Abstract
Free full text

Wild-Type Puumala Hantavirus Infection Induces Cytokines, C-Reactive Protein, Creatinine, and Nitric Oxide in Cynomolgus Macaques
Abstract
Hantaviruses cause two severe human diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Approximately 200,000 cases are reported annually, and there is to date no specific treatment available. A major obstacle in studying the medical aspects of HFRS and HPS has been the lack of an adequate animal model. Here we show that infection of cynomolgus macaques by wild-type Puumala hantavirus resulted in typical signs of HFRS including lethargy, anorexia, proteinuria, and/or hematuria, in addition to cytokine (interleukin 6 [IL-6], IL-10, and tumor necrosis factor alpha), C-reactive protein, creatinine, and nitric oxide responses. Viral RNA was detected in plasma from days 3 to 7 postinoculation until days 24 to 28 postinoculation, infectious virus was recovered, and the virus-specific immune responses (immunoglobulin M [IgM], IgG, and neutralizing antibodies) mimicked those seen in humans. The results indicated that the monkey model will provide a valuable tool for studies of pathogenesis, candidate vaccines, and antivirals for hantavirus disease.
Hantaviruses, members of the family Bunyaviridae, are known to cause two serious and often fatal human diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). The clinical symptoms of HFRS are characterized by fever, thrombocytopenia, renal failure, and, in severe cases, hemorrhage caused by capillary leak syndrome (13, 31). Puumala hantavirus (PUUV) causes a milder form of HFRS named nephropathia epidemica (NE), which occurs in northern and central Europe. The more severe forms of HFRS are caused by Hantaan (Asia) and Dobrava (Europe) viruses, while Seoul virus (worldwide) causes an intermediate form (3, 17). Hantaviruses are carried by specific rodent hosts, and virus transmission to humans occurs via inhalation of aerosolized animal excreta (5, 32). The mortality of HFRS varies between <0.1 and 12%, depending on the causative virus (31, 32). Sin Nombre virus, Andes virus, and related hantaviruses cause HPS in the Americas, mainly characterized by acute respiratory dysfunction and with a mortality rate of approximately 40% (26, 27).
In the rodent reservoirs, hantaviruses cause persistent and subclinical infections (31). Although successfully used, e.g., for studies of immune responses and for evaluation of vaccine candidates and/or therapeutic reagents, rodent models are of limited value for studying the pathogenesis of human hantavirus infections. Only two earlier studies of nonhuman primates have been reported, and both described models with certain limitations (9, 40). We have previously shown phenotypic and genetic changes of PUUV when propagated in cell culture, resulting in a decrease of infectivity for its natural host, the bank vole (Clethrionomys glareolus) (22). We therefore speculated that wild-type PUUV (strain Kazan-wt) might infect nonhuman primates in a way different from previously reported studies. Here we report the first successful experimental infection of cynomolgus macaques (Macaca fascicularis), resulting in an infection that in all investigated parameters mirrored NE in humans.
Cynomolgus macaques were kept in biological safety rooms. The housing, maintenance, and care of the animals used in the present study were in compliance with the relevant guidelines and requirements. Animals (three females, aged 4 to 6 years) were inoculated intravenously with approximately 105 bank vole 50% infective doses of PUUV strain Kazan-wt (22) in 1 ml of phosphate-buffered saline. After inoculation, the monkeys were monitored daily for behavioral changes and clinical signs.
Monkey plasma samples were analyzed for C-reactive protein (CRP) and creatinine by the Laboratory for Clinical Chemistry, Huddinge Hospital, Stockholm, Sweden. Plasma nitric oxide (NO) was analyzed by a commercial assay as described by the manufacturer (R & D Systems). Proteinuria and hemoglobinuria were measured with dipsticks (Bayer Corporation).
Plasma, collected on day 7 after virus inoculation of monkey 59, was inoculated intraperitoneally and subcutaneously at reciprocal dilutions of 10, 100, and 1,000 (300 μl) in Hanks balanced salt solution, supplemented with 2% HEPES and 2% fetal calf serum, into hantavirus-free colonized bank voles (22).
RNA was isolated by Tripure (Roche Diagnostics) according to the manufacturer’s instructions. To ensure uniform quality of the RNA extracted from organs, reverse transcription-PCR (RT-PCR) with primers specific for human housekeeping protein glyceraldehyde-3-phosphate dehydrogenase was performed in parallel: all samples revealed a product of the expected size (approximately 500 bp). RT-PCR of nucleotides 799 to 1106 from the viral S segment was performed as described earlier (28). Amplicons were purified and sequenced automatically.
