov 21;279(1-2):258–261. [Abstract] [Google Scholar]
- Bickford ME, Günlük AE, Van Horn SC, Sherman SM. GABAergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. J Comp Neurol. 1994 Oct 22;348(4):481–510. [Abstract] [Google Scholar]
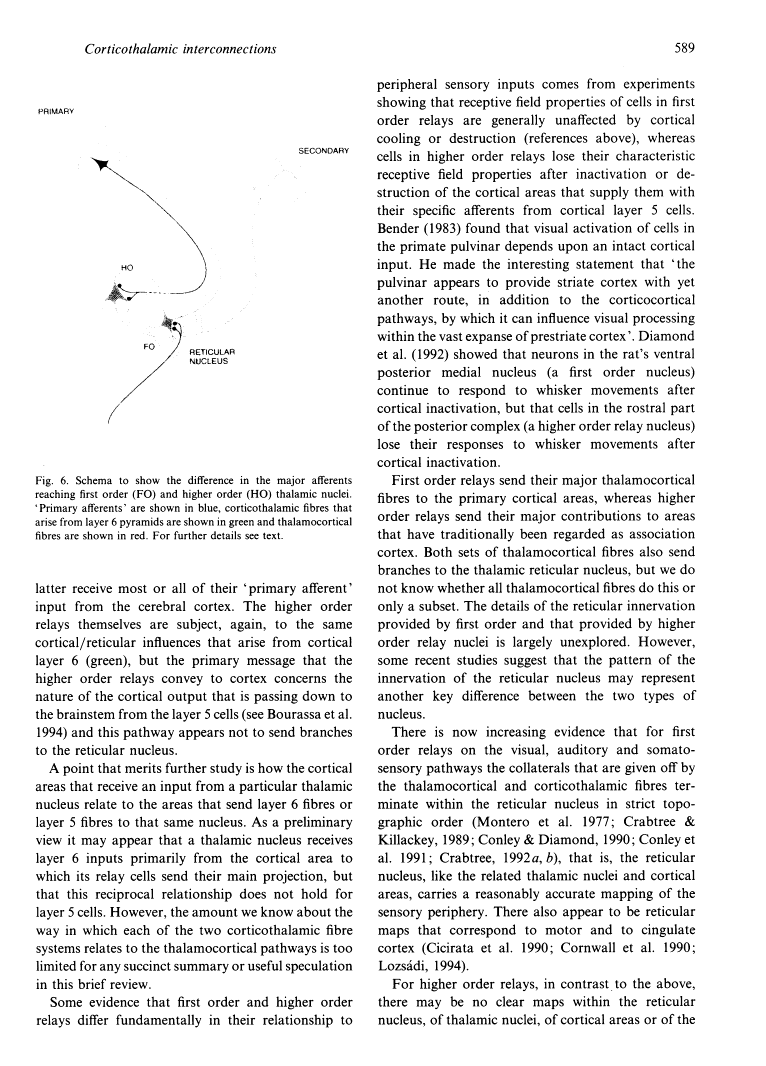
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995 Jan 1;7(1):19–30. [Abstract] [Google Scholar]
- Chen S, Bentivoglio M. Nerve growth factor receptor-containing cholinergic neurons of the basal forebrain project to the thalamic reticular nucleus in the rat. Brain Res. 1993 Mar 26;606(2):207–212. [Abstract] [Google Scholar]
- Cicirata F, Angaut P, Serapide MF, Panto MR. Functional organization of the direct and indirect projection via the reticularis thalami nuclear complex from the motor cortex to the thalamic nucleus ventralis lateralis. Exp Brain Res. 1990;79(2):325–337. [Abstract] [Google Scholar]
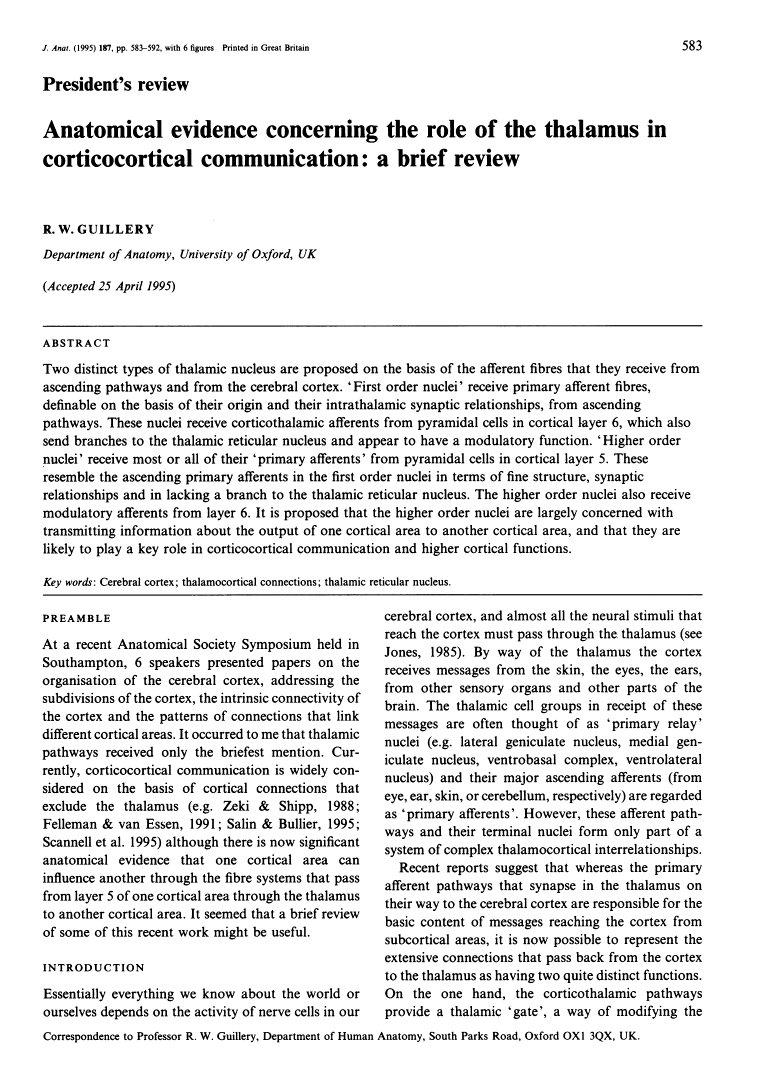
- COLONNIER M, GUILLERY RW. SYNAPTIC ORGANIZATION IN THE LATERAL GENICULATE NUCLEUS OF THE MONKEY. Z Zellforsch Mikrosk Anat. 1964 Apr 9;62:333–355. [Abstract] [Google Scholar]
- Conley Michael, Diamond Irving T. Organization of the Visual Sector of the Thalamic Reticular Nucleus in Galago. Eur J Neurosci. 1990;2(3):211–226. [Abstract] [Google Scholar]
- Conley Michael, Kupersmith Andrew C, Diamond Irving T. The Organization of Projections from Subdivisions of the Auditory Cortex and Thalamus to the Auditory Sector of the Thalamic Reticular Nucleus in Galago. Eur J Neurosci. 1991 Oct;3(11):1089–1103. [Abstract] [Google Scholar]
- Conley M, Raczkowski D. Sublaminar organization within layer VI of the striate cortex in Galago. J Comp Neurol. 1990 Dec 8;302(2):425–436. [Abstract] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Projections to the rostral reticular thalamic nucleus in the rat. Exp Brain Res. 1990;80(1):157–171. [Abstract] [Google Scholar]
- Crabtree John W. The Somatotopic Organization Within the Rabbit's Thalamic Reticular Nucleus. Eur J Neurosci. 1992;4(12):1343–1351. [Abstract] [Google Scholar]
