Abstract
Free full text

Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury
Abstract
Diverse functions have been reported for lipocalin 2. To investigate these functions in vivo, we generated gene-targeted lipocalin 2-deficient mice (Lcn2−/− mice). In vitro studies have suggested that lipocalin 2 is important for cellular apoptosis induced by IL-3 withdrawal, and for the induction of kidney differentiation during embryogenesis. Analysis of Lcn2−/− mice showed normal cell death upon IL-3 withdrawal and normal kidney development. However, we found that Lcn2−/− mice exhibited an increased susceptibility to bacterial infections, in keeping with the proposed function of lipocalin 2 in iron sequestration. Neutrophils isolated from Lcn2−/− mice showed significantly less bacteriostatic activity compared with WT controls. The bacteriostatic property of the WT neutrophils was abolished by the addition of exogenous iron, indicating that the main function of lipocalin 2 in the antibacterial innate immune response is to limit this essential element. Another important function ascribed to lipocalin 2 has been its protective role against kidney ischemia-reperfusion injury. We analyzed Lcn2−/− mice using a mouse model for severe renal failure and could not detect any significant differences compared with their WT littermates.
Lipocalins comprise a family of diverse proteins that exhibit only limited amino acid sequence similarity but share a common tertiary structure (1). Lipocalins are typically small (160–180 aa) secreted proteins that have several common molecular properties. Lipocalins bind with high affinity to lipophilic molecules (such as retinoids, fatty acids, cholesterols, and prostaglandins); and they form covalent and noncovalent complexes with other soluble macromolecules (e.g., neutrophil gelatinase) (2). Initially characterized as transporter proteins, it is now well established that the lipocalins perform a variety of important functions. For instance, several lipocalins (including apolipoprotein D, quiescence-specific protein, purpurin, and α1-microglobulin) have been implicated in the modulation of cell growth and metabolism, whereas others (α1-microglobulin, glycodelin, and lipocalin 2) appear to regulate the immune response 3–6.
Lipocalin 2 was originally described as an acute-phase protein in the liver (7) but is also up-regulated after LPS stimulation in macrophages (8) and in the lungs of LPS-treated mice (9). In addition, lipocalin 2 may be involved in tissue involution, a highly organized process of tissue restructuring. During involution, lipocalin 2 levels can reach as high as 0.2–0.5% of the total extractable protein of the breast tissue (9). To date, there has been only speculation about the role lipocalin 2 might play in this process. One hypothesis is that, because lipocalin 2 expression coincides with a high degree of apoptosis in an involuting tissue, lipocalin 2 may promote the cell death of invading neutrophils and thus delay neutrophil entry into a tissue until the second phase of involution (9). A proapoptotic function for lipocalin 2 has also been reported by Devireddy et al. (10). However, the most important function of lipocalin 2 may be as an iron-sequestering protein in the antibacterial innate immune response. Lipocalin 2 binds to iron when this element is associated with a small microbial adapter protein called a siderophore. Siderophores are synthesized and secreted by bacteria under iron-limiting conditions, such as occur during infection. They scavenge iron from various host iron-binding proteins (such as transferrin and ferritin) that tightly regulate iron metabolism in the host. The siderophores then facilitate bacterial uptake of this iron via a receptor-mediated mechanism. To combat this bacterial salvage process, an infected host secretes lipocalin 2, which binds to the bacterial siderophores and prevents bacterial iron uptake. Most recently, lipocalin 2 has been reported to bind enterochelin, a bacterial catecholate-type ferric siderophore (6).
Lipocalin 2 also plays an important role as an early marker for kidney damage (11, 12). Administration of purified recombinant lipocalin 2 in mice resulted in a rapid uptake of the protein into mainly proximal tubule cells and a marked amelioration of ischemic acute renal injury in a mouse model of renal ischemia-reperfusion injury (13, 14).
To investigate the physiological functions of lipocalin 2, we generated and analyzed gene-targeted lipocalin 2-deficient mice. Our results confirm the crucial role of lipocalin 2 in the innate immune response to bacterial infection in vivo (6). We show that the ability of WT neutrophils to significantly reduce bacterial growth in vitro depends on the ambient iron level, and that this level is controlled by lipocalin 2. We analyzed the role of lipocalin 2 in protection against ischemic acute renal injury in a well established murine model of acute renal injury and could not find any significant differences between lipocalin 2-deficient mice and WT littermates. Contrary to previous reports, we find no evidence that lipocalin 2 is essential for apoptosis in IL-3-dependent primary bone marrow mast cells or in thymocytes, or that lipocalin 2 is required for kidney development in vivo.
Results
Targeted Disruption of the lipocalin 2 Gene in Mice.
