Abstract
Free full text

Growth differentiation factor 11 signals through the transforming growth factor-β receptor ALK5 to regionalize the anterior–posterior axis
Abstract
Growth differentiation factor 11 (GDF11) contributes to regionalize the mouse embryo along its anterior–posterior axis by regulating the expression of Hox genes. The identity of the receptors that mediate GDF11 signalling during embryogenesis remains unclear. Here, we show that GDF11 can interact with type I receptors ALK4, ALK5 and ALK7, but predominantly uses ALK4 and ALK5 to activate a Smad3-dependent reporter gene. Alk5 mutant embryos showed malformations in anterior–posterior patterning, including the lack of expression of the posterior determinant Hoxc10, that resemble defects found in Gdf11-null mutants. A heterozygous mutation in Alk5, but not in Alk4 or Alk7, potentiated Gdf11−/−-like phenotypes in vertebral, kidney and palate development in an Acvr2b−/− background, indicating a genetic interaction between the two receptor genes. Thus, the transforming growth factor-β (TGF-β) receptor ALK5, which until now has only been associated with the biological functions of TGF-β1 to TGF-β3 proteins, mediates GDF11 signalling during embryogenesis.
Introduction
The transforming growth factor-β (TGF-β) superfamily comprises a large group of structurally related ligands with pleiotropic functions. Many TGF-β superfamily members have key roles in patterning processes during embryogenesis (Chang et al, 2002). A large body of in vitro work has been devoted to the characterization of TGF-β superfamily signalling through heteromeric complexes of type I and type II receptors. Their signalling specificity in vivo, however, remains largely unknown (Chang et al, 2002; Shi & Massague, 2003). Type I receptors act as the main intracellular effectors of TGF-β superfamily signalling by the phosphorylation of different sets of Smad proteins. Although more than 30 ligands are known in the TGF-β family, only seven type I receptors (ALK1–7) have been identified. ALK4, ALK5 and ALK7 activate Smad2 and Smad3 in response to activin, TGF-β and Nodal ligands. Conversely, ALK1, ALK2, ALK3 and ALK6 phosphorylate Smad1, Smad5 and Smad8 in response to TGF-β and bone morphogenetic protein (BMP) ligands.
Growth differentiation factor 11 (GDF11) is a member of the TGF-β superfamily that has been shown to regulate several patterning events, including anterior–posterior regionalization, kidney development and closure of the palate (Gad & Tam, 1999; McPherron et al, 1999; Esquela & Lee, 2003). Previous biochemical experiments have demonstrated that GDF11 can activate Smad2 in ectodermal explants from Xenopus embryos (Oh et al, 2002), suggesting the involvement of ALK4, ALK5 or ALK7 receptors in GDF11 signalling. In agreement with this, GDF11 was also shown to be capable of interacting with ALK4 when coexpressed with either Acvr2 (also known as ActRIIA) or Acvr2b (also known as ActRIIB; Oh et al, 2002). The possible roles of these type I receptors were not tested in that study, and the identity of the type I receptors that mediate GDF11 signalling in vivo remains unknown. In this work, we set out to investigate GDF11 signalling and ligand–receptor interactions to determine which type I receptor—that is, ALK4, ALK5 or ALK7—mediates GDF11 signalling both in vitro and in vivo.