- Crabtree John W. The Somatotopic Organization Within the Cat's Thalamic Reticular Nucleus. Eur J Neurosci. 1992;4(12):1352–1361. [Abstract] [Google Scholar]
- Crabtree John W, Killackey Herbert P. The Topographic Organization and Axis of Projection within the Visual Sector of the Rabbit's Thalamic Reticular Nucleus. Eur J Neurosci. 1989 Jan;1(1):94–109. [Abstract] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol. 1992 Apr 22;318(4):462–476. [Abstract] [Google Scholar]
- Famiglietti EV, Jr, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972 Mar;144(3):285–334. [Abstract] [Google Scholar]
- Feig S, Harting JK. Ultrastructural studies of the primate lateral geniculate nucleus: morphology and spatial relationships of axon terminals arising from the retina, visual cortex (area 17), superior colliculus, parabigeminal nucleus, and pretectum of Galago crassicaudatus. J Comp Neurol. 1994 May 1;343(1):17–34. [Abstract] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991 Jan-Feb;1(1):1–47. [Abstract] [Google Scholar]
- Geisert EE, Langsetmo A, Spear PD. Influence of the cortico-geniculate pathway on response properties of cat lateral geniculate neurons. Brain Res. 1981 Mar 16;208(2):409–415. [Abstract] [Google Scholar]
- Gilbert CD, Kelly JP. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. [Abstract] [Google Scholar]
- Graybiel AM, Berson DM. Histochemical identification and afferent connections of subdivisions in the lateralis posterior-pulvinar complex and related thalamic nuclei in the cat. Neuroscience. 1980;5(7):1175–1238. [Abstract] [Google Scholar]
- Grofová I, Rinvik E. Cortical and pallidal projections to the nucleus ventralis lateralis thalami. Electron microscopical studies in the cat. Anat Embryol (Berl) 1974;146(2):113–132. [Abstract] [Google Scholar]
- Guillery RW, Geisert EE, Jr, Polley EH, Mason CA. An analysis of the retinal afferents to the cat's medial interlaminar nucleus and to its rostral thalamic extension, the "geniculate wing". J Comp Neurol. 1980 Nov 1;194(1):117–142. [Abstract] [Google Scholar]
