Abstract
Free full text

Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays
Abstract
Nephrophilic autoantibodies dominate the seroprofile in lupus, but their fine specificities remain ill defined. We constructed a multiplexed proteome microarray bearing about 30 antigens known to be expressed in the glomerular milieu and used it to study serum autoantibodies in lupus. Compared with normal serum, serum from B6.Sle1.lpr lupus mice (C57BL/6 mice homozygous for the NZM2410/NZW allele of Sle1 as well as the FASlpr defect) exhibited high levels of IgG and IgM antiglomerular as well as anti–double-stranded DNA/chromatin Abs and variable levels of Abs to α-actinin, aggrecan, collagen, entactin, fibrinogen, hemocyanin, heparan sulphate, laminin, myosin, proteoglycans, and histones. The use of these glomerular proteome arrays also revealed 5 distinct clusters of IgG autoreactivity in the sera of lupus patients. Whereas 2 of these IgG reactivity clusters (DNA/chromatin/glomeruli and laminin/myosin/Matrigel/vimentin/heparan sulphate) showed association with disease activity, the other 3 reactivity clusters (histones, vitronectin/collagen/chondroitin sulphate, and entactin/fibrinogen/hyaluronic acid) did not. Human lupus sera also displayed 2 distinct IgM autoantibody clusters, one reactive to DNA and the other apparently polyreactive. Interestingly, the presence of IgM polyreactivity in patient sera was associated with reduced disease severity. Hence, the glomerular proteome array promises to be a powerful analytical tool for uncovering novel autoantibody disease associations and for distinguishing patients at high risk for end-organ disease.
Introduction
Renal disease is a leading cause of mortality in murine and human lupus, and autoantibodies constitute important contributors to renal damage in this disease (1). In particular, anti-DNA and glomerulophilic Abs have been accorded a pathogenic role in this disease (2–8). Adoptive transfer studies of purified mAbs and hybridomas have revealed that whereas anti-histone and anti-nucleosome Abs may not be pathogenic, anti–double-stranded DNA (anti-dsDNA) and antiglomerular Abs may be pathogenic (9–12). In particular, correlative evidence and adoptive transfer studies have appended a greater degree of pathogenic potential to antiglomerular autoantibodies than to nonglomerular binding anti-DNA Abs (3, 10–12). Using conventional immunoassays, several investigators have highlighted the potential importance of Abs specific for various glomerular or basement membrane antigens (Ags) as being the targets for such antiglomerular, or “nephrophilic,” autoantibodies. These Ags include laminin, various types of proteoglycans, heparin, collagen, α-actinin, etc. (13–28). In some instances, these antiglomerular reactivities have been demonstrated to be dependent upon nuclear Ag bridges, whereas in other studies, the nephrophilic Abs have been shown to be anti-DNA Abs with direct crossreactive potential to glomerular Ags (3, 9, 22–31). In addition, antiglomerular Abs that were not DNA reactive have also been documented in murine and human lupus nephritis (32, 33).
In contrast to the above studies, which focus on a couple of selected glomerular target Ag specificities, the recent advent of large-scale immunoproteomic approaches has allowed the screening of disease sera in a more comprehensive manner, against a vast array of potential target Ags (34–39). In particular, Robinson et al. have fabricated autoantigen microarrays, in which a large spectrum of autoantigens was spotted onto glass slides (40–42). Sera can then be added to these slides and developed with fluorescent-labeled secondary Abs to uncover the spectrum of antigenic specificities targeted in different autoimmune disease states. The purpose of the present study was to adopt a similar multiplexed approach to define the spectrum of autoantibodies reactive with different glomerular or glomerular basement membrane (GBM) Ags, which best correlate with disease activity and may be present in lupus. To this end, a panel of Ags documented to be present in the glomeruli/GBM (43–45) was spotted onto specially precoated glass slides; these arrays have been termed glomerular proteome arrays. These novel proteome microarrays were then used to analyze the nephrophilic autoantibody profiles in murine and human lupus.
Interestingly, lupus mice were noted to harbor a wide spectrum of antibodies, including autoantibodies to α-actinin, aggrecan, collagen, entactin, fibrinogen, hemocyanin, heparan sulphate, laminin, myosin, proteoglycans, DNA, and histones. In addition, the use of these arrays has also helped to uncover 2 nonoverlapping IgG autoantibody clusters that distinguish lupus patients with more severe disease activity.
Results
First, a panel of glomerular/GBM Ags as well as control nuclear Ags was assembled as shown on the array map portrayed in Figure Figure1A.1A. The initial studies were focused on selecting the optimal slide chemistry for Ag spotting. The panel of Ags assembled did not bind well to untreated glass slides, but adhered fairly well to poly-L-lysine, superaldehyde, and HydroGel coated slides. Among the different slide surface chemistries tested, HydroGel coated slides yielded the best results in terms of the evenness and consistency of the spot dimensions as well as the signal-to-noise ratios, as illustrated in Supplemental Figure 1A (supplemental material available online with this article; 10.1172/JCI23587DS1). We also verified that whereas the omission of a blocking step resulted in high-fluorescence background, preincubating the slides in PBS, 0.1% Tween 20, and 0.5% BSA yielded optimal blocking, as depicted in Supplemental Figure 1B. Next, each Ag was spotted at 4 serial dilutions (from 0.025 to 1 μg/ml) to determine the optimal coating concentration for each Ag, as exemplified in Supplemental Figure 1C. For almost all Ags, the optimal signal-to-noise ratios were observed when 1 μg/ml was used for coating. An exception was cardiolipin, for which the optimal coating concentration was noted to be 0.1 μg/ml. Hence, all further studies were performed using HydroGel slides coated with the different Ags at the optimal coating concentrations.