Results and Discussion
We first characterized GDF11 signalling in vitro in receptor binding and reporter gene assays. Soluble Fc-fusion proteins of the extracellular domains of ALK4, ALK5, ALK7 and Acvr2b were incubated with haemagglutinin (HA)-tagged GDF11 and used in pull-down assays. GDF11 could only bind directly to Acvr2b but not to any type I receptor (Fig 1A). Several TGF-β superfamily ligands engage in a complex with a cognate type I receptor only after they have bound to a type II receptor (Shi & Massague, 2003). We therefore crosslinked 125I-labelled GDF11 to COS cells that had been transfected with different combinations of HA-tagged ALK4, ALK5 or ALK7, together with Acvr2b receptors. Robust binding of 125I-GDF11 to all three type I receptors was observed in the presence but not in the absence of co-transfected Acvr2b (Fig 1B), indicating that GDF11 can interact with ALK4, ALK5 and ALK7 in an Acvr2b-dependent manner. We then examined the ability of GDF11 to elicit intracellular signals through distinct receptors in receptor reconstitution experiments using the Smad3-dependent gene reporter CAGA-Luc. To examine which type II receptors are able to mediate GDF11 signalling, we used HepG2 cells, which are highly sensitive to addition of type II receptors and endogenously express ALK4 and ALK5 (Reissmann et al, 2001). GDF11 had a modest effect on CAGA-Luc activity in these cells (Fig 2A). Transfection of Acvr2b greatly increased responsiveness to GDF11, whereas the TGF-β type II receptor and BMP type II receptor did not affect GDF11 signalling (Fig 2A). An increase in reporter activity could also be seen after transfection of Acvr2, which is consistent with previous studies (Oh et al, 2002). To investigate which type I receptors are able to mediate GDF11 signalling, we used R4-2 cells, which express low levels of type I receptors. As expected, TGF-β1 activated the CAGA-Luc reporter only in cells that received ALK5, whereas Xnr-1 (Xenopus nodal related 1) did so in cells that received either ALK4 or ALK7 together with the Nodal co-receptor Cripto (Fig 2B). Although GDF11 could generate signals through all three type I receptors, it was significantly more potent when either ALK4 or ALK5 was expressed (Fig 2B). We further analysed GDF11 signalling by combinations of type I and Acvr2 receptors in R4-2 cells (Fig 2C). Both Acvr2 and Acvr2b were able to potentiate GDF11 signalling through ALK4, ALK5 or ALK7 in these cells, indicating that these type I receptors might use either Acvr2 or Acvr2b receptors to transmit GDF11 signalling in transfected cells. Together, these experiments demonstrated that GDF11 can use type II receptors Acvr2 and Acvr2b and the type I receptors ALK4 and ALK5 to mediate intracellular signalling.
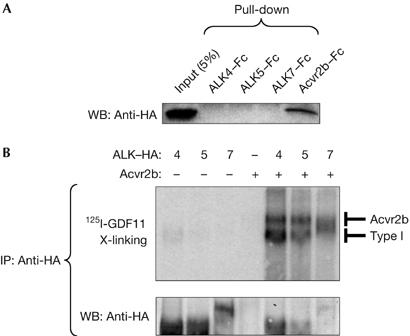
GDF11 binding to Acvr2b and type I receptors ALK4, ALK5 and ALK7. (A) Pull-down assay of haemagglutinin (HA)-tagged GDF11 with soluble Fc-fusion proteins of Acvr2b, ALK4, ALK5 and ALK7. The first lane in the western blot (WB) corresponds to 5% input of HA-tagged GDF11 (50 μl conditioned media). (B) Crosslinking binding assay in COS cells transfected with the indicated constructs and incubated with 125I-GDF11. After crosslinking, receptor complexes were immunoprecipitated (IP) with anti-HA antibodies. The lower panel shows reprobing with anti-HA antibodies. GDF11, growth differentiation factor 11.
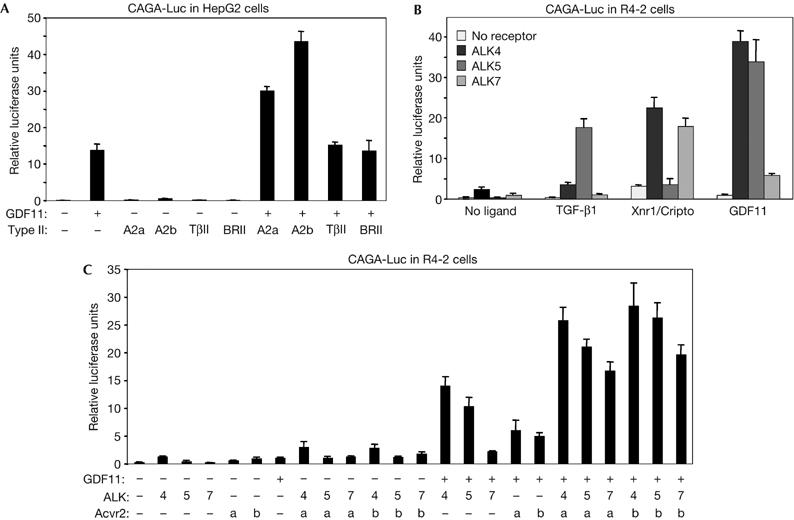
Characterization of GDF11 signalling through type I and type II receptors. Gene reporter assays in (A) HepG2 and (B,C) R4-2 cells. The results are relative luciferase activity of triplicate determinations ±s.d. A2a, Acvr2; A2b, Acvr2b; BRII, bone morphogenetic protein type II receptor; GDF11, growth differentiation factor 11; TβII, transforming growth factor-β type II receptor.