- Hámori J, Pasik T, Pasik P, Szentágothai J. Triadic synaptic arrangements and their possible significance in the lateral geniculate nucleus of the monkey. Brain Res. 1974 Nov 22;80(3):379–393. [Abstract] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol. 1987 May 8;259(2):165–192. [Abstract] [Google Scholar]
- Harding BN. An ultrastructural study of the termination of afferent fibres within the ventrolateral and centre median nuclei of the monkey thalamus. Brain Res. 1973 May 17;54:341–346. [Abstract] [Google Scholar]
- Hoogland PV, Welker E, Van der Loos H. Organization of the projections from barrel cortex to thalamus in mice studied with Phaseolus vulgaris-leucoagglutinin and HRP. Exp Brain Res. 1987;68(1):73–87. [Abstract] [Google Scholar]
- Hoogland PV, Wouterlood FG, Welker E, Van der Loos H. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L). Exp Brain Res. 1991;87(1):159–172. [Abstract] [Google Scholar]
- Jones EG. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975 Aug 1;162(3):285–308. [Abstract] [Google Scholar]
- Jones EG, Powell TP. Electron microscopy of synaptic glomeruli in the thalamic relay nuclei of the cat. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):153–171. [Abstract] [Google Scholar]
- Jones EG, Powell TP. An electron microscopic study of the mode of termination of cortico-thalamic fibres within the sensory relay nuclei of the thalamus. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):173–185. [Abstract] [Google Scholar]
- Kalil RE, Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol. 1970 May;33(3):459–474. [Abstract] [Google Scholar]
- Kultas-Ilinsky K, Ilinsky IA, Young PA, Smith KR. Ultrastructure of degenerating cerebellothalamic terminals in the ventral medial nucleus of the cat. Exp Brain Res. 1980 Jan;38(2):125–135. [Abstract] [Google Scholar]
- Majorossy K, Kiss A. Specific patterns of neuron arrangement and of synaptic articulation in the medial geniculate body. Exp Brain Res. 1976 Aug 27;26(1):1–17. [Abstract] [Google Scholar]
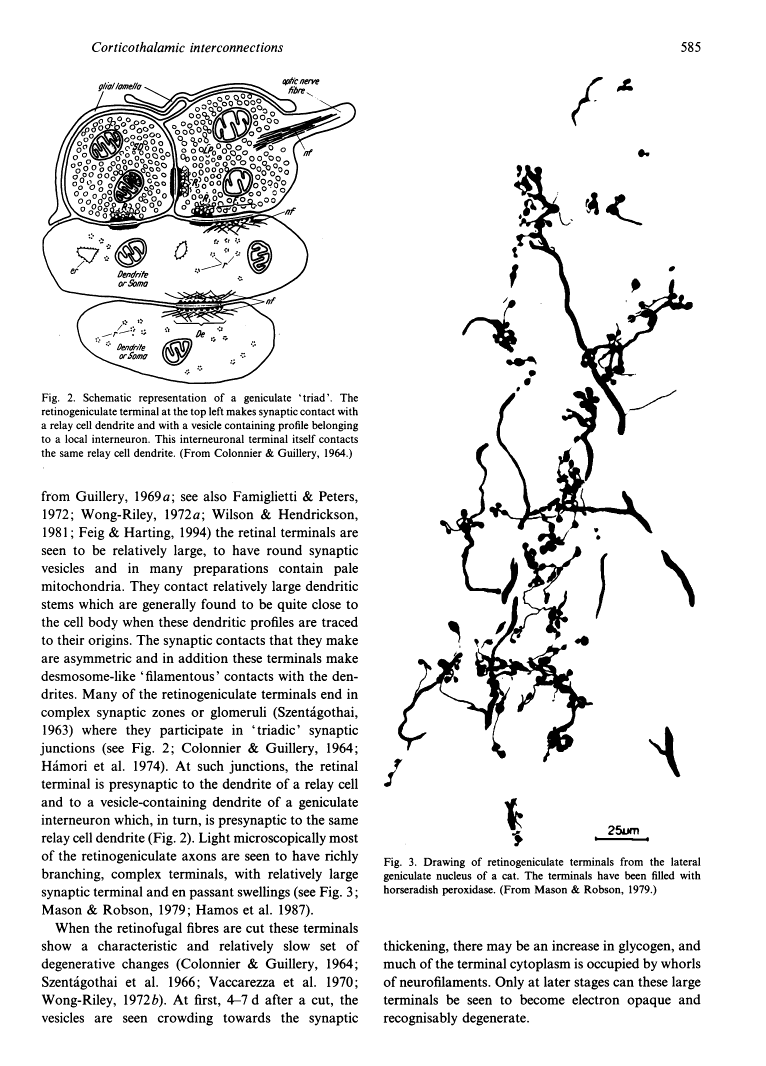