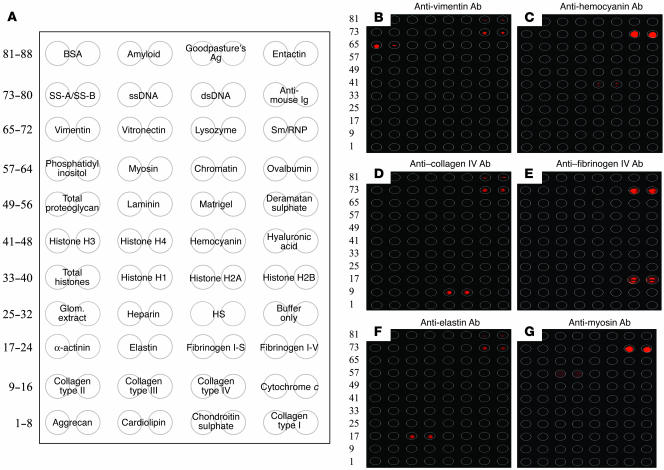
Target Ags and specificity profiles of glomerular proteome arrays. (A) HydroGel slides were coated with different glomerular/GBM and nuclear Ags in duplicate as shown. Deramatan sulphate, deramatan sulphate proteoglycan; glom. extract, glomerular extract; HS, heparan sulphate. (B–G) Six commercially available mAbs specific for vimentin (B), hemocyanin (C), collagen IV (D), fibrinogen IV (E), elastin (F), and myosin (G) were added to 6 separate glomerular proteome arrays and developed using Cy5-labeled goat anti-mouse IgG/IgM in order to gauge the specificity of the Ag/Ab interactions on the glomerular proteome arrays. The Ags in B–G were arrayed as shown in A.
Next, we titrated the test sera and commercially available mAbs of defined specificities to ascertain the dynamic performance range of the arrays and their sensitivities. The titration curve for the anti-elastin mAb is depicted in Supplemental Figure 2A and is compared with the corresponding ELISA readings observed at the same dilutions (Supplemental Figure 2B). It was clear that diluting the mAb more than 25,000-fold yielded an array signal that was still significantly above the background; in contrast, diluting the mAb beyond 3,000-fold dropped the OD values to the ELISA background levels. Similar titration curves were derived using 5 additional mouse mAbs as well as lupus sera. Whereas the arrays performed significantly better than the conventional ELISA approach in the case of the anti-elastin and anti-myosin Abs (in being able to detect reactivity at far lower Ab dilutions), the anti-vimentin, anti–collagen IV, anti-hemocyanin, and anti–fibrinogen IV mAbs performed similarly in both the ELISA and array methodologies (data not shown). Likewise, as portrayed in Supplemental Figure 2C, serum from B6.Sle1.lpr lupus mice (C57BL/6 mice homozygous for the NZM2410/NZW allele of Sle1 as well as the FASlpr defect) showed significant IgG and IgM reactivity to dsDNA, chromatin, and several glomerular Ags, even when diluted beyond 25,000-fold. Hence, the glomerular proteome array appears to be particularly sensitive, with a significant dynamic range, yielding a fairly linear readout spanning 4 logs of fluorescence intensity and corresponding to 4–5 logs of Ab dilution.
The specificity of the assay was next gauged using commercially available mAbs. Figure Figure1,1, B–G, depicts the Ag specificity profiles of 6 commercially available mouse mAbs, with specificities for vimentin, hemocyanin, collagen IV, fibrinogen IV, myosin, and elastin. All 6 Abs reacted specifically with their target Ags but not with the other Ags on the arrays, with 1 exception. Interestingly, some of the tested Abs demonstrated a low degree of binding to the entactin preparation, perhaps reflecting the presence of some contaminating proteins in the entactin preparation. Reactivity to the mouse Ig spotted on all arrays served as a positive control and also allowed for interslide normalization.
Having optimized the slide precoating chemistry, Ag coating concentrations, Ab/serum dilutions to use, and the specificity and sensitivity profiles of the array, sera from a healthy mouse strain and sera drawn from a lupus-afflicted strain were compared with respect to their reactivity profiles to the different glomerular Ags. For this purpose, we selected C57BL/6 (B6) mice as the negative control and B6.Sle1.lpr as the disease strain, since they both share the same genetic background but differ vastly in their clinical phenotypes. Importantly, B6.Sle1.lpr mice are known to exhibit highly penetrant lupus nephritis, accompanied by high titers of anti-DNA and antiglomerular Abs (46). With both sets of sera, negligible reactivity was observed against the control Ags ovalbumin and lysozyme as well as against the spots that were coated with no Ags, which typically yielded 10–60 normalized fluorescence intensity units (nfi). In contrast, the lupus sera, but not the control sera, reacted strongly to several glomerular and nuclear Ags, as illustrated in Figure Figure2A;2A; indeed, the sera from these 2 strains clustered apart from each other almost perfectly, as illustrated by the heat map in Figure Figure22B.
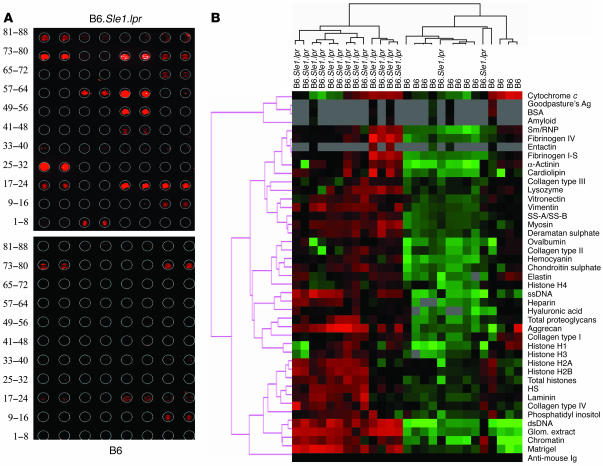
The use of glomerular proteome arrays to uncover autoantibodies in murine lupus sera. Dilutions (1:200) of various sera were applied to HydroGel slides coated with different glomerular/GBM and nuclear Ags as shown in Figure Figure1A.1A. (A) Representative glomerular proteome arrays hybridized with B6 (bottom) or B6.Sle1.lpr sera (top) and developed with Cy5-coupled anti-mouse IgG. In these arrays, the intensity of the fluorescence signal ranged from none (black) to high (red), as scanned at 635 nM. (B) A total of 12 B6 sera and 15 B6.Sle1.lpr sera (10 females, 5 males) were studied similarly, and the data summarized in a heat map which shows the relative IgG seroreactivities of each of these 27 serum samples to the respective Ags on the arrays. For all Ags, the reactivity intensities are depicted on a relative scale, where reactivities above the array mean are colored red, reactivities below are colored green, and reactivities close to the mean are colored black. In addition, a clustering algorithm was used to group together sera that exhibited similar reactivity patterns (dendrogram at top) and to cluster together Ags that were similarly targeted by the different test sera (dendrogram at left). Data in B are representative of at least 3 independent experiments (using the same sera, but independent arrays).