Inactivation of the Gdf11 gene in mice leads to defects in anterior–posterior patterning and in kidney and palate development (McPherron et al, 1999). To identify the type I receptors that mediate GDF11 signalling in vivo, we investigated the occurrence of Gdf11-null phenotypes in mice that carry inactivating mutations in the Alk4, Alk5 or Alk7 genes. Unfortunately, the early embryonic lethality of Alk4−/− and Alk5−/− homozygous mutants prevented us from directly assessing phenotypes in these mice (Gu et al, 1998; Larsson et al, 2001; Seki et al, 2006). However, we hypothesized that haploinsufficiency of either Alk4 or Alk5, which per se results in no visible defects, might potentiate the phenotypes observed in Acvr2b−/− mice, which show malformations that are similar to, but less severe than, those seen in Gdf11−/− mutants (Oh & Li, 1997). Unlike Acvr2b−/− mutants, Acvr2−/− animals show no overt abnormalities in axial patterning (Oh et al, 2002), which suggests a less prominent role for this receptor in GDF11 signalling in vivo. Although Alk7−/− single mutants are viable, fertile and do not show any axial patterning malformations (Jörnvall et al, 2004), Alk7−/−;Acvr2b−/− double knockouts were also generated and analysed.
Wild-type mice normally have 26 presacral vertebrae in a characteristic pattern that comprises seven cervical (C), 13 thoracic (T) and six lumbar (L) vertebrae, herein referred to as C7/T13/L6. By contrast, and owing to defects in anterior–posterior axis formation, most Gdf11−/− mutants have 33 presacral vertebrae in a C7/T18/L8 pattern (McPherron et al, 1999). Conversely, Acvr2b−/− mice most often showed 29 presacral vertebrae in a C7/T16/L6 pattern (Fig 3A; Table 1), consistent with what has previously been described (Oh & Li, 1997). Interestingly, the severity of this phenotype was potentiated in Alk5+/−;Acvr2b−/− mutants, in which the number of presacral vertebrae were increased to 30 (Table 1). Potentiation of this homeotic transformation contributed to an increase in either thoracic or lumbar segments, which manifested as either C7/T16/L7 or C7/T17/L6 vertebral patterns in the compound mutants (Fig 3B; Table 1). The anterior transformation was most clear at the thoracic level, at which there was an increased number of ribs. Thus, whereas wild-type mice typically presented seven vertebrosternal ribs—that is, the ribs that articulate the vertebral column with the sternum—Acvr2b−/− mutants most often had nine vertebrosternal ribs and Alk5+/−;Acvr2b−/− mutants had ten (Fig 3C,D; Table 1). In addition, the incidence of a related Gdf11−/− phenotype—that is, fusion of the two most anterior thoracic ribs (T1/T2 fusion)—was also augmented in Alk5+/−;Acvr2b−/− compound mutants (83%, n=24) compared with Acvr2b−/− single mutant littermate (57%, n=33). In contrast to these observations, neither Alk4+/−;Acvr2b−/− nor Alk7−/−;Acvr2b−/− compound mutants showed any enhanced homeotic transformation compared with Acvr2b−/− single mutant littermate (Table 1), which suggests that these type I receptors do not have a role in vertebral column patterning. Together, these data indicate that ALK5 is important in anterior–posterior skeletal patterning and support a role for this type I receptor in mediating GDF11 activities in vivo.
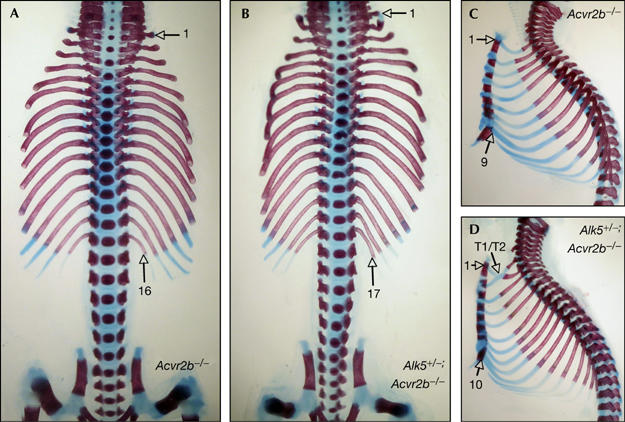
Genetic interaction between Acvr2b and Alk5 during vertebral patterning. Representative skeleton preparations of (A,C) Acvr2b−/− and (B,D) Alk5+/−;Acvr2b−/− littermate mutants in frontal (A,B) and lateral (C,D) views. The numbers of thoracic vertebrae (A,B) and vertebrosternal ribs (C,D) are indicated. T1/T2 denotes fused first and second ribs.