- Mason CA, Robson JA. Morphology of retino-geniculate axons in the cat. Neuroscience. 1979;4(1):79–97. [Abstract] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. [Abstract] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate "metabotropic" receptors. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2774–2778. [Europe PMC free article] [Abstract] [Google Scholar]
- Mathers LH. Ultrastructure of the pulvinar of the squirrel monkey. J Comp Neurol. 1972 Sep;146(1):15–42. [Abstract] [Google Scholar]
- Mathers LH. The synaptic organization of the cortical projection to the pulvinar of the squirrel monkey. J Comp Neurol. 1972 Sep;146(1):43–60. [Abstract] [Google Scholar]
- Montero VM, Guillery RW, Woolsey CN. Retinotopic organization within the thalamic reticular nucleus demonstrated by a double label autoradiographic technique. Brain Res. 1977 Dec 23;138(3):407–421. [Abstract] [Google Scholar]
- Ogren MP, Hendrickson AE. The morphology and distribution of striate cortex terminals in the inferior and lateral subdivisions of the Macaca monkey pulvinar. J Comp Neurol. 1979 Nov 1;188(1):179–199. [Abstract] [Google Scholar]
- Ralston HJ., 3rd The synaptic organization of lemniscal projections to the ventrobasal thalamus of the cat. Brain Res. 1969 Jun;14(1):99–115. [Abstract] [Google Scholar]
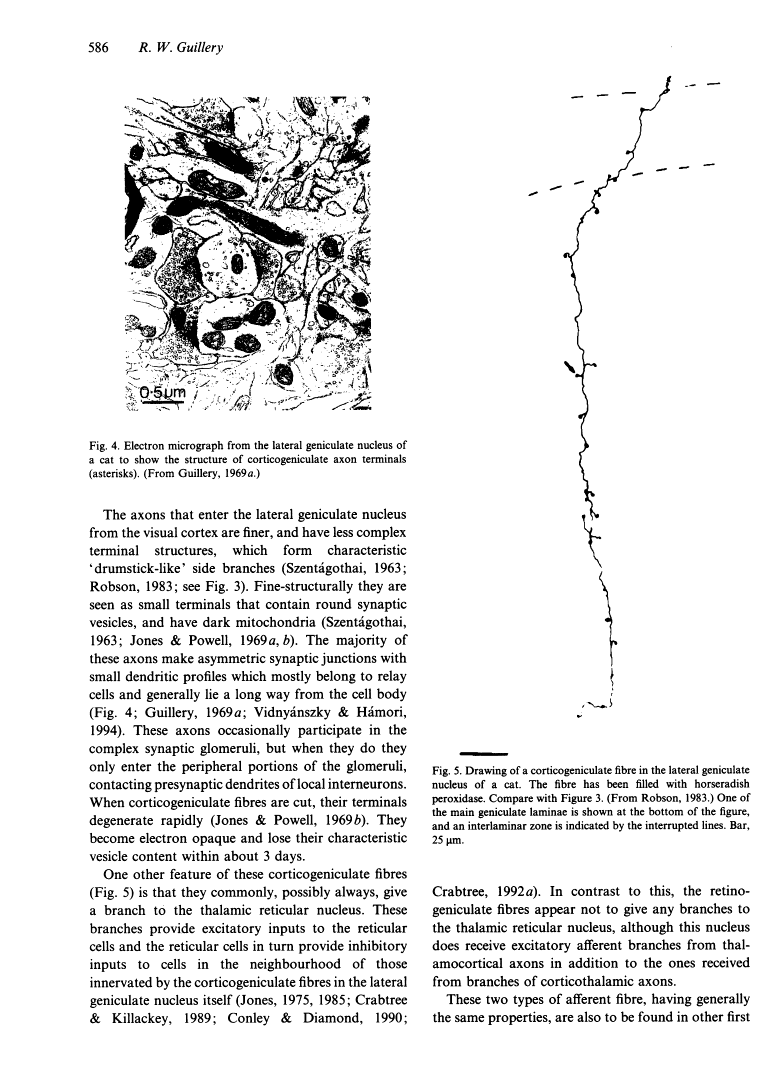
- Robson JA. The morphology of corticofugal axons to the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1983 May 1;216(1):89–103. [Abstract] [Google Scholar]
- Robson JA, Hall WC. The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). II. Synaptic organization and comparisons with the dorsal lateral geniculate nucleus. J Comp Neurol. 1977 May 15;173(2):389–416. [Abstract] [Google Scholar]
- Salin PA, Bullier J. Corticocortical connections in the visual system: structure and function. Physiol Rev. 1995 Jan;75(1):107–154. [Abstract] [Google Scholar]
- Scannell JW, Blakemore C, Young MP. Analysis of connectivity in the cat cerebral cortex. J Neurosci. 1995 Feb;15(2):1463–1483. [Abstract] [Google Scholar]