To determine the physiological role of lipocalin 2 in vivo, we used homologous recombination in embryonic stem (ES) cells to disrupt the Lcn2 gene and generate Lcn2−/− knockout mice (Fig. 4A, which is published as supporting information on the PNAS web site). The targeting vector was designed to replace most of the lipocalin 2 coding region, including exons 1–5, with the PGK-neo cassette. The 5′ probe in Fig. 4A recognizes a 9.8-kb fragment from the 129/Ola C57BL6/J WT Lcn2 allele and an 8.5-kb fragment from the targeted Lcn2 allele. We obtained 20 independent ES cell clones carrying the mutated Lcn2 allele, and 5 of these were injected into C57BL/6J blastocysts to generate chimeric mice. F1 heterozygotes originating from the two clones were intercrossed to produce the F2 progeny analyzed in this report. Fig. 4B shows the Southern blot analysis of DNA from 129/Ola ES cells, 129/Ola/C57BL6/J F1 offspring, and F2 offspring from F1 intercrosses.
To confirm that the knockout Lcn2 allele was a null mutation, neutrophils were prepared from Lcn2+/+ (WT), Lcn2+/−, and Lcn2−/− littermate mice, and lysates were subjected to Western blot analysis to detect lipocalin 2 expression. Lcn2−/− neutrophils do not express the lipocalin 2 protein (Fig. 4C). The Southern analysis of F2 genotypes was confirmed by PCR (Fig. 4D). Of 609 F2 pups, 162 were WT (26.6%), 316 were heterozygous for the mutation (51.9%), and 131 were homozygous mutants (21.5%), consistent with the expected Mendelian ratio. Both male and female Lcn2−/− mice were healthy, developed normally, and did not exhibit any obvious macroscopic or microscopic morphological abnormalities. Neither did aged Lcn2−/− mice (>12 months of age) show any sign of anomalies or an increase in tumorigenesis (data not shown).
Although Lcn2−/− males appeared to be fertile, Lcn2−/− females showed significantly (P < 0.05) decreased fertility compared with WT littermates when crossed to WT or Lcn2+/− males. Whereas 95% of WT females (n = 19) had their first litter within 8 weeks of mating, only 68% of Lcn2−/− females (n = 25) had any offspring within the same time frame (data not shown).
Because lipocalin 2 has been implicated in kidney organogenesis, we analyzed this organ in WT and Lcn2−/− embryos from the earliest stages of kidney formation. Kidneys of WT and Lcn2−/− embryos from embryonic day 10.5 until embryonic day 12.5 were harvested, fixed, sectioned, and stained with hematoxylin/eosin. Three embryos of each genotype were examined for a possible delay in kidney development, or any other observable kidney anomaly, but no differences between WT and Lcn2−/− embryos could be detected (data not shown).
Because lipocalin 2 is known to be involved in antibacterial immunity, we carried out an extensive flow-cytometric analysis comparing thymocyte, splenocyte, lymph node, and bone marrow cell populations in WT and Lcn2−/− littermate mice. As shown in Table 1, no significant differences were observed in any of the cell populations analyzed.
Table 1.
Analysis of immune system cell populations in Lcn2−/− and WT littermate mice
| +/+ mean | −/− mean | +/+ SD | −/− SD | +/+, n | −/−, n | t test | ||
|---|---|---|---|---|---|---|---|---|
| THY | CD4/8 | 82.57 | 83.68 | 2.76 | 1.41 | 7 | 7 | 0.398 |
| CD4 | 11.62 | 10.69 | 2.44 | 1.35 | 7 | 7 | 0.432 | |
| CD8 | 2.88 | 2.72 | 0.82 | 0.56 | 7 | 7 | 0.708 | |
| SPL | CD4 | 39.27 | 41.98 | 14.75 | 15.49 | 7 | 7 | 0.762 |
| CD8 | 15.71 | 14.30 | 2.03 | 2.23 | 7 | 7 | 0.275 | |
| B220 | 50.05 | 50.85 | 2.55 | 4.97 | 7 | 7 | 0.730 | |
| LN | CD4 | 48.19 | 48.71 | 3.00 | 3.45 | 7 | 6 | 0.796 |