Table 1
Analysis of skeletal patterning in compound mutants
| Genotype | ||||||
|---|---|---|---|---|---|---|
| Alk4+/+;Acvr2b−/− | Alk4+/−;Acvr2b−/− | Alk5+/+;Acvr2b−/− | Alk5+/−;Acvr2b−/− | Alk7+/+;Acvr2b−/− | Alk7−/−;Acvr2b−/− | |
| Number of presacral vertebrae | ||||||
| 28 | 3/33 (9%) | 2/25 (8%) | 2/22 (9%) | |||
| 29 | 17/17 (100%) | 16/16 (100%) | 29/33 (88%) | 1/24*** (4%) | 23/25 (92%) | 20/22 (91%) |
| 30 | 1/33 (3%) | 20/24*** (83%) | ||||
| 31 | 3/24 (13%) | |||||
| Vertebral patterning | ||||||
| C7/T16/L5 | 3/33 (9%) | 2/25 (8%) | 2/22 (9%) | |||
| C7/T16/L6 | 16/17 (94%) | 16/16 (100%) | 28/33 (85%) | 1/24 (4%) | 23/25 (92%) | 20/22 (91%) |
| C7/T16/L7 | 7/24 (29%) | |||||
| C7/T17/L5 | 1/17 (6%) | 1/33 (3%) | ||||
| C7/T17/L6 | 1/33 (3%) | 13/24 (54%) | ||||
| C7/T17/L7 | 3/24 (13%) | |||||
| Number of vertebrosternal ribs | ||||||
| 9 | 16/17 (94%) | 14/16 (88%) | 26/33 (79%) | 2/24*** (8%) | 24/25 (96%) | 22/22 (100%) |
| 10 | 1/17 (6%) | 2/16 (12%) | 7/33 (21%) | 22/24*** (92%) | 1/25 (4%) | |
Potentiation of homeotic transformation was determined by comparing compound mutant mice with Acvr2b−/− single mutant littermates from the same cross, to circumvent effects caused by mixed genetic backgrounds. Note that the increase of presacral vertebrae in Alk5+/−;Acvr2b−/− contributed to either thoracic or lumbar segments. ***P<0.001.
To clarify the mechanism by which Alk5 affects anterior–posterior patterning, we examined the induction of specific Hox genes. This class of transcription factors comprises 39 members, organized into four genomic clusters, that act together to regionalize the embryo along the anterior–posterior axis (Dubrulle & Pourquie, 2004; Deschamps & van Nes, 2005). The induction of specific domains of Hox gene expression is determined by opposing gradients of retinoic acid from the anterior end of the embryo and fibroblast growth factor (FGF) and GDF11 from the posterior. GDF11 has been shown to cooperate with FGF in the induction of Hoxc10 expression in explants from chick embryos (Liu et al, 2001), and in Gdf11−/− mouse embryos, the onset of expression of genes in the Hoxc cluster was delayed and shifted posteriorly (McPherron et al, 1999). Alk5−/− mutant mice have retarded growth at embryonic day (E)9.5, and die around E10 owing to impaired vasculogenesis of the yolk sac (Larsson et al, 2001; Seki et al, 2006). Hox gene expression was therefore examined up to E9 in these mutants. Hoxb1 is the earliest expressed Hox gene, first present in the posterior part of the primitive streak during gastrulation (Forlani et al, 2003). At E7.5, Alk5−/− mutants showed normal expression of Hoxb1 in the paraxial mesoderm along the primitive streak (n=7; Fig 4A). At E8.5, the expression of Hoxc8 along the posterior part of Alk5−/− embryos was also normal (n=6; Fig 4B). Interestingly, however, expression of Hoxc10 was absent in the posterior paraxial mesoderm of Alk5−/− mutant embryos at E9 (n=6; Fig 4C), resembling the phenotype observed in Gdf11−/− embryos and suggesting a common mechanism for the defects observed in both mutants. Consistent with a role for ALK5 in Hoxc10 induction, robust expression of this receptor was detected in the posterior part of the paraxial mesoderm of wild-type embryos between E9 and E10.5 (Fig 4D; data not shown). Importantly, Gdf11 expression was normal in Alk5−/− mutant embryos (Fig 4E), indicating that Alk5 does not function upstream of Gdf11 in anterior–posterior patterning. By contrast, neither Alk4 (Fig 4F) nor Alk7 (O.A. and C.F.I., unpublished observations) showed any significant expression in structures implicated in axial patterning at those ages. Together with previous work, these findings support the idea that GDF11 signals through ALK5 at posterior levels to regionalize the mouse embryo along its anterior–posterior axis.