Among the B6.Sle1.lpr lupus sera, the strongest autoreactivity was noted against dsDNA, chromatin, total glomerular sonicate, and the Matrigel mix of basement membrane Ags; these can be seen tightly clustered toward the bottom of the heat map in Figure Figure2B.2B. In particular, the reactivities to dsDNA, chromatin, and total glomerular sonicate ranged from 10,000–30,000 nfi in female B6.Sle1.lpr lupus mice (Figure (Figure3A).3A). Weaker but significant reactivity (>1,000 nfi) was also noted against fibrinogen, cardiolipin, aggrecan, and SS-A/SS-B (also known as Ro/La Ags) in lupus, but not control, sera (Figure (Figure3A).3A). Compared with the B6 sera, the lupus sera also exhibited stronger reactivities against myosin, α-actinin, collagen IV, hemocyanin, and heparan sulphate as well as the nuclear Ags, single-stranded DNA (ssDNA), and histones, though these signals were somewhat weaker (100–1,000 nfi; Figure Figure3A).3A). Reactivity to most of the other glomerular Ags examined (e.g., the other collagens, hyaluronic acid, etc.) was either absent or barely above the background. Interestingly, most of the observed seroreactivities were significantly higher in B6.Sle1.lpr females compared with males (Figure (Figure3A),3A), as has been noted previously (46).
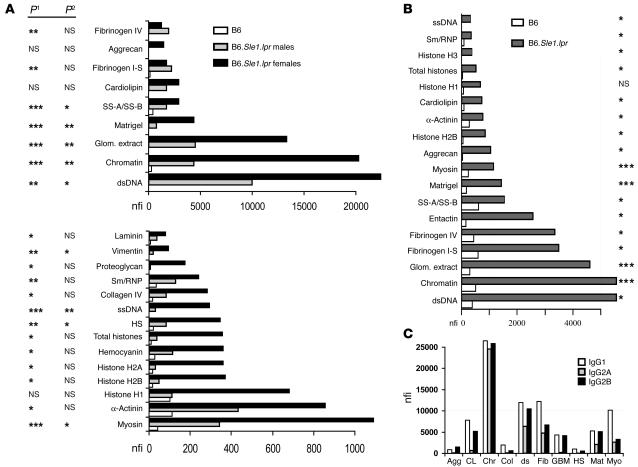
The strongest IgG and IgM antiglomerular reactivities in B6.Sle1.lpr lupus sera. (A) IgG seroreactivities to various glomerular and nuclear Ags assayed in B6 (n = 12) and B6.Sle1.lpr mice (n = 15, 10 females and 5 males) are partitioned according to whether the observed reactivities in the lupus sera were stronger than 1,000 nfi (top) or 100–1,000 nfi (bottom). Among the B6 sera, there were no significant differences between genders; therefore data from B6 males and females have been pooled. P values at left compare B6.Sle1.lpr with the corresponding B6 values (P1) and differences between gender in B6.Sle1.lpr sera (P2). *P < 0.05; **P < 0.01; ***P < 0.001. Note that the reactivity levels of B6.Sle1.lpr female sera to chromatin and dsDNA exceeded 20,000 nfi. (B) The strongest IgM seroreactivities (>300 nfi) noted in 15 B6.Sle1.lpr sera (10 females, 5 males) using glomerular proteome arrays are compared with the corresponding B6 levels (n = 12). P values at right compare the 2 strains. (C) Some of the highest fluorescence reactivities observed in B6.Sle1.lpr sera, categorized according to their IgG subclass. In similar assays, the reactivities observed in B6 control sera ranged from 20–100 nfi (data not plotted). Agg, aggrecan; CL, cardiolipin; Chr, chromatin; Col, collagen type IV; ds, dsDNA; Fib, fibrinogen IV; GBM, total glomerular lysate; Mat, Matrigel; Myo, myosin.
The presence of serum IgM autoantibodies was also examined. As depicted in Figure Figure3B,3B, the strongest reactivity was again noted against dsDNA, chromatin, and total glomerular sonicate in lupus sera but not control sera. Weaker but significant levels of IgM autoantibodies were also noted against the same subset of Ags targeted by the IgG Abs, including α-actinin, aggrecan, cardiolipin, fibrinogen, and myosin. Unlike the IgG autoantibodies, no difference was noted in IgM autoantibody levels between the genders in B6.Sle1.lpr sera except in the levels of various anti-histone specificities (data not shown).
The utility of the glomerular proteome array was extended to study the isotype subclasses of antiglomerular Abs. The simultaneous use of cyanine 3–coupled (Cy3-coupled) anti-mouse IgG1 and Cy5-coupled anti-mouse IgG2A (or anti-mouse IgG2B) to develop the slides allowed us to quantitate the relative amounts of glomerular Ag-specific Abs of different isotypes in any given serum sample. As depicted in Figure Figure3C,3C, B6.Sle1.lpr sera exhibited high levels of IgG1, IgG2B, and IgG2A Abs to various targets, including dsDNA and chromatin. Consistent with the data shown in Figure Figure3A,3A, B6 sera showed negligible levels of Abs to these Ags (data not shown).
The above findings indicate that lupus sera harbor IgM and IgG reactivities to several nuclear as well as GBM Ag preparations. The fact that the total glomerular sonicate and the Matrigel preparations represented relatively crude Ag mixes, as well as the observation that the reactivities to these complex antigenic mixes were closely paralleled by their respective strengths of reactivity to chromatin/DNA (Figure (Figure3),3), triggered us to examine this further. Indeed, when the reactivity profile of each individual control as well as lupus mouse serum was analyzed, a strong correlation was observed between the antinuclear reactivity and the antiglomerular reactivity in both the IgG and the IgM Abs (Figure (Figure4,4, A and B). We next examined whether the observed reactivity profiles to the glomerular Ags were mediated at least in part by residual, or contaminating, nuclear material in the glomerular/Matrigel preparations or within the Ag-binding pockets of the serum Abs. To directly test this, the immunoproteome assays were repeated after DNAse-I pretreatment of the glomerular proteome array slides alone, the lupus sera alone, or both components.