PUUV-specific monkey and bank vole antibodies were analyzed by enzyme-linked immunosorbent assays (ELISAs) and PEPSCAN as previously described (7, 8, 21). A focus reduction neutralization assay was performed as previously described (22).
Cytokines (interleukin 1β [IL-1β], IL-6, IL-10, IL-12, alpha interferon [IFN-α], IFN-γ, and tumor necrosis factor alpha [TNF-α]) were measured from samples of undiluted monkey serum or plasma with commercially available ELISAs according to the manufacturer’s instructions (Endogen). Since the assays were developed for measurement of human cytokines, the calculated values of concentrations in serum or plasma are not absolute.
Clinical signs and chemistry.
After infection, the three monkeys lost their appetite and became clearly apathetic during days 7 to 14. During this period, two monkeys (no. 53 and 59) stopped eating completely for 2 to 4 days. Clear differences in terms of general affectedness were observed among the three monkeys: no. 59 had the most pronounced symptoms, no. 53 had intermediate symptoms, and no. 25 had less pronounced symptoms. The measurements of body temperatures could not be optimally carried out, and due to the anesthesia, great variations were observed. However, for the most affected monkey, no. 59, the temperature clearly peaked on day 10 (39.4°C [Fig. 1]). After day 14, until termination of the experiment on day 28, no clinical signs were observed.
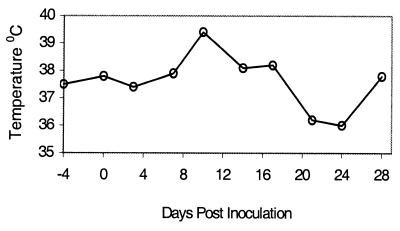
Temperature curve of monkey 59 infected by wild-type PUUV. Temperature was measured in the ear after anesthetization.
Plasma samples showed elevated values of CRP and creatinine in the two most affected monkeys, no. 53 and 59 (Fig. 2A and B). Elevated levels of NO were observed for all three monkeys at days 14 to 24 postinfection; monkey 53 also showed a single peak on day 3 (Fig. (Fig.2C2C).
Clinical chemistry of monkeys infected by wild-type PUUV. (A) Plasma CRP; (B) plasma creatinine; (C) NO.
Urine samples collected at day 7 showed proteinuria for the no. 25 and 53 samples, while the urine sample from no. 59 was completely colorless, indicating polyuria. Urine samples collected at days 21 and 28 were all negative for proteinuria. Clear hemoglobinuria was observed for the no. 25 and 53 samples on day 7.
Detection of PUUV RNA in monkey plasma and virus reisolation in bank voles.
Subsequently drawn monkey plasma and serum samples were analyzed by RT-PCR for the presence of viral RNA. All three monkeys had detectable levels of PUUV-S RNA in the plasma samples for a prolonged time, between days 3 and 24 (no. 25), days 7 and 24 (no. 53), and days 7 and 28 (no. 59), while all serum samples were found to be negative. All plasma and serum samples drawn prior to virus inoculation were negative. Thus, the results indicated the presence of replicating virus, cleared from the circulation by two of the monkeys. Sequences that were recovered (nucleotides 799 to 1106 of the S segment) were 100% identical to those of the original virus (PUUV, strain Kazan-wt). Tissue samples (heart, liver, lung, spleen, and kidney), collected at the termination of the experiment (day 28), were analyzed for the presence of PUUV-S RNA by RT-PCR. All five tissues from all monkeys, except the lung samples from monkeys 25 and 53, were found to be positive. Since plasma samples from that day were negative for monkeys 25 and 53, the results indicated a prolonged presence of the virus in certain tissues, after the clearance of viremia.
Virus isolation attempts were made with colonized bank voles with plasma samples from monkey 59 drawn 7 days postinfection. Four of the inoculated bank voles had detectable levels of immunoglobulin G (IgG) to recombinant PUUV-N, while all eight were PUUV-S RNA positive in their lungs when sacrificed 26 days post-plasma inoculation.
Development of antibody responses.
All three monkeys had clearly detectable IgM responses on day 10, peaking on day 14. All remaining samples were IgM positive, although with declining titers, until the termination of the experiment (Fig. (Fig.3A).3A). Maximum IgM endpoint titers varied between 25,600 and ≥102,400 at day 14 (Table (Table11).
(A) Kinetics of IgM and IgG responses in monkeys infected by wild-type PUUV as determined by ELISA. (B) Kinetics of neutralizing antibody responses as determined by focus reduction neutralization assay (FRNT).