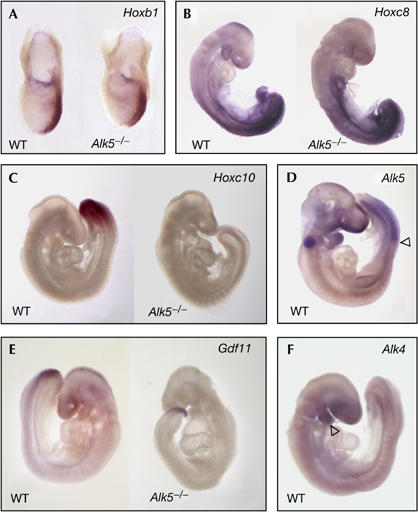
Expression of Hox genes, ALK5, GDF11 and ALK4 in Alk5−/− mutants. (A) Hoxb1 messenger RNA expression in the posterior streak of wild-type (WT) and Alk5−/− mutant embryos at embryonic day (E)7.5. (B) Expression of Hoxc8 mRNA in E8.5 WT and Alk5−/− mutant embryos. (C) Hoxc10 mRNA expression in the paraxial mesoderm of an E9 WT embryo (left). Note the lack of expression in the Alk5−/− mutant embryo (right). (D) Alk5 mRNA expression in the paraxial mesoderm in a WT E9.5 embryo. The arrowhead marks the anterior limit of Alk5 mRNA expression. The signal in the otic vesicle is background staining caused by trapping of antibody. (E) Expression of Gdf11 mRNA in WT and Alk5−/− mutant embryos at E9.5. (F) Lack of Alk4 mRNA expression in the paraxial mesoderm in a WT E9.5 embryo. Note the expression of Alk4 mRNA in the first branchial arch (arrowhead). GDF11, growth differentiation factor 11.
Genetic evidence has also linked GDF11 to kidney organogenesis and palate closure (McPherron et al, 1999; Esquela & Lee, 2003). GDF11 has been shown to direct the initial outgrowth of the ureteric bud during E11, and therefore mice lacking Gdf11 show kidney agenesis at birth (Esquela & Lee, 2003). Acvr2b−/− mutant mice also show malformations in those structures but, again, to a lesser extent than Gdf11−/− mutants, probably owing to redundancy between Acvr2 and Acvr2b receptors (Oh & Li, 1997; Oh et al, 2002). We found that the incidences of kidney agenesis and cleft palate were increased in Alk5+/−;Acvr2b−/− mutants compared with Acvr2b−/− single mutants (Fig 5A–D; Table 2). In head skeletal preparations, the secondary palate was not fused, whereas all other bones of the head developed normally (Fig 5E,F). By contrast, no significant increase in kidney agenesis or cleft palate was found in either Alk4+/−;Acvr2b−/− or Alk7+/−;Acvr2b−/− mutants, indicating that these type I receptors do not have a role in kidney formation or palate closure (Table 2). Collectively, our data indicate that Alk5 genetically interacts with Acvr2b to mimic several phenotypes found in Gdf11 mutant mice.