| CD8 | 26.24 | 26.04 | 1.93 | 3.86 | 7 | 6 | 0.911 | |
| B220 | 22.43 | 22.02 | 3.66 | 3.98 | 7 | 6 | 0.861 | |
| BM | CD4 | 2.93 | 2.66 | 0.64 | 0.98 | 7 | 7 | 0.587 |
| CD8 | 3.71 | 3.19 | 1.12 | 1.22 | 7 | 7 | 0.453 | |
| B220 | 58.04 | 58.90 | 9.95 | 11.23 | 7 | 7 | 0.891 | |
| SPL CD25+ | CD4 | 8.81 | 8.02 | 1.36 | 0.43 | 7 | 7 | 0.199 |
| LN CD25+ | CD4 | 7.63 | 7.94 | 0.97 | 1.03 | 7 | 7 | 0.602 |
| SPL CD69+ | CD4 | 8.23 | 7.01 | 1.81 | 1.58 | 7 | 7 | 0.237 |
| LN CD69+ | CD4 | 8.66 | 10.40 | 1.33 | 2.01 | 7 | 7 | 0.102 |
| SPL memory | CD4 | 25.08 | 19.92 | 5.01 | 3.81 | 7 | 7 | 0.067 |
| LN memory | CD4 | 8.36 | 8.07 | 1.73 | 2.71 | 7 | 7 | 0.824 |
| BM IgM/IgD | B220 | 17.33 | 24.58 | 3.28 | 5.88 | 5 | 5 | 0.063 |
| SPL IgM/IgD | B220 | 92.75 | 93.44 | 1.85 | 2.01 | 5 | 5 | 0.627 |
| LN IgM/IgD | B220 | 91.48 | 90.58 | 3.29 | 4.73 | 5 | 5 | 0.761 |
| SPL NK | IL-2Rb + CD3 − | 4.98 | 3.50 | 1.36 | 0.30 | 5 | 5 | 0.067 |
| BM NK | IL-2Rb + CD3− | 4.47 | 5.22 | 1.19 | 2.27 | 7 | 7 | 0.489 |
| BM PMN | GR-1 + CD11b+ | 44.11 | 45.72 | 7.60 | 7.99 | 7 | 7 | 0.727 |
| SPL PMN | GR-1 + CD11b+ | 1.72 | 1.15 | 0.70 | 0.42 | 5 | 5 | 0.206 |
BM, bone marrow; LN, lymph node; NK, natural killer cell; PMN, polymorphonuclear cell (neutrophil); SPL, spleen; THY, thymus. Data shown are means ± SD of three independent experiments with a total of at least five mice per genotype.
Lipocalin 2 Is Not Essential for Apoptosis.
Lipocalin 2 has been strongly implicated as a proapoptotic factor. For example, in FL5.12 cells, an IL-3-dependent pro-B cell line, IL-3 withdrawal induced lipocalin 2 expression and secretion leading to apoptosis induction (10). To further investigate the involvement of lipocalin 2 in apoptosis, we isolated IL-3-dependent primary bone marrow mast cells from the femurs of Lcn2−/− and WT mice and cultured them in complete medium containing 3 ng/ml recombinant IL-3. Upon IL-3 withdrawal, both Lcn2−/− and WT cells underwent apoptosis in a time-dependent manner at a comparable rate (Fig. 1A). After 96 h, 29% and 39% of cells remained viable in Lcn2−/− and WT cultures, respectively (Fig. 1A). These data suggest that lipocalin 2 is not essential for apoptosis induced by IL-3 withdrawal.
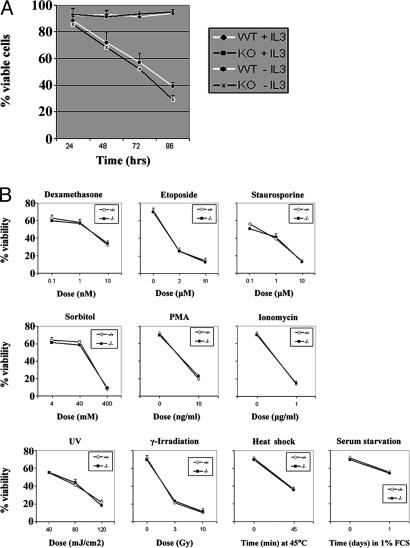
Lipocalin 2-deficient cells respond normally to IL-3 withdrawal and a variety of apoptotic stimuli. (A) Cultured bone marrow cells from WT and Lcn2−/− littermates were subjected to IL-3 withdrawal, and the percentage of viable cells remaining was measured by annexin–propidium iodide staining at the indicated time points. Results shown are the mean percentage of viable cells (±SD) of three independent experiments involving at least two mice for each genotype. (B) Thymocytes from WT and Lcn2−/− littermate mice were treated with the indicated chemical and physical stimuli, and cell viability was assessed by annexin–propidium iodide staining after 24 h.
We next examined a range of chemical and physical agents to determine whether lipocalin 2 might be involved in programmed cell death only in response to certain apoptotic stimuli. Thymocytes treated with dexamethasone, etoposide, staurosporine, sorbitol, PMA, or ionomycin underwent normal apoptosis (Fig. 1B). Apoptosis induced by UV-irradiation, γ-irradiation, heat shock, or serum starvation was also indistinguishable between Lcn2−/− and WT thymocytes (Fig. 1B). Thus, despite the reported involvement of lipocalin 2 in programmed cell death, loss of lipocalin 2 has no effect on the apoptosis of these cell types under these conditions.
Lipocalin 2 Has an Important Role in Innate Immunity in Vitro and in Vivo.