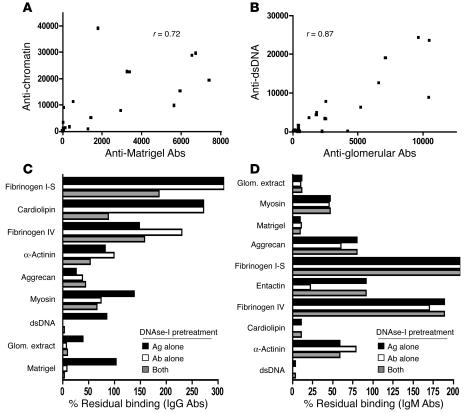
DNA dependence of glomerular-reactive autoantibodies in lupus. For all the B6 and B6.Sle1.lpr mouse sera studied (total, n = 27; A and B), the reactivity to DNA/chromatin was compared to the reactivities to total glomerular lysate or Matrigel, within the same serum samples. Shown are the scatter plots (and correlation coefficients) relating mouse IgG reactivities against chromatin versus Matrigel (A) and mouse IgM reactivity against dsDNA versus glomerular extract (B). (C and D) Mean remnant IgG (C) and IgM (D) seroreactivities to dsDNA or to the different glomerular Ags following DNAse-I pretreatment of the glomerular proteome array slides, the test sera alone, or both, expressed as a percentage of the fluorescence intensities recorded in sham-treated controls, arbitrarily set at 100%. Each bar represents the mean value derived from 3 individual B6.Sle1.lpr serum samples that had expressed high reactivity to the depicted glomerular targets. All IgG seroreactivities remaining after both the sera and the Ag arrays were DNAse-I treated (C) were significantly less than the sham-treated controls (P < 0.05 for fibrinogen IV; P < 0.01 for myosin; and P < 0.001 for all the other Ags), with the exception of α-actinin (P > 0.05). Likewise, all IgM seroreactivities remaining after both the sera and the Ag arrays were DNAse-I treated (D) were significantly less than the sham-treated controls (P < 0.01 for aggrecan and P < 0.001 for the other Ags), with the exceptions of fibrinogen IV and α-actinin (P > 0.05).
DNAse-I pretreatment of the lupus sera reduced IgG and IgM binding to the glomerular lysates and Matrigel by >80% (Figure (Figure4,4, C and D), indicating that the DNA dependence of the glomerular reactivity was largely due to DNA-bearing Ags bound within the serum Abs. Although DNAse-I pretreatment abrogated antiglomerular reactivity, it was interesting to observe that the reactivity to certain Ags, such as fibrinogen, was unaltered or even increased following DNAse-I pretreatment. On the other hand, IgG reactivity to α-actinin, myosin, and aggrecan were partially impaired by the DNAse-I pretreatment of the sera and slides (Figure (Figure4C),4C), indicating that not all target Ag specificities are absolutely DNA dependent. An almost identical pattern was observed when the DNA dependence of IgM autoantibodies was examined — whereas reactivity to glomerular sonicates and Matrigel were almost obliterated and reactivity to fibrinogen was paradoxically elevated, the reactivity to α-actinin, myosin, and aggrecan were partially impaired (Figure (Figure44D).
The usefulness of the arrays in studying patient samples was next evaluated. Figure Figure55 shows the IgG seroreactivities to the different glomerular and nuclear Ags noted in normal human sera (n = 11), sera from lupus patients with varying degrees of disease activity (n = 37; Table Table1),1), and sera from 5 RA controls. It was evident from the heat map in Figure Figure55 that the sera from lupus patients exhibited significantly higher levels of IgG Abs to several different nuclear and glomerular Ags compared with sera from RA patients and healthy adults. Even more striking was the uncovering of 5 or more Ag reactivity clusters, shown along the left margin in Figure Figure5.5. To confirm the clustering tree automatically generated by the Cluster/TreeView algorithm (http://rana.lbl.gov/EisenSoftware.htm) in Figure Figure5,5, pairwise correlations were next performed for the different Ags tested using Excel software (Microsoft Office 2003; Microsoft). As denoted in Figure Figure6,6, A–E, 5 distinct IgG autoantibody clusters surfaced, marked by a high degree of correlation between the Ab specificities within each cluster (with r typically exceeding 0.6 between any 2 given specificities; Figure Figure6,6, A–E), and low correlation (r < 0.2) among Ab specificities drawn from different clusters.
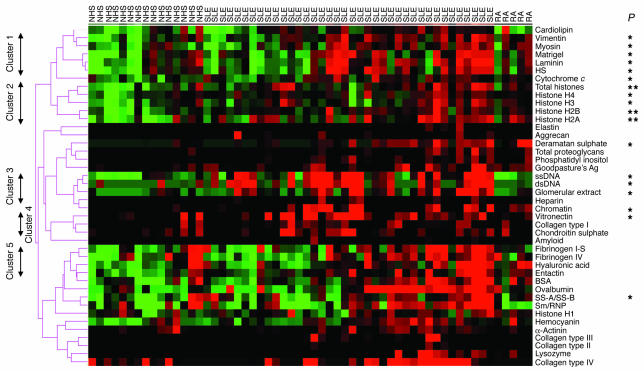
The strongest IgG antiglomerular reactivities in human lupus sera. Sera from 11 healthy adults (NHS), 37 lupus patients (SLE) with varying degrees of disease (see Table Table1),1), and 5 RA patients were applied to the glomerular proteome arrays as shown in Figure Figure1A1A and developed using Cy5-labeled anti-human IgG. The relative fluorescence intensities for each Ag are depicted using a green/black/red heat map and clustered Ag-wise as described in the legend to Figure Figure2B.2B. Indicated on the left margin are 5 distinct groups of Ags, the reactivities to which were noted to cluster together in the tested samples. Depicted results are representative of 2 independent experiments using the same sera but fresh arrays. P values indicated at right were the result of comparing the lupus sera against the normal controls. An additional SLE column has been included which shows results from 1 duplicated sample. *P < 0.05; **P < 0.01.