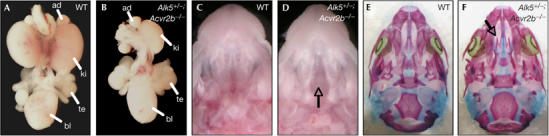
Genetic interaction between Acvr2b and Alk5 during kidney development and palate closure. (A,B) Whole-mount urogenital system from wild type (WT) (A) and Alk5+/−;Acvr2b−/− mutant (B). Note the unilateral kidney agenesis in the Alk5+/−;Acvr2b−/− mutant. (C,D) Macroscopic view of WT (C) and Alk5+/−;Acvr2b−/− mutant (D) heads. Arrow in (D) points at the cleft palate. (E,F) Skeleton staining of skulls from embryos shown in (C,D). Arrow in (F) points to the cleft palate, which leads to absence of secondary palate. ad, adrenal gland; bl, bladder; ki, kidney; te, testis.
Table 2
Analysis of kidney agenesis and cleft palate in compound mutants
| Genotype | ||||||
|---|---|---|---|---|---|---|
| Alk4+/+;Acvr2b−/− | Alk4+/−;Acvr2b−/− | Alk5+/+;Acvr2b−/− | Alk5+/−;Acvr2b−/− | Alk7+/+;Acvr2b−/− | Alk7−/−;Acvr2b−/− | |
| Kidney agenesis | 8/17 (47%) | 6/16 (38%) | 11/33 (33%) | 19/24** (79%) | 8/25 (32%) | 5/22 (23%) |
| Cleft palate | 6/17 (35%) | 3/16 (19%) | 3/33 (9%) | 13/24*** (54%) | 9/25 (36%) | 8/22 (36%) |
Significant increases in the incidence of kidney agenesis and cleft palate were seen only in Alk5+/−;AcrIIb−/− compound mutant mice when compared with Acvr2b−/− single mutant littermates from the same cross. **P<0.01, ***P<0.001.
The fact that a mutation in Alk5 had an effect in the Acvr2b-null background indicates that Alk5 must also be coupling to another type II receptor, most probably Acvr2, to mediate the effects of GDF11 on embryonic development. In support of this idea, mutations in Acvr2 have been shown to augment the phenotypes observed in Acvr2b−/− animals (Oh et al, 2002). Unlike Acvr2b−/− mutants, however, Acvr2−/− animals lack any vertebral patterning defects, indicating that although the Acvr2b−/− strain is genetically sensitized for those phenotypes, the Acvr2−/− strain is not. Together with the absence of effects of ALK4 and ALK7 in axial patterning, the lack of ALK4 and ALK7 expression in relevant patterning structures and the fact that GDF11, similar to TGF-β, signals through Smad2 and Smad3, our results strongly indicate that GDF11 uses the TGF-β receptor ALK5 in vivo to control several developmental events. Our study has not addressed the possible participation of other ALK5 ligands in the regionalization of the anterior–posterior axis of the mouse embryo. Although ALK5 has been shown to mediate signalling by TGF-β1 to TGF-β3 and myostatin, mice lacking all of these ligands have been generated and, unlike Gdf11−/− mutants, they do not show any abnormalities in anterior–posterior patterning (Chang et al, 2002). It is nevertheless possible that other as-yet-unknown ligands of the TGF-β superfamily could potentially have a role in axial patterning through the ALK5 receptor.
Whereas ALK4 has been shown to interact with many members of the TGF-β ligand family, including activins A and B, inhibins A and B, Nodal, Vg1, GDF1, GDF3, GDF11 and myostatin (Reissmann et al, 2001; Chang et al, 2002; Shi & Massague, 2003; Lee, 2004; Chen et al, 2006), most previous studies on ALK5—the best studied type I receptor of the TGF-β superfamily—have mainly focused on the canonical TGF-β1 to TGF-β3 ligands. One previous study has shown that myostatin can signal through both ALK4 and ALK5 when these receptors are overexpressed in vitro (Rebbapragada et al, 2003), but the receptors that mediate myostatin actions in vivo remain to be defined. Although GDF11 and myostatin have divergent functions, their amino-acid sequence is highly related. The close sequence identity of GDF11 and myostatin is consistent with similar receptor specificity.
Ligand–receptor interactions in the TGF-β superfamily have been extensively studied in vitro, and several possible complexes between ligands and type I and type II receptors have been proposed on the basis of those studies. By contrast, much less is known about the in vivo receptor specificities and functions in this important family of molecules. Signalling specificity in vivo is restricted by both temporal and spatial expression patterns and the ability of a certain ligand–receptor combination to confer signalling. Establishing the in vivo functions of ligands and receptors in the TGF-β superfamily has been hampered by embryonic lethal phenotypes, redundancy and compensatory effects. Studying gene-dosage effects in compound mutant mice is one way to circumvent such limitations (Chang et al, 2002; Andersson et al, 2006). Using this approach, we have shown novel and important roles for the TGF-β receptor ALK5 in anterior–posterior patterning, kidney development and palate closure, as a mediator of GDF11 signalling.