To test the function of lipocalin 2 in the innate immune response in vivo, we used a murine model of an acute lethal bacterial infection. Lcn2−/− and WT littermates were i.p. challenged with a lethal dose of Escherichia coli [ATCC 25922; 1.5 × 108 colony-forming units (cfu)/30 g], and the survival rates of the mice were compared (Fig. 2A). Lcn2−/− mice showed a significant acceleration of lethality such that 74% (14 of 19) of the knockout mice had died after 16 h compared with 21% (5 of 24) of the WT controls. Only 21% of the Lcn2−/− mice survived and recovered completely from the infection, whereas 54% of the WT mice recovered (P < 0.005; log-rank test).

Antibacterial effects of lipocalin 2 in vivo and in vitro. (A) Lipocalin 2 protects against bacterial infection in vivo. Shown are survival curves of Lcn2−/− and WT mice that were i.p. injected with 1.5 × 108 cfu/30 g of E. coli (ATCC 25922). Results shown are the percentage survival from three independent experiments of a total of 19 Lcn2−/− mice and 24 WT mice (P > 0.005; log-rank test). (B) Lcn2−/− neutrophils exhibit impaired bacteriostatic activity in vitro. Neutrophils isolated from Lcn2−/− mice (black bars) and WT littermates (white bars) were incubated with E. coli at a multiplicity of infection of 1. Gray bars indicate cultures of bacteria and WT neutrophils to which 50 μM iron was added. Bacterial growth was determined at the indicated time points as described in Materials and Methods. Data shown are the mean cfu of bacteria (±SD) of triplicate measurements from three independent experiments. Significant differences in bacterial growth between WT and Lcn2−/− cultures were found at 30 min ( , P < 0.001) and at 60 and 120 min (
, P < 0.001) and at 60 and 120 min (
 , P < 0.0002).
, P < 0.0002).
Several reports have indicated that lipocalin 2 mediates a bacteriostatic effect by sequestering iron that has associated with bacterial siderophores (6, 15). To further analyze this mechanism, we isolated neutrophils from Lcn2−/− and WT littermates and incubated them in cultures to which E. coli was added at a multiplicity of infection of 1. The in vitro bacteriostatic effects of Lcn2−/− and WT neutrophils were then compared. As shown in Fig. 2B, the number of viable bacteria remaining in the cultures of Lcn2−/− neutrophils was significantly higher than the number of viable bacteria remaining in WT cultures (P < 0.001 at 30 min, and P < 0.0002 at 60 min and 120 min). After 120 min of incubation, we measured a 2.5-fold increase in the number of bacteria in the Lcn2−/− neutrophil cultures compared with the WT. This striking difference in bacterial growth was completely abolished by the addition of 50 μM iron to the cultures containing WT neutrophils and E. coli (Fig. 2B).
Lipopolysaccharides are characteristic components of the cell walls of Gram-negative bacteria and are the major causative agent of sepsis. We therefore examined whether the difference in survival after bacterial infection is due to an altered response to LPS. Although the reaction of Lcn2−/− mice to septic shock induced by i.p. injection of 50 mg/kg LPS was delayed, there was no significant difference in overall mortality in Lcn2−/− mice compared with WT littermates (data not shown). We also analyzed the levels of TNF-α, IL-6, IL-12, and IFN-γ in the sera of LPS-treated WT and Lcn2−/− mice and could not detect any differences in the levels of any of these cytokines (data not shown).
Taken together, these data clearly indicate that lipocalin 2 has an essential role in the antibacterial innate immune response, and that this function can be abrogated by an increase in the local iron concentration. Our results confirm that the sequestration of iron is likely the primary function of lipocalin 2 during bacterial infections.
Lipocalin 2 Deficiency Does Not Enhance ATN.
Because it has been reported that administration of recombinant lipocalin 2 significantly decreases the extent of renal injury after ischemia-reperfusion injury, we hypothesized that mice lacking the protective effect of lipocalin 2 would have a more severe response to ischemic renal injury. We therefore subjected Lcn2−/− mice and WT littermate controls to a common model of renal damage (14). The renal pedicle was clamped for 30 min, and the contralateral kidney was removed. After 24 h of reperfusion, the serum creatinine level rose from 0.22 ± 0.05 mg/dl (n = 5) in sham-operated mice to 2.26 ± 0.34 mg/dl in WT mice and to 2.36 ± 0.27 mg/dl in Lcn2−/− mice (n = 7; P > 0.5, for WT vs. lipocalin 2−/−) showing no significant difference between Lcn2−/− mice and WT mice (Fig. 3A). Similar results were obtained by measurement of blood urea nitrogen, which rose from 26 ± 8 mg/dl in sham-operated mice (n = 5) to 167 ± 25 mg/dl in WT mice and 202 ± 31 mg/dl in Lcn2−/− mice (n = 7; P > 0.05, for WT mice vs. Lcn2−/− mice), resulting in no significant difference between Lcn2−/− mice and WT controls (Fig. 3B). Similarly severe kidney damage was also observed in the histological analysis (Fig. 3C). No significant difference was measurable after scoring of the sections according to the Jablonski scale (Fig. 3D). One important event of ischemic kidney damage is tubule cell apoptosis. We therefore counted the percentage of tubules with at least one transferase-mediated dUTP nick-end labeling (TUNEL)-positive cell, 24 h after reperfusion. Ischemic WT mice showed that 10.3 ± 3.5% of cortical tubules had TUNEL-positive cells compared with 10.5 ± 4.0% in Lcn2−/− mice (n = 7; P > 0.9), again showing no significant difference between Lcn2−/− mice and WT controls (Fig. 3E). Taken together, these results demonstrate that, under the conditions reported here, the lack of endogenous lipocalin 2 does not enhance the severity of ischemic kidney damage.