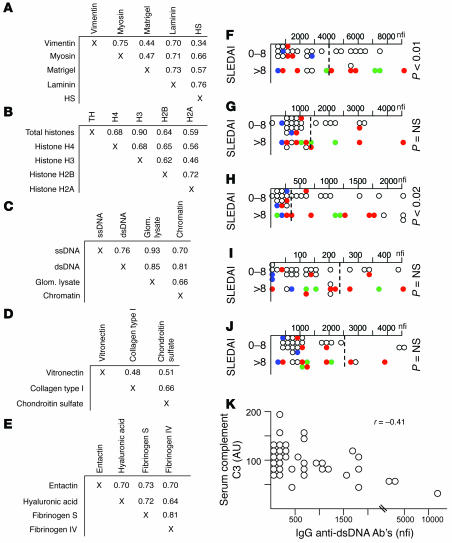
Five distinct clusters of IgG autoreactivity in lupus sera. (A–E) The autoantigen seroreactivities that apparently clustered together in Figure Figure55 were reassessed for correlation in a pairwise fashion. Indicated within each matrix are the corresponding correlation coefficients when seroreactivity to the different Ags were compared for concordance. For array Ags not listed in A, the seroreactivity correlation coefficients between any 2 Ags were r < 0.2, with the exception of concordance between SS-A/SS-B and Sm/RNP seroreactivity. (F–J) Seroreactivity levels noted in lupus sera (n = 37) against the 5 clusters of targeted Ags, parsed according to their total SLEDAI scores. When available, glomerular pathology class was indicated (red, grade IV GN; green, grade II/III GN; blue, grade V GN; white, no biopsy done). (F) For cluster 1 Ags, reactivity to laminin is plotted as the cluster’s representative. (G) For cluster 2 Ags, reactivity to total histone is plotted as the cluster’s representative. (H) For cluster 3 Ags, reactivity to dsDNA is plotted as the cluster’s representative. (I) For cluster 4 Ags, reactivity to chondroitin sulphate is plotted as the cluster’s representative. (J) For cluster 5 Ags, reactivity to fibrinogen IV is plotted as the cluster’s representative. The dotted line within each plot pertains to the cutoff for normality, representing mean ± 2 SD noted in the 11 normal control sera studied. (K) Scatter-plotted serum concentrations of complement C3 (y axis) versus IgG anti-dsDNA Ab levels (representative of cluster 3 seroreactivity; x axis).
Table 1
Human subjects studied using glomerular proteome arrays
Reactivity to vimentin, myosin, Matrigel, laminin, and heparan sulphate clustered together (Figure (Figure6A),6A), whereas reactivity to the different core histones (but not histone H1) clustered separately (Figure (Figure6B).6B). Reactivity to DNA-bearing Ags (ssDNA, dsDNA, and chromatin), as well as to total glomerular lysate, clustered together (Figure (Figure6C).6C). Reactivity to chondroitin sulphate, collagen I, and vitronectin clustered somewhat weakly (Figure (Figure6D),6D), whereas reactivity to entactin, hyaluronic acid, and the fibrinogens clustered together strongly (Figure (Figure6E).6E). In contrast to these clusters, reactivity to the other Ags studied displayed unique distribution profiles, with the exception of SS-A/SS-B and Smith Ag/ribonucleoprotein (Sm/RNP), which displayed concordant seroreactivity profiles in the study subjects as shown in Figure Figure55.
We next asked whether the above reactivity clusters were useful in distinguishing lupus patients with differing disease activity. Interestingly, cluster 1 reactivity (IgG anti-laminin, anti-myosin, etc.) and cluster 3 reactivity (anti-DNA, antiglomerular Abs) were significantly higher in patients with higher total systemic lupus erythematosus (SLE) disease activity scores (SLEDAI scores; Figure Figure6,6, F and H) as well as higher renal SLEDAI scores (data not shown). In contrast, cluster 2, cluster 4, and cluster 5 specificities failed to distinguish patients with low SLEDAI scores from those with high disease activity (Figure (Figure6,6, G, I, and J). When cluster 1 and cluster 3 specificities were examined further, it was interesting to note that a substantial fraction of patients with high IgG autoantibody levels had grade III or IV glomerulonephritis (GN), but not grade V GN (Figure (Figure6,6, F and H, and Table Table1).1). Interestingly, of the 3 patients with grade IV GN (based on past biopsy reports) but low total SLEDAI scores, 2 were free of active renal disease; therefore, the levels of cluster 1 and cluster 3 seroreactivities appeared to correlate better with concurrent renal disease/flares rather than past history of pathology. When the absolute SLEDAI scores (or any of the other disease parameters listed in Table Table1)1) were used as continuous variables to ascertain correlation with any of the Abs assayed, only the cluster 3 specificities (e.g., anti-dsDNA, antiglomerular Abs, etc.) showed a positive correlation with total SLEDAI score (r = 0.34; data not plotted) and a negative correlation with serum C3 (r = –0.41 for IgG anti-dsDNA; Figure Figure6K).6K). Cluster 1 specificities, as well as the other specificities assayed on the arrays, did not correlate well with the absolute SLEDAI scores, proteinuria, or hypocomplementemia (data not shown).
Figure Figure77 shows the IgM seroreactivities to the different glomerular Ags in the same group of lupus patients and controls. In general, when the Ag reactivity profiles of all 53 study subjects were considered together, the IgM reactivity profiles to the different Ags demonstrated a fairly good correlation with their respective IgG reactivities. However, this was not because of any crossreactivity of the anti-IgM detection Ab (see Methods) or because of rheumatoid factor, which was verified to be absent in most of the study subjects. Interestingly, however, in contrast to the IgG seroreactivities portrayed in Figure Figure5,5, the IgM reactivity displayed 2 broad clusters. On the one hand, IgM reactivity to ssDNA, dsDNA, and chromatin were strongly clustered together (Figure (Figure7A).7A). In contrast, about 60% of the Ags tested clustered together broadly (Figure (Figure7A,7A, arrow); this broad cluster included anti-histone Abs and reactivity to several control Ags such as lysozyme, BSA, and ovalbumin, indicating that these are likely to represent IgM polyreactive Abs. Interestingly, 3–4 of the RA patients also exhibited these polyreactive Abs in the absence of anti-DNA Abs (Figure (Figure7A).7A). Besides these 2 broad clusters, the other specificities in between were only weakly clustered together (Figure (Figure77A).