Methods
Mouse strains and PCR primers. All mutant mice used in this study have previously been described in detail (Oh & Li, 1997; Gu et al, 1998; Larsson et al, 2001; Jörnvall et al, 2004). Acvr2b and Alk4 were made in the Sv129 sub-strain J1, whereas Alk7 was made in Sv129 OlaHsd. Alk5 mutants were maintained in an Sv129;C57BL/6 hybrid strain. Littermates were used as controls in all experiments. Acvr2b, Alk5 and Alk7 mutant mice were genotyped by PCR as described previously (Oh & Li, 1997; Larsson et al, 2001; Jörnvall et al, 2004), and Alk4 was genotyped with the following set of primers: 5′-CTTGTCTGCAGCCAGTGGT-3′ and 5′-CCTCTGAGCCCAGAAAGCGAAGG-3′.
Histology, ligand–receptor interactions and receptor reconstitution experiments. Whole-mount in situ hybridization was performed according to standard protocols. All markers were RNA probes labelled with digoxigenin (DIG RNA Labeling Kit, Roche, Penzberg, Germany). Skeletal staining was performed as described (Oh & Li, 1997). Vertebrae that had different characteristics on each side were scored according to the more posterior identity. Significant differences between groups of mutants were examined using Fisher's exact test. A DNA fragment encoding the mature domain of mouse GDF11 was cloned into a pCDNA3.1 vector backbone consisting of an upstream activin B pro-domain and an HA tag that, after processing, remained fused to the amino terminus of mature GDF11. COS cells were transfected with HA-tagged GDF11, and conditioned media were collected for subsequent pull-down experiments performed in solution. GDF11 was processed and secreted as a mature protein of the expected size (data not shown). A 1 μg portion of soluble receptors (Acvr2b–Fc, ALK4–Fc, ALK5–Fc and ALK7–Fc; R&D Systems, Minneapolis, MN, USA) was added to 1 ml of conditioned media and incubated overnight before pull-down with Protein-G–Sepharose beads. Ligand–receptor interactions were detected by western blotting with anti-HA antibodies (Covance, Denver, PA, USA, HA.11). Crosslinking experiments were performed using purified recombinant human GDF11 (R&D Systems) and COS cells transiently transfected with different receptor combinations. GDF11 was radioactively labelled with 125I-Na using lactoperoxidase. Binding and subsequent crosslinking were performed in cell culture plates by addition of the chemical crosslinker ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysulfosoccinimide (S-NHS). Receptor complexes were immunoprecipitated from cell lysates with anti-HA antibodies, separated with SDS–polyacrylamide gel electrophoresis, blotted to polyvinylidine difluoride membranes and visualized in a STORM840 phosphorimager. Receptor reconstitution and reporter gene experiments were performed in cells cultured in 24-well plates, as described previously (Reissmann et al, 2001). The amount of plasmid DNA transfected into cells used for reporter assay experiments was 10 ng per three wells for type I receptors, 2 ng per three wells for type II receptors, 30 ng per three wells for Cripto and 750 ng per three wells for all ligands.
Acknowledgments
We are grateful to P. Oh, E. Li and S. Karlsson for providing, respectively, Acvr2b, Alk4 and Alk5 mutant mice and to E. De Robertis, C. Mummery, P. Tam, M. Capecchi, A. Boulet, L. Attisano and P. ten Dijke for providing plasmids. This work was supported by grants from the Swedish Foundation for Strategic Research, the Swedish Cancer Society (04 0235) and the Swedish Research Council (K2006-33X-10908-13-3).