Lipocalin 2 deficiency does not increase kidney damage after ischemia-reperfusion injury. In a murine model for acute tubular necrosis (ATN), the renal pedicle was clamped for 30 min and the contralateral kidney was removed. (A) After 24 h of reperfusion, the serum creatinine level rose from 0.22 ± 0.05 mg/dl (n = 5) in sham-operated mice to 2.26 ± 0.34 mg/dl in WT mice and to 2.36 ± 0.27 mg/dl in Lcn2−/− mice (n = 7; P > 0.5, for WT vs. Lcn2−/−), demonstrating no difference. (B) Similarly, blood urea nitrogen levels showed no significant difference between Lcn2−/− mice and WT mice, rising from 26 ± 8 mg/dl in sham-operated mice (n = 5) to 167 ± 25 mg/dl in WT mice and 202 ± 31 mg/dl in Lcn2−/− mice (n = 7; P > 0.05, for WT mice vs. Lcn2−/− mice). (C) Ischemic kidneys in both Lcn2−/− and WT mice showed similar loss of tubular nuclei (ATN, Bottom) and an equal presence of cortical and medullary intratubular casts (ATN, Middle). (D) The area of the proximal convoluted tubular necrosis was evaluated by the Jablonski scale, which also demonstrated no significant difference (0, no necrosis; 1, isolated necrosis; 2, focal necrosis in inner cortex; 3, diffuse necrosis in inner cortex; 4, necrosis involving whole cortex). (E) Percentage of apoptotic tubules containing at least one apoptotic nucleus. Ischemia-reperfusion injury resulted in a similar increase of apoptotic tubules in Lcn2−/− and WT mice.
Discussion
Targeted disruption of the murine lipocalin 2 gene allowed us to analyze the role of lipocalin 2 in the innate immune response against bacterial infection. In this report, we show that lipocalin 2 has an essential bacteriostatic role under iron-limiting conditions, and that it acts as an iron-depleting factor in the innate immune response against enterobacteria.
Pathogenic microorganisms (including E. coli) require iron to survive in human or animal hosts. Although the tissues of human and animal hosts contain plenty of iron, the amount available to a microorganism may be extremely limited because most of this iron is bound either intracellularly by heme and ferritin or extracellularly by transferrin and lactoferrin. As a result, free iron is generally present in mammalian tissues at a concentration below that required to support the growth of E. coli. To remedy this difficulty, E. coli (like many potential pathogens) secretes siderophores under conditions of iron limitation. The high-affinity iron-binding capability of siderophores permits them to remove iron from host iron-binding proteins and transport it into the bacterial cell via specific receptors (16). Siderophores produced by E. coli include the phenolate iron chelator enterobactin (also known as enterochelin) and aerobactin 17–19. Both are produced by most pathogenic E. coli strains (20, 21). The genes for aerobactin biosynthesis can also be plasmid-encoded, and the presence of such plasmids in E. coli has been associated with virulent extraintestinal infections.
Our results have shown that isolated Lcn2−/− neutrophils incubated in vitro with Gram-negative bacteria were significantly less able to inhibit bacterial growth compared with WT neutrophils. This effect depended on the iron level in the incubation medium. Addition of iron to cultures containing WT neutrophils and bacteria abolished the bacteriostatic effect and allowed an increase in bacterial number similar to that reached in cultures containing Lcn2−/− neutrophils and bacteria. We then verified the involvement of lipocalin 2 in the innate immune response to Gram-negative bacterial infections in vivo. Lcn2−/− mice challenged i.p. with Gram-negative bacteria showed an accelerated and increased mortality rate compared with WT littermates. Our findings are consistent with previous reports demonstrating that lipocalin 2 acts as a bacteriostatic factor preventing bacterial siderophore-mediated iron acquisition (6, 15).