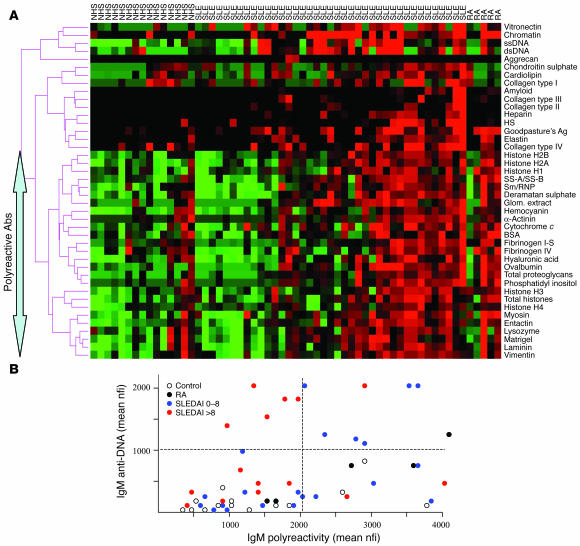
The strongest IgM antiglomerular reactivities in human lupus sera. (A) Sera from 11 healthy adults, 37 lupus patients with varying degrees of disease (see Table Table1),1), and 5 RA patients were applied to the glomerular proteome arrays as shown in Figure Figure1A1A and developed using Cy3-labeled anti-human IgM. The relative fluorescence intensities for each Ag are depicted using a green/black/red heat map and clustered Ag-wise as described in the legend to Figure Figure2B.2B. Indicated on the left are a panel of Ags that clustered together, most likely serving as targets for polyreactive Abs. Depicted results are representative of 2 independent experiments using the same sera but fresh arrays. Two additional SLE columns have been included which show results from 2 duplicated samples. (B) For each normal control (n = 11; white dots), RA control (n = 5; black dots), and lupus patient (n = 37; blue dots, total SLEDAI score 0–8; red dots, total SLEDAI score >8), the mean serum IgM anti-DNA reactivity (y axis) was derived by averaging the observed reactivity to ssDNA, dsDNA, and chromatin on the arrays and scatter plotted against the average extent of serum IgM polyreactivity (x axis) to the bottom-most 26 array Ags clustered together in the heat map shown in A. The dotted lines were arbitrarily set to distinguish patients with high IgM anti-DNA Abs and/or high IgM polyreactivity in their sera.
We next asked how the degree of IgM anti-DNA reactivity and/or the extent of IgM polyreactivity correlated with disease. The former was represented by the average nfi for anti-ssDNA, anti-dsDNA, and anti-chromatin assayed for each sample, whereas the latter was represented by the mean nfi derived by averaging the IgM reactivity to the 26-Ag cluster described above (Figure (Figure7A).7A). Interestingly, whereas the healthy controls had low to modest levels of anti-DNA and polyreactive IgM Abs, RA patients had significantly higher levels of IgM polyreactive Abs (Figure (Figure7B,7B, black dots). Among SLE patients, it was intriguing to observe that patients with low disease activity (Figure (Figure7B,7B, blue dots) tended to have higher levels of IgM polyreactive Abs than patients with more severe active disease (Figure (Figure7B,7B, red dots), who tended to have higher levels of IgM anti-DNA Abs. Viewed from a different perspective, among SLE patients who possessed high serum IgM anti-DNA Abs, the co-presence of IgM polyreactive Abs in their serum (Figure (Figure7B,7B, upper right quadrant) was associated with lower disease activity.
Discussion
This work contributes several novel perspectives to our understanding of antiglomerular Ab specificities in lupus. First and foremost, it allows for an unbiased comparison of different antiglomerular and antinuclear fine specificities in lupus nephritis in parallel. Since all specificities were assayed using the same conditions on the same slides and normalized in an identical manner, this allowed us to compare the relative levels of the different Ab specificities in a very reliable fashion. Through this parallel comparison using murine and human lupus sera, we observed that the reactivity to dsDNA and total glomerular lysate was far stronger than the reactivity to any of the other nuclear Ags — including ssDNA, histones, Sm/RNP, and SS-A/SS-B — or the other glomerular Ags tested. DNA/glomerular autoreactivity was not only profound in the sera from the lupus mice, it also constituted a distinct IgG autoantibody cluster that distinguished lupus patients with more severe disease activity and renal disease (Figure (Figure6,6, H and K) from patients with low disease activity. Hence, these studies reaffirm the prominence and potential pathogenic significance of anti-dsDNA and antiglomerular Abs in lupus proposed in previous studies (2–12).
It was clear from the DNAse-I pretreatment studies that the majority of glomerular-reactive Abs were really anti-dsDNA (or anti-dsDNA/protein) Abs that had acquired nephrophilicity due to the nuclear material complexed within their Ag-binding pockets. This resonates well with previous reports demonstrating that nephrophilicity can be mediated by DNA-containing bridges (3, 11, 29–31). It was interesting, however, to observe that the reactivity to several of these glomerular/GBM Ags were only partially abrogated by DNAse-I pretreatment. A significant fraction of the reactivity to α-actinin, myosin, entactin, and aggrecan, for instance, appears to be independent of nuclear antigenic bridges. On the other hand, it remains possible that the observed “direct” binding to these Ags is mediated, at least in part, by anti-DNA Abs that are crossreactive to these other Ags, as has been demonstrated previously with a couple of glomerular Ags (22–29). It will be important to firmly establish the extent to which reactivity to these glomerular Ags is mediated by crossreactive anti-DNA Abs in future studies.
Both the DNAse-I pretreatment studies as well as the emergence of distinct autoantibody clusters in the lupus sera point to the importance of additional autoantibody specificities in lupus. A second important IgG autoantibody cluster uncovered using the glomerular proteome arrays was composed of laminin, myosin, heparan sulphate, Matrigel, and vimentin (Figure (Figure55 and Figure Figure6,6, A–E). Previous Ab transfer studies by other investigators (3, 13, 14, 16, 21–24), as well as the apparent association of this Ab cluster with more severe disease (Figure (Figure6F),6F), underline the potential pathogenic significance of these autoantibodies. Although anti-laminin, anti-myosin, and anti–heparan sulphate Abs have previously been implicated in lupus nephritis, vimentin constitutes a novel addition to this cluster. The inclusion of Matrigel in this cluster may not be a surprise, since laminin and heparan sulphate constitute 2 dominant constituents of Matrigel.