References
- Andersson O, Reissmann E, Jörnvall H, Ibáñez CF (2006) Synergistic interaction between Gdf1 and Nodal during anterior axis development. Dev Biol 293: 370–381 [Abstract] [Google Scholar]
- Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23: 787–823 [Abstract] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW (2006) The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133: 319–329 [Abstract] [Google Scholar]
- Deschamps J, van Nes J (2005) Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development 132: 2931–2942 [Abstract] [Google Scholar]
- Dubrulle J, Pourquie O (2004) Coupling segmentation to axis formation. Development 131: 5783–5793 [Abstract] [Google Scholar]
- Esquela AF, Lee SJ (2003) Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol 257: 356–370 [Abstract] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J (2003) Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development 130: 3807–3819 [Abstract] [Google Scholar]
- Gad JM, Tam PP (1999) Axis development: the mouse becomes a dachshund. Curr Biol 9: R783–R786 [Abstract] [Google Scholar]
- Gu Z, Nomura M, Simpson BB, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe PK, Li E (1998) The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev 12: 844–857 [Europe PMC free article] [Abstract] [Google Scholar]
- Jörnvall H, Reissmann E, Andersson O, Mehrkash M, Ibáñez CF (2004) ALK7, a receptor for nodal, is dispensable for embryogenesis and left–right patterning in the mouse. Mol Cell Biol 24: 9383–9389 [Europe PMC free article] [Abstract] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S (2001) Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J 20: 1663–1673 [Europe PMC free article] [Abstract] [Google Scholar]
- Lee SJ (2004) Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 20: 61–86 [Abstract] [Google Scholar]
- Liu JP, Laufer E, Jessell TM (2001) Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron 32: 997–1012 [Abstract] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ (1999) Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet 22: 260–264 [Abstract] [Google Scholar]
- Oh SP, Li E (1997) The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev 11: 1812–1826 [Abstract] [Google Scholar]
- Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E (2002) Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev 16: 2749–2754 [Europe PMC free article] [Abstract] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L (2003) Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol 23: 7230–7242 [Europe PMC free article] [Abstract] [Google Scholar]
- Reissmann E, Jörnvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibáñez CF, Brivanlou AH (2001) The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev 15: 2010–2022 [Europe PMC free article] [Abstract] [Google Scholar]
- Seki T, Hong KH, Oh SP (2006) Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Lab Invest 86: 116–129 [Abstract] [Google Scholar]
- Shi Y, Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700 [Abstract] [Google Scholar]
Articles from EMBO Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/sj.embor.7400752
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1525155?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Novel insights into the pleiotropic health effects of growth differentiation factor 11 gained from genome-wide association studies in population biobanks.
BMC Genomics, 25(1):837, 06 Sep 2024
Cited by: 0 articles | PMID: 39237910 | PMCID: PMC11378601
Cell type-specific transforming growth factor-β (TGF-β) signaling in the regulation of salivary gland fibrosis and regeneration.
J Oral Biol Craniofac Res, 14(3):257-272, 21 Mar 2024
Cited by: 0 articles | PMID: 38559587 | PMCID: PMC10979288
Tgfbr1 controls developmental plasticity between the hindlimb and external genitalia by remodeling their regulatory landscape.
Nat Commun, 15(1):2509, 20 Mar 2024
Cited by: 2 articles | PMID: 38509075 | PMCID: PMC10954616
State- and stimulus-specific dynamics of SMAD signaling determine fate decisions in individual cells.
Proc Natl Acad Sci U S A, 120(10):e2210891120, 01 Mar 2023
Cited by: 2 articles | PMID: 36857347 | PMCID: PMC10013741
Deficiency of GDF-11 Accelerates TAC-Induced Heart Failure by Impairing Cardiac Angiogenesis.
JACC Basic Transl Sci, 8(6):617-635, 22 Feb 2023
Cited by: 1 article | PMID: 37426531 | PMCID: PMC10322730
Go to all (94) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5.
Mol Endocrinol, 18(3):653-665, 18 Dec 2003
Cited by: 124 articles | PMID: 14684852
Growth differentiation factor 11 is an encephalic regionalizing factor in neural differentiated mouse embryonic stem cells.
BMC Res Notes, 7:766, 29 Oct 2014
Cited by: 8 articles | PMID: 25352416 | PMCID: PMC4228095
Growth differentiation factor 11 locally controls anterior-posterior patterning of the axial skeleton.
J Cell Physiol, 234(12):23360-23368, 10 Jun 2019
Cited by: 8 articles | PMID: 31183862 | PMCID: PMC6772169
Growth and differentiation factor 11 (GDF11): Functions in the regulation of erythropoiesis and cardiac regeneration.
Pharmacol Ther, 156:26-33, 30 Oct 2015
Cited by: 38 articles | PMID: 26523637
Review