Compared with a recent study by Flo et al. (6), our results show a less dramatic difference in survival between WT and Lcn2−/− mice after i.p. E. coli infection. This discrepancy may be explained by the fact that the E. coli strain used in our study (ATCC 25922) not only produces enterochelin but also aerobactin and the aerobactin receptor complex (data not shown). Lipocalin 2 is unable to bind to aerobactin, so that the E. coli used in our study may have acquired low amounts of iron by means of this siderophore. In contrast, the E. coli strain used by Flo et al. (6) produces only enterochelin and not aerobactin. Nevertheless, our results reinforce the importance of lipocalin 2 in sequestering iron out of the reach of pathogenic enterobacteria that produce multiple classes of siderophores.
We used a murine model of acute renal injury to investigate the role of lipocalin 2 as a factor ameliorating ischemia-induced kidney damage. Against our initial hypothesis, we could not show any significant difference in the extent of kidney damage observed between Lcn2−/− mice and WT mice. We analyzed the serum creatinine and blood urea nitrogen levels, the percentage of apoptotic tubules, and the histological damage in the kidney 24 h after reperfusion. None of these parameters showed a significant difference between Lcn2−/− and WT mice. These data are in contrast to two published reports (13, 14) showing that recombinant protein administered up to 1 h after reperfusion rescues the kidney from ischemia-reperfusion injury. A possible explanation could be the kinetics involved in the expression of endogenous lipocalin 2 after ischemia-reperfusion injury. We could detect endogenous lipocalin 2 at the earliest time point 3 h after reperfusion (data not shown). In contrast, recombinant lipocalin 2 was only effective when given within 1 h of reperfusion; it therefore seems likely that the up-regulation of lipocalin 2 after severe ischemia-reperfusion injury is too late to have a significant protective effect. Nevertheless, our results do not exclude the possibility that a less severe kidney damage induced by a shorter ischemia time and a prolonged reperfusion time will show a more pronounced and prolonged ischemic damage in Lcn2−/− mice.
Lipocalin 2 has also been implicated in the induction of apoptosis (10). However, our results clearly show that lipocalin 2 deficiency does not affect apoptosis induced by a broad range of stimuli. We found that Lcn2−/− and WT primary bone marrow cells underwent apoptosis at a similar rate in response to IL-3 withdrawal, as did Lcn2−/− and WT thymocytes treated with a variety of proapoptotic stimuli. We therefore conclude that lipocalin 2 does not have an essential role in apoptosis induction, at least not for the stimuli and cell types tested here.
Another postulated function for lipocalin 2 is the induction of epithelium formation in the embryonic kidney (22). However, we have shown in vivo that lipocalin 2 does not have an obligatory role in mouse embryonic kidney development. The gross phenotype of Lcn2−/− mice and the Mendelian distribution of F2 offspring from heterozygous F1 crosses immediately suggested that organogenesis and development to adulthood are normal in Lcn2−/− mice. We nevertheless analyzed kidney development in WT and Lcn2−/− embryos from embryonic day 10.5 onwards but observed no differences. Our data suggest that other molecules may be able to substitute for lipocalin 2 in the iron delivery pathway operating during embryonic kidney development.
Lipocalin 2 is secreted into the uterine fluid during the proestrous phase of mice. There have been reports implicating lipocalin 2 as an enhancer of sperm motility (23), through the delivery and internalization of ferric ion into mouse spermatozoa (24). Consistent with these findings, we observed that female Lcn2−/− mice were delayed in litter-bearing. It is possible that a lipocalin 2-free environment in the uterus reduces sperm motility, resulting in the apparent decreased fertility of our female Lcn2−/− mice.
In summary, targeted disruption of the murine lipocalin 2 gene has demonstrated its essential role in the early stages of the innate immune response to bacterial infection. An absence of lipocalin 2 readily leads to death of the host because of loss of bacteriostatic action and accelerated bacterial growth. Our data have important implications for improvements to clinical treatment of bacterial infections.
Materials and Methods
ES Cell Culture.
E14K ES cells from 129/Ola mice were maintained on a layer of mitomycin C-treated mouse embryonic fibroblasts (MEFs) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with leukemia inhibitory factor, 15% heat-inactivated FCS (HyClone), l-glutamine, and 2-mercaptoethanol.
Generation of Lcn2−/− Mice.