TABLE 1.
Summary of clinical chemistry, humoral, and cytokine responses in cynomolgus macaques infected by wild-type PUUVa
| Result for monkey:
| % of NE patients (reference) | |||
|---|---|---|---|---|
| 25 | 53 | 59 | ||
| Clinical chemistry | ||||
 Creatinine Creatinine | − | + | + | 85–100 (34) |
 CRP CRP | − | + | + | 89 (25) |
 NO NO | + | + | + | 100 (18) |
 Proteinuria Proteinuria | + | + | − | 84–100 (34) |
 Hematuria Hematuria | + | + | − | 38–85 (34) |
| Viral RNA in plasmab | 3–24 | 7–24 | 7–28 | NAc |
| Humorald | ||||
 IgM IgM | 25,600 | ≥102,400 | ≥102,400 | |
 IgG IgG | 1,600 | 6,400 | 6,400 | |
 Neutralizing antibody Neutralizing antibody | ≥10,240 | ≥10,240 | ≥10,240 | |
| Cytokine | ||||
 IL-1β IL-1β | − | − | + | 33 (33) |
 IL-6 IL-6 | + | + | + | 100 (19) |
 IL-10 IL-10 | + | − | + | 87 (19) |
 IL-12 IL-12 | − | − | − | NA |
 IFN-α IFN-α | + | − | − | NA |
 IFN-γ IFN-γ | − | − | + | 47 (19) |
 TNF-α TNF-α | − | + | + | 100 (19) |
All monkeys had detectable IgG responses on days 14 to 21, with increasing levels at the end of the experiment (Fig. 3A). Maximum IgG endpoint titers varied between 1,600 and 6,400 at the end of the experiment (Table (Table11).
The monkeys had significant levels of virus-neutralizing antibodies on days 7 to 10 with rapidly increasing titers. From day 14, the titers varied between 2,560 and ≥10,240 until the end of the experiment (Fig. (Fig.3B3B and Table Table11).
Sera of the three monkeys drawn on day 28 were analyzed by PEPSCAN to locate IgG-reactive peptides within the sequence of PUUV-N. The results showed that several antigenic regions in N were recognized by the IgG response, similar to what has been earlier found for NE patients (21, 38; data not shown).
Development of cytokines.
In general, significantly increased levels (>2-fold cutoff-baseline values) of IL-1β, IL-6, IL-10, IFN-α, IFN-γ, and TNF-α were demonstrated for at least one monkey, while the levels of IL-12 remained undetectable or very low in all cases until the termination of the experiment.
A marked increase of serum IL-1β was observed for monkey 59 (maximum, 209 ng/liter on day 14), while the two other monkeys remained negative until the end of the experiment (Table (Table11 and Fig. Fig.4A4A).
Kinetics of cytokine responses in monkeys after infection by wild-type PUUV. Cytokines were measured in sequentially drawn serum or plasma samples. (A) Serum IL-1β; (B) serum IL-6; (C) serum IL-10; (D) plasma IFN-α; (E) serum IFN-γ; (F) plasma TNF-α; (G) serum IL-12. The lower limit of detection for each assay is indicated by a baseline (test values lower than the lower limit of detection are indicated at the baseline).
Increases of IL-6 (maximum, 2.6- to 230-fold increases) were detected in the serum samples from all monkeys, with peak values on day 14. Monkey 59 showed a rapid increase of serum IL-6 between days 7 and 10 after infection (maximum, 230 ng/liter on day 17) with continuously high and stable values (Table (Table11 and Fig. Fig.4B4B).
Increases of serum IL-10 were observed for monkeys 25 and 59 with similar patterns: maximum peak values (10.0 to 11.9 ng/liter) were detected on days 14 to 21, followed by mainly stable values for approximately 1 week and a slight decrease until the end of the experiment. A low increase (<2-fold the baseline value) was detected for monkey 53 (Table (Table11 and Fig. Fig.4C4C).
Monkey 25 showed a significant increase in plasma IFN-α concentration (>3-fold increase; maximum, 192 ng/liter on day 14), while the levels for the other monkeys remained at the baseline values or decreased slightly (Table (Table11 and Fig. Fig.4D4D).
Monkey 59 developed a pronounced serum IFN-γ response (maximum, 679 ng/liter on day 17), with levels remaining high until the end of the experiment. Monkeys 25 and 53 remained close to negative for IFN-γ until the end of the experiment (Table (Table11 and Fig. Fig.4E4E).