- Schwartz ML, Dekker JJ, Goldman-Rakic PS. Dual mode of corticothalamic synaptic termination in the mediodorsal nucleus of the rhesus monkey. J Comp Neurol. 1991 Jul 15;309(3):289–304. [Abstract] [Google Scholar]
- Sillito AM, Jones HE, Gerstein GL, West DC. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994 Jun 9;369(6480):479–482. [Abstract] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. [Abstract] [Google Scholar]
- Singer W. Neurobiology. A new job for the thalamus. Nature. 1994 Jun 9;369(6480):444–445. [Abstract] [Google Scholar]
- Somogyi G, Hajdu F, Tömböl T. Ultrastructure of the anterior ventral and anterior medial nuclei of the cat thalamus. Exp Brain Res. 1978 Mar 15;31(3):417–431. [Abstract] [Google Scholar]
- SZENTAGOTHAI J. THE STRUCTURE OF THE SYNAPSE IN THE LATERAL GENICULATE BODY. Acta Anat (Basel) 1963;55:166–185. [Abstract] [Google Scholar]
- Szentágothai J, Hámori J, Tömböl T. Degeneration and electron microscope analysis of the synaptic glomeruli in the lateral geniculate body. Exp Brain Res. 1966;2(4):283–301. [Abstract] [Google Scholar]
- Vaccarezza OL, Reader TA, Pasqualini E, Pecci-Saavedra J. Temporal course of synaptic degeneration in the lateral geniculate nucleus. Its dependence on axonal stump length. Exp Neurol. 1970 Aug;28(2):277–285. [Abstract] [Google Scholar]
- Vidnyánszky Z, Hámori J. Quantitative electron microscopic analysis of synaptic input from cortical areas 17 and 18 to the dorsal lateral geniculate nucleus in cats. J Comp Neurol. 1994 Nov 8;349(2):259–268. [Abstract] [Google Scholar]
- Wilson JR, Hendrickson AE. Neuronal and synaptic structure of the dorsal lateral geniculate nucleus in normal and monocularly deprived Macaca monkeys. J Comp Neurol. 1981 Apr 10;197(3):517–539. [Abstract] [Google Scholar]
- Wong-Riley MT. Neuronal and synaptic organization of the normal dorsal lateral geniculate nucleus of the squirrel monkey, Saimiri sciureus. J Comp Neurol. 1972 Jan;144(1):25–59. [Abstract] [Google Scholar]