The targeting strategy used is outlined in Fig. 4A. A genomic DNA clone containing exons 1–6 of the murine Lcn2 gene was isolated from a 129/J mouse genomic library. A targeting vector was designed to replace a 2.5-kb genomic fragment containing Lcn2 exons 1–5 with the PGK-neo cassette. The diphtheria toxin A gene (DT-A) driven by the pMC1 promoter was incorporated into the 3′ end of the vector to allow for negative selection (25). The targeting vector was linearized with NotI and electroporated into 129/Ola ES cells (Bio-Rad Gene Pulser; 0.34 kV, 250 μF). The transfected cells were then cultured in the presence of 300 μg/ml G418 (Sigma-Aldrich) for 10 days. Homologous recombinants were identified by PCR and verified by Southern blot with a PCR-generated 5′ flanking probe (primers: 5′-ATAGCCCTGGCTGTCCTGAA-3′ and 5′-TAAGGTCCCCCTCTAAACC-3′). Correctly targeted clones were injected into C57BL/6J blastocysts, and the generation of chimeras was performed as described in ref. 26. Two independently targeted ES cell clones transmitted the Lcn2 mutation into the germ line. Male chimeric mice were mated to C57BL/6 female mice, and the heterozygous F1 progeny were intercrossed to generate lipocalin 2-deficient mice. The WT littermates from these crosses were used as controls for all experiments.
All mice were maintained in the animal facility of the Ontario Cancer Institute in accordance with established ethical care regulations of the Canadian Council in Animal Care.
Analysis of Lcn2 Genotypes.
Genotypes of mutant mice and embryos were determined by PCR and confirmed by Southern blot analysis of genomic DNA from tail biopsy samples as described in ref. 27 with the external 5′ probe as depicted in Fig. 4A. For PCR, primers a and b (see Fig. 4A), which are specific for an Lcn2 intron sequence located 5′ and 3′ of the deleted exon 2, were used to detect the WT Lcn2 allele. Primer c, specific for an Lcn2 5′ intron sequence, and primer d, specific for Neo, were used to detect the mutant Lcn2 allele. Primer sequences were as follows: a, 5′-GGT TTG GTG GCA GGC TAT TA-3′; b, 5′-CAG AGT GGC TTT CCC CAT AA-3′; c, 5′-TGC TCC TGC CGA GAA AGT AT-3′; d, 5′-CTC TTC CTC CTC CAG CAC AC-3′.
Immune System Cell Populations.
Populations of thymocytes, splenocytes, lymph node cells, and bone marrow cells in mice were identified by staining with fluorochrome-labeled primary antibodies and quantitated by flow cytometry using a FACSCalibur FACS machine and cellquest software (Becton Dickinson).
Apoptosis in Primary Bone Marrow Cells and Thymocytes.
Bone marrow cells were prepared from femurs of WT or Lcn2−/− mice and cultured for 14 days in OptiMEM medium containing 10% FCS and 3 ng/ml recombinant IL-3 (R & D Systems). The nonadherent cells were transferred into a new culture flask every 2 days for the first 6 days. To assay apoptosis, the cells were washed twice in PBS to remove any residual IL-3, resuspended at 0.5 × 106 cells per ml in fresh IL-3-free medium, and cultured for 24–96 h in the presence or absence of exogenous IL-3 (3 ng/ml). At each time point, the cells were stained with Annexin V (BD Bioscience) plus propidium iodide and analyzed by flow cytometry.
Thymocytes were prepared from WT or Lcn2−/− mice by standard procedures (28), plated at 1 × 106 cells per ml in 24-well plates in RPMI medium 1640 containing 10% FCS, and treated as shown in Fig. 1B. Cell viability was determined after 24 h as for bone marrow cells. All chemicals were purchased from Sigma-Aldrich.
Isolation and Purification of Neutrophils.
Isolation of peritoneal exudate cells (PECs) after casein injection was carried out as described in ref. 29. Neutrophils were purified by discontinuous Percoll gradient centrifugation as described in ref. 30. Neutrophil purity was typically >93%, as judged by flow cytometric analysis of CD11b- and Gr-1-positive cells (data not shown).
Neutrophil Bacteriostatic Activity.
The bacteriostatic activity of isolated neutrophils was determined by an in vitro killing assay as described in ref. 31. Briefly, purified neutrophils (5 × 105) were resuspended in 1 ml of warmed PBS containing 10% fresh serum obtained from syngeneic mice and 0.1% gelatin. These cells were mixed with log phase E. coli (ATCC 25922) in culture tubes coated with silicon (Sigma-Aldrich). The ratio of bacteria to neutrophils was adjusted to 1:1 (multiplicity of infection of 1). The culture tubes were incubated in a shaking water bath at 37°C for 2 h. Triplicate samples were removed for analysis at 0, 30, 60, and 120 min of incubation. The number of viable bacteria left in each sample was determined by plating 0.1 ml of an appropriate dilution of the sample on LB plates. The results were expressed as the mean cfu/ml (±SD) of triplicate cultures.
Bacterial Infection.
Log-phase E. coli (ATCC 25922) were washed, resuspended in 0.5 ml of PBS to the desired concentration, and i.p. injected into Lcn2−/− and WT littermate mice. Each mouse received 1.5 × 108 cfu E. coli per 30 g of body weight. Injected mice were observed twice daily and were not left alone for >3–4 h during survival experiments. The log-rank test was used for statistical analysis of the survival curve.
Embryonic Kidney Histology.