Marked increases of plasma TNF-α were observed for monkey 59 (maximum, 113 ng/liter on day 17) and monkey 53 (maximum, 20.4 ng/liter on day 17), while a low increase (<2-fold the baseline value) was detected for monkey 25 (Table (Table11 and Fig. Fig.4F4F).
A low increase (<2-fold the baseline value) of serum IL-12 was detected only for monkey 59 (Table (Table11 and Fig. Fig.4G4G).
A major obstacle in hantavirus research is the absence of an animal model that mirrors human infection. Albeit a limited number of monkeys could be included in the present study, our results clearly indicated a successful disease model. Therefore, we believe that further development of monkey models based on wild-type hantaviruses will greatly facilitate studies of the medical aspects of HFRS-HPS.
Although rodent models have been extensively explored in hantavirus research, they do not allow studies mimicking the pathogenesis of hantavirus infections in humans. Rodent hantavirus models have, however, been proven most valuable for studies concerning humoral and cellular immune responses (4, 8) and studies of reagents with potential therapeutic effects (29, 41), as well as for vaccine candidate studies (15, 23, 29, 30). In addition, rodent models may provide valuable information on the mechanisms underlying viral persistence, which leads to virus spread to humans.
In 1987, Dalrymple and coworkers made the first attempts to induce HFRS in monkeys using prototype Hantaan virus and blood from a Korean HFRS patient; these attempts were, however, unsuccessful (see reference 10). Two other studies of hantavirus infections of nonhuman primates have been reported, neither of them describing efficient infections (9, 40). Yanagihara and coworkers were the first to report experimental hantavirus infections of nonhuman primates (40). They inoculated 38 animals belonging to 19 different Old World and New World monkey species and 2 anthropoid apes by the intravenous route with either Prospect Hill hantavirus (PHV) or PUUV. Asymptomatic infections were recorded for nearly all animals, except for one of two capuchin monkeys (Cebus nigriviltatus) inoculated with PHV, one of two cynomolgus macaques inoculated with PHV or PUUV, respectively, and a chimpanzee (Pan troglodytes) inoculated with PHV, which developed a mild proteinuria. Although this study demonstrated the susceptibility of three primate species in principle, there were several drawbacks, e.g., only some of the animals from each species became infected and none of the animals showed any general signs of disease. Most important, there was no difference in the outcome of the infection between PUUV and PHV, the latter known to be apathogenic for humans (31).
In 1995, Groen et al. reported a study of PUUV infection of cynomolgus macaques (9). Although most of the monkeys became antibody positive, no clinical signs that mirrored human hantavirus infection were observed.
The present study revealed the successful hantavirus infection of cynomolgus macaques. The animals developed antibody responses very similar to what has been described for human patients, including rapidly increasing levels of neutralizing antibodies (12, 24). The monkeys exhibited typical clinical signs of NE, including lethargy, anorexia, proteinuria, and/or hemoglobinuria (Table (Table1).1). In addition, elevated levels of CRP and creatinine in plasma, parameters that are both typical for NE, were detected in the two most affected monkeys. Elevated levels of NO, which have recently been reported for NE patients (11, 18) and which have been suggested as important for pathogenesis, were seen for all three animals. Studies of the plasma cytokine responses revealed elevated levels of IL-6, IL-10, and TNF-α, all typical for human PUUV infection and which subsequently correlated with the clinical signs (for a review, see reference 34). Thus, all investigated parameters mirrored human PUUV infection. Here, it should be noted that only about 5% of patients with human PUUV infections are admitted to a hospital (2, 6); i.e., the majority of the infections are either asymptomatic or very mild.
We believe that the major reason for the differences between the two earlier reports and our study was the selection of the virus strain. We have previously shown that cell adaptation of PUUV strain Kazan alters the phenotype of the virus and that the infectivity for the natural reservoir, the bank vole, was dramatically decreased when the virus had been fully adapted to cell culture (22).
The mechanisms of hantavirus pathogenesis are poorly understood. The viruses grow only slowly in cell culture and cause little or no cytopathology. For the Old World HFRS-causing hantaviruses (which include the European PUUV), much of the research concerning pathogenicity has been focused on the renal involvement. Only little attention has so far been paid to vascular changes, although changes of the microvasculature of the medulla have been suggested to contribute to tubular dysfunction (16). The general assumption is that increased capillary permeability is a key factor in hantavirus pathogenesis, but the specific mediators have not yet been defined.