- Wong-Riley MT. Terminal degeneration and glial reactions in the lateral geniculate nucleus of the squirrel monkey after eye removal. J Comp Neurol. 1972 Jan;144(1):61–91. [Abstract] [Google Scholar]
- Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988 Sep 22;335(6188):311–317. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Anatomy are provided here courtesy of Anatomical Society of Great Britain and Ireland
Citations & impact
Impact metrics
Citations of article over time
Article citations
Thalamo-cortical neural mechanism of sodium salicylate-induced hyperacusis and anxiety-like behaviors.
Commun Biol, 7(1):1346, 18 Oct 2024
Cited by: 0 articles | PMID: 39420035 | PMCID: PMC11487285
Convergent direct and indirect cortical streams shape avoidance decisions in mice via the midline thalamus.
Nat Commun, 15(1):6598, 04 Aug 2024
Cited by: 0 articles | PMID: 39097600 | PMCID: PMC11297946
A Reconsideration of the Core and Matrix Classification of Thalamocortical Projections.
J Neurosci, 44(24):e0163242024, 12 Jun 2024
Cited by: 2 articles | PMID: 38866538
Review
Involvement of the claustrum in the cortico-basal ganglia circuitry: connectional study in the non-human primate.
Brain Struct Funct, 229(5):1143-1164, 14 Apr 2024
Cited by: 0 articles | PMID: 38615290 | PMCID: PMC11147942
Mediodorsal thalamus projection to medial prefrontal cortical mediates social defeat stress-induced depression-like behaviors.
Neuropsychopharmacology, 49(8):1318-1329, 04 Mar 2024
Cited by: 2 articles | PMID: 38438592
Go to all (195) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system.
Neuron, 33(2):163-175, 01 Jan 2002
Cited by: 388 articles | PMID: 11804565
Review
Functional anatomy of thalamus and basal ganglia.
Childs Nerv Syst, 18(8):386-404, 26 Jul 2002
Cited by: 337 articles | PMID: 12192499
Review
Synchrony in the interconnected circuitry of the thalamus and cerebral cortex.
Ann N Y Acad Sci, 1157:10-23, 01 Mar 2009
Cited by: 135 articles | PMID: 19351352
Review
Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey.
J Comp Neurol, 344(1):1-19, 01 Jun 1994
Cited by: 186 articles | PMID: 7914894