Embryos and tissues were dissected on ice, fixed with 4% paraformaldehyde (PFA), paraffin-embedded, and sectioned according to standard procedures. Hematoxylin/eosin staining was performed as described in ref. 32.
Mouse Model of Renal Ischemia-Reperfusion Injury.
Male lipocalin 2-deficient mice and WT control littermates (25–30 g) were anesthetized with i.p. pentobarbital (50 mg/kg) and placed on a warming table to maintain 37°C core body temperature. The kidneys were exposed through an abdominal section, and the left kidney was removed after ligation of its vascular pedicle and ureter. The vascular pedicle of the right kidney was clamped with a nontraumatic vascular clamp (Roboz Surgical Instrument, Rockville, MD) for 30 min, during which time the kidney was kept warm and moist. The clamp was then removed, the kidney was observed for return of blood flow, and the incision was sutured with 5-0 nylon. After 24 h of reperfusion, heparinized blood was obtained for measurement of serum creatinine and urea (Vita-Tech, Markham, ON, Canada). The kidney was harvested, and sagittal sections were fixed in 4% formalin. Paraffin-embedded sections (4 μm) were stained with hematoxylin/eosin or by in situ end-labeling of fragmented DNA for detection of apoptotic nuclei and examined histologically.
Histopathology Scoring.
Hematoxylin/eosin-stained kidney sections were scored for histopathologic damage to the tubules in a blinded manner. The area of proximal convoluted tubular necrosis was evaluated by the Jablonski scale (33) ranging from 0 to 4, with no necrosis (0), isolated necrotic cells (1), focal necrosis in inner cortex (2), diffuse necrosis in inner cortex (3), and necrosis involving the whole cortex (4).
Apoptosis Assay.
The extent of apoptosis in kidney sections was determined by the TUNEL assay (34). TUNEL-positive apoptotic cells were detected by intense staining of the nucleus. Only cells that displayed additional characteristic morphological features of apoptosis, such as nuclear condensation and fragmentation were counted as apoptotic. TUNEL-positive cells that did not display any morphological apoptotic features were not considered apoptotic. Slides were analyzed in a blinded manner, and apoptosis was quantified by counting the number of TUNEL-positive nuclei per 100 cells counted in an average of 10 high-power fields (×40) in each section.
Acknowledgments
We thank M. Nilsen-Hamilton (Iowa State University, Ames) for providing the Lcn 2 antibody; J. Dembowy for technical assistance; and M. Saunders for scientific editing. T.B. is the recipient of a postdoctoral fellowship from the Cancer Research Institute, New York. C.C.C. is supported by Canadian Institutes of Health Research.
Footnotes
Conflict of interest statement: No conflicts declared.
Freely available online through the PNAS open access option.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.0510847103
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/103/6/1834.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101940555
Article citations
Lipocalin-2 drives neuropsychiatric and cutaneous disease in MRL/lpr mice.
Front Immunol, 15:1466868, 27 Sep 2024
Cited by: 0 articles | PMID: 39399497 | PMCID: PMC11466786
Interrelationships among metabolic syndrome, bone-derived cytokines, and the most common metabolic syndrome-related diseases negatively affecting bone quality.
Diabetol Metab Syndr, 16(1):217, 06 Sep 2024
Cited by: 0 articles | PMID: 39238022 | PMCID: PMC11378428
Review Free full text in Europe PMC
Evaluation of diagnostic accuracy of urine neutrophil gelatinase-associated lipocalin in patients with symptoms of urinary tract infections: a meta-analysis.
Front Pediatr, 12:1368583, 22 May 2024
Cited by: 0 articles | PMID: 38840804
Review
Loss of SREBP-1c ameliorates iron-induced liver fibrosis by decreasing lipocalin-2.
Exp Mol Med, 56(4):1001-1012, 16 Apr 2024
Cited by: 1 article | PMID: 38622198 | PMCID: PMC11058876
Structure, Functions, and Implications of Selected Lipocalins in Human Disease.
Int J Mol Sci, 25(8):4290, 12 Apr 2024
Cited by: 1 article | PMID: 38673873 | PMCID: PMC11050150
Review Free full text in Europe PMC
Go to all (312) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2.
Proc Natl Acad Sci U S A, 103(44):16502-16507, 23 Oct 2006
Cited by: 193 articles | PMID: 17060628 | PMCID: PMC1637611
Lipocalin 2 is protective against E. coli pneumonia.
Respir Res, 11:96, 15 Jul 2010
Cited by: 60 articles | PMID: 20633248 | PMCID: PMC2912245
Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron.
Nature, 432(7019):917-921, 07 Nov 2004
Cited by: 1124 articles | PMID: 15531878
Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia.
Curr Opin Nephrol Hypertens, 15(4):442-449, 01 Jul 2006
Cited by: 138 articles | PMID: 16775460
Review