With both the above clusters (cluster 1 and cluster 3), these Abs were more prominent in patients with higher SLEDAI scores. Since almost all patients with SLEDAI scores greater than 8 also had significant renal SLEDAI scores, one can surmise that these autoantibody clusters may actually be reflective of renal flares. It would be important in future studies to examine how these clusters fluctuate in longitudinal studies and how they relate to the degree of renal pathology. Though some of the studied patients had biopsy information, these numbers were quite limited; clearly, these studies need to be expanded and confirmed in additional data sets.
Using the glomerular proteome arrays, this study has uncovered several additional IgG autoantibody clusters in lupus sera, including anti-histone Abs as well as several previously unreported specificities: vitronectin, collagen I, chondroitin sulphate, entactin, hyaluronic acid, fibrinogen, etc. However, these did not appear to associate with disease severity (Figure (Figure6).6). An additional insight of this study revolves around the IgM autoantibody specificities in lupus. Whereas some lupus patients, as well as the B6.Sle1.lpr lupus mouse strain, possessed very high levels of IgM anti-DNA Abs, a substantial fraction of the lupus and RA patients were noted to possess IgM reactivity to a broad cluster of 26 Ags, including several foreign (control) Ags. This apparent polyreactivity was not simply the consequence of rheumatoid factors, since most of the patients and controls were verified to be negative for rheumatoid factors. Caution should, however, be exercised in labeling these Abs as being polyreactive, since it is still possible that these may indeed represent multireactivity rather than polyreactivity. Clearly, future absorption studies and examination at the monoclonal level will help make this distinction.
Assuming these are indeed polyreactive IgM Abs, our results lend support to 2 novel notions, both of which warrant further experimental verification. First, it appears that among patients with serum anti-DNA Abs, the co-presence of polyreactive IgM Abs in the serum may confer protection against disease (Figure (Figure7B),7B), although this needs to be formally demonstrated. This correlates well with the demonstrated protective role of IgM in lupus (47). Second, IgM polyreactivity appears to be a common denominator of several autoimmune diseases, including lupus and RA, even if these Abs are not directly pathogenic. This observation is consistent with the recent description of polyreactive Abs in the early repertoire of lupus and RA patients based on single-cell B cell repertoire studies (48, 49).
As illustrated in this study, immunoproteome arrays offered the advantage of massive multiplexing compared with conventional ELISA and Western blot approaches. Moreover, this approach also compares superbly to the latter approaches in terms of its sensitivity and specificity (34–40). Since 1 set of test samples can be Cy3-labeled, a second set Cy5-labeled, and both samples cohybridized onto the same substrate spots, the glomerular proteome arrays and similar target organ Ag arrays lend themselves handsomely to several additional comparisons: predisease versus disease-phase sera; IgM Abs versus IgG Abs to different end-organ targets; paired serum–renal eluate, serum-CSF, and serum–synovial fluid comparisons; etc. In addition, the panel of Ags spotted onto these arrays can be readily modified or expanded to examine the potential disease contribution of RNA/DNA-containing immune complexes as well as to study serum and/or fluids from related connective tissue diseases. The autoantigen proteome array originally introduced by Robinson and colleagues (40–42), together with the more focused tissue-targeted arrays such as the glomerular proteome array described in this study, are likely to expand our serodiagnostic horizon in the coming years.
Methods
Antigens.
The literature was first surveyed to identify Ags that were known to be expressed in the glomerular milieu and/or GBM (43–45). Total glomerular sonicates were obtained by harvesting glomeruli from B6 mouse kidneys and sonicating them as described previously (46, 50). Total chromatin was prepared from sheep rbcs as described previously (51). Entactin (nidogen) and Goodpasture’s Ag (the NC1 domain of collagen IV) were kind gifts from J. Wieslander (Lund University, Lund, Sweden). All other Ags were purchased from Sigma-Aldrich, INOVA Diagnostics Inc., BD Biosciences — Pharmingen, Roche Diagnostics, or Chondrex Inc. The optimal coating concentration for all Ags was determined to be 1 μg/ml except for cardiolipin, for which the optimal concentration was determined to be 0.1 μg/ml.
Slide manufacture.
Ags were dissolved in PBS (or other buffers, as recommended by the manufacturer) and diluted from 1 μg/ml to 0.025 μg/ml using the “printing” buffer (0.06 M sodium bicarbonate, pH 9.5). Poly-L-lysine, super-aldehyde, or HydroGel coated slides were purchased from PerkinElmer. All slides were prewashed using PBS and double-deionized water (3 5-minute washes) and spin dried for storage. A BioRobotics MicroGrid II spotter (Genomic Solutions) was used to print the proteins in duplicate or triplicate onto the precoated slides. After printing, the slides were incubated in a humid chamber, rinsed with PBS, spin dried, and stored at 4°C.
Hybridization.
On the day of hybridization, the Ag-coated slides were washed using 0.1% Tween-20 in PBS, blocked with wash buffer containing 0.5% BSA, rinsed, and spin dried. Up to 100 μl of the appropriately diluted serum sample (optimal dilution, 1:200) was applied to the slide, and slides were placed in a hybridization chamber at 37°C for 1 hour. The slides were then washed and spin dried. Cy3- or Cy5-labeled anti-IgM, anti-IgG and various isotype-specific detection Abs (5 μg/ml; Jackson ImmunoResearch Laboratories) were next applied to the slides. We verified that the anti-IgM detection Ab used did not crossreact with array-bound IgG. Following 1 hour of incubation at 37°C, the slides were washed and spin dried. Finally, the slides were scanned using a GenePix 4000B scanner (Molecular Devices). Whereas the Cy3 signal (green) was scanned at 532 nM, the Cy5 signal (red) was scanned at 635 nM. All fluorescence intensities were normalized using mouse/human total Ig (Sigma-Aldrich) spotted onto the same slide. To derive the nfi, the absolute fluorescence intensity against any given Ag was divided by the absolute fluorescence intensity for the Ig control spots, and the resulting ratio was multiplied by a factor of 1,000. For each Ag, data obtained from duplicate or triplicate spots were averaged prior to any statistical comparison.
Murine Abs and sera.