Recent data have indicated the importance of certain cytokines in the pathogenesis of NE. Linderholm and coworkers showed that, of 15 hospitalized NE patients (PUUV infections), 13 to 15 developed increased levels of IL-6, IL-10, and TNF-α in plasma (19) (Table (Table1).1). TNF-α is one of the most important proinflammatory cytokines. When intravenously injected into cancer patients, TNF-α causes changes reminiscent of clinical characteristics seen in NE: fever, chills, headache, myalgia, and a relative hypotension have been observed (35). Further, the more rare TNF2 allele, associated with higher TNF-α production by mononuclear cells, has been found previously to be significantly more frequent in hospitalized NE patients than in healthy controls (14), and increased expression of TNF-α has been shown elsewhere in NE patient kidney biopsy specimens (36). The genetically determined high-TNF-α-production phenotype could thus play an important role in severe NE. Increased levels of IL-6 and IL-10 are in accordance with the well-recognized interregulation of these cytokines with TNF-α. IL-10 and TNF-α are believed to form an autoregulatory loop, in which TNF-α is an inducer of IL-10 and IL-10 is a down regulator of TNF-α (37). Therefore, it is highly interesting that the most affected monkey (no. 59) developed high levels of TNF-α and that all three animals developed significantly increased levels of IL-6.
Significantly increased levels of IL-1β and IFN-γ were detected only in the most affected monkey (no. 59). Previous studies of PUUV-infected patients have indicated increased levels of IL-1β in only a minority of the patients and only a brief IFN-γ response during the acute phase of the disease. Both these cytokines are known to augment the proinflammatory effects of TNF-α in animal models of septic shock (1, 39), and their general absence in NE patients has therefore been interpreted as a possible explanation for the benign course of NE (20). Thus, the finding of these two cytokines only in monkey 59 was in line with the various levels of affectedness seen among the infected monkeys. Interestingly, IFN-α, which is one of the most potent antiviral cytokines, increased only in the less affected monkey (no. 25).
In conclusion, our results indicated that the monkey–PUUV Kazan-wt model will be valuable for further studies, e.g., of the pathogenesis of HFRS and of virus-host interactions and for evaluation of potential therapeutic reagents (antivirals, anticytokines, and immunoglobulins) and vaccine candidates.
. . . .
Acknowledgments
We thank Pär Bierke, Kjell Eklund, and Christel Werner for excellent animal care and sampling and for most valuable discussions.
The work was supported by grants from the Swedish Medical Research Council (projects no. 12177 and 12642), the Swedish Society of Medicine, and the European Community (contract nos. BMH4-CT97-2499 and QLK2-1999-01119).
REFERENCES
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.76.1.444-449.2002
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc135710?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Bunyavirales: Scientific Gaps and Prototype Pathogens for a Large and Diverse Group of Zoonotic Viruses.
J Infect Dis, 228(suppl 6):S376-S389, 01 Oct 2023
Cited by: 3 articles | PMID: 37849397 | PMCID: PMC10582323
Hemorrhagic Fever with Renal Syndrome in Asia: History, Pathogenesis, Diagnosis, Treatment, and Prevention.
Viruses, 15(2):561, 18 Feb 2023
Cited by: 17 articles | PMID: 36851775 | PMCID: PMC9966805
Review Free full text in Europe PMC
Puumala Hantavirus Infections Show Extensive Variation in Clinical Outcome.
Viruses, 15(3):805, 22 Mar 2023
Cited by: 4 articles | PMID: 36992513 | PMCID: PMC10054505
Coagulopathy in Acute Puumala Hantavirus Infection.
Viruses, 13(8):1553, 06 Aug 2021
Cited by: 11 articles | PMID: 34452419 | PMCID: PMC8402851
Review Free full text in Europe PMC
Immune response during hantavirus diseases: implications for immunotherapies and vaccine design.
Immunology, 163(3):262-277, 18 Mar 2021
Cited by: 15 articles | PMID: 33638192
Review
Go to all (63) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Passive immunization protects cynomolgus macaques against Puumala hantavirus challenge.
Antivir Ther, 13(1):125-133, 01 Jan 2008
Cited by: 23 articles | PMID: 18389907
Delayed viremia and antibody responses in Puumala hantavirus challenged passively immunized cynomolgus macaques.
Arch Virol, 150(1):79-92, 21 Sep 2004
Cited by: 7 articles | PMID: 15449139
Andes virus infection of cynomolgus macaques.
J Infect Dis, 186(12):1706-1712, 22 Nov 2002
Cited by: 20 articles | PMID: 12447754
Hantaviruses: clinical, microbiologic, and epidemiologic aspects.
Crit Rev Clin Lab Sci, 32(5-6):469-508, 01 Jan 1995
Cited by: 50 articles | PMID: 8561891
Review