The anti–collagen IV, anti-elastin, anti-hemocyanin, anti–fibrinogen IV, anti-myosin, and anti-vimentin mouse mAbs were purchased from Sigma-Aldrich, reconstituted to 1 mg/ml, and used to gauge the sensitivity and specificity of the glomerular proteome arrays. B6 mice were purchased from Jackson ImmunoResearch Laboratories. B6.Sle1.lpr mice, homozygous for the NZM2410/NZW allele of Sle1 as well as the FASlpr defect, were bred in our animal facility; the lupus phenotypes in these mice have been described previously (52). Sera were obtained from both strains at 6–9 months of age for use in this study. All animal studies were approved by the University of Texas Southwestern Medical School Animal Use Review Committee.
Patient recruitment and sera.
Sera were drawn from 11 healthy adults who were seronegative for anti-DNA Abs, 5 RA patients, and 37 SLE patients at the Albert Einstein College of Medicine in accordance with institutional review board–approved guidelines. Subjects gave informed consent for the study. With regard to the SLE patients, all subjects who fulfilled at least 4 American College of Rheumatology criteria for diagnosis were recruited for the study. From this collection, blood samples from 37 patients were used for the glomerular proteome studies so as to include patients with a wide spread of SLEDAI scores (53). The age and ethnicity of the study subjects as well as their SLEDAI scores, ANA titers, ELISA-determined anti-DNA Ab levels, serum complements (C3 and C4 levels), and severity of renal inflammation graded using the WHO classification (54) are detailed in Table Table1.1. Renal SLEDAI score was derived from the total SLEDAI score by totaling only the renal-specific components of the SLEDAI score. For some analyses, the patients were divided into 2 groups based on whether they had mild disease (SLEDAI score, 0–8) or more severe lupus (SLEDAI score, >8) based on the threshold used to classify severe lupus flares for which the SLEDAI scores typically exceed 12 (55, 56). For most patients, the time between clinical disease assessment and blood draw was 1 week. On the other hand, the kidney biopsy information presented in Table Table11 was obtained 0–29 months prior to blood draw. Most patients were undergoing treatment for their disease; however, any potential impact of particular immunosuppressives on autoantibody titers or patterns were not specifically examined in this study.
DNAse-I pretreatment studies.
For some of the depicted studies, either the human or mouse serum alone, the Ag-coated slides alone, or both were pretreated with DNAse-I for 30 minutes at 37°C, using 200 U/ml for treating the serum and 50 U/ml for treating the arrays (in buffer containing 50 mM Tris-HCl, 75 mM KCl, and 3 mM MgCl2; pH 8.3) before hybridizing the sera to the slides. The resulting fluorescence intensities were expressed as a ratio relative to the fluorescence intensities derived using sham (PBS) pretreatment.
Data analysis.
For intergroup comparisons, the Student’s t test was used (SigmaStat version 2.0; Jandel Scientific). Heat map diagrams with row-wise and columnwise clustering were generated using Cluster and TreeView software (versions 2.2 and 1.6, respectively; http://rana.lbl.gov/EisenSoftware.htm). In these diagrams, fluorescence intensities that were higher than the row mean were colored red, those that fell below the row mean were colored green, and cells with signals close to the mean were left black. Missing data was denoted using gray.
Acknowledgments
This work was supported by grants from the NIH (R01 AI47460, to C. Mohan; R01 AR486912 and PO1 AI51392, to C. Putterman). We would also like to acknowledge Mei Yan and Desi Kresak for technical assistance, Ramesh Saxena for helpful feedback, and Jorgen Wieslander for his generous gift of antigens.
Footnotes
Nonstandard abbreviations used: Ag, antigen; B6, C57BL/6; Cy, cyanine; dsDNA, double-stranded DNA; GBM, glomerular basement membrane; GN, glomerulonephritis; nfi, normalized fluorescence intensity unit(s); SLE, systemic lupus erythematosus; SLEDAI score; SLE disease activity score; Sm/RNP, Smith Ag/ribonucleoprotein; ssDNA, single-stranded DNA.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci23587
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/23587/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci23587
Article citations
Dietary docosahexaenoic acid supplementation inhibits acute pulmonary transcriptional and autoantibody responses to a single crystalline silica exposure in lupus-prone mice.
Front Immunol, 15:1275265, 01 Feb 2024
Cited by: 0 articles | PMID: 38361937 | PMCID: PMC10867581
Sirtuin 3 is required for the dexmedetomidine-mediated alleviation of inflammation and oxidative stress in nephritis.
Immun Inflamm Dis, 12(1):e1135, 01 Jan 2024
Cited by: 1 article | PMID: 38270316 | PMCID: PMC10777884
Lupus Nephritis Risk Factors and Biomarkers: An Update.
Int J Mol Sci, 24(19):14526, 25 Sep 2023
Cited by: 11 articles | PMID: 37833974 | PMCID: PMC10572905
Review Free full text in Europe PMC
Cystic fibrosis autoantibody signatures associate with Staphylococcus aureus lung infection or cystic fibrosis-related diabetes.
Front Immunol, 14:1151422, 11 Sep 2023
Cited by: 0 articles | PMID: 37767091 | PMCID: PMC10519797
Antibodies Produced by CLL Phenotype B Cells in Patients With Myasthenia Gravis Are Not Directed Against Neuromuscular Endplates.
Neurol Neuroimmunol Neuroinflamm, 10(2):e200087, 08 Feb 2023
Cited by: 2 articles | PMID: 36754834 | PMCID: PMC9909583
Go to all (196) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Murine lupus strains differentially model unique facets of human lupus serology.
Clin Exp Immunol, 168(2):178-185, 01 May 2012
Cited by: 15 articles | PMID: 22471278
Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes.
Clin Exp Immunol, 147(1):60-70, 01 Jan 2007
Cited by: 146 articles | PMID: 17177964
Characterization of autoantibody activities in sera anti-DNA antibody and circulating immune complexes from 12 systemic lupus erythematosus patients.
J Clin Lab Anal, 10(6):451-457, 01 Jan 1996
Cited by: 8 articles | PMID: 8951619
Autoantigen Microarray for High-throughput Autoantibody Profiling in Systemic Lupus Erythematosus.
Genomics Proteomics Bioinformatics, 13(4):210-218, 01 Aug 2015
Cited by: 60 articles | PMID: 26415621 | PMCID: PMC4610965
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: P01 AI051392
Grant ID: R01 AI47460
Grant ID: P01 AI51392
Grant ID: R01 AI047460
NIAMS NIH HHS (1)
Grant ID: R01 AR486912





