Abstract
Free full text

Promoter-Specific Roles for Liver X Receptor/Corepressor Complexes in the Regulation of ABCA1 and SREBP1 Gene Expression
Abstract
Liver X receptors (LXRs) regulate the expression of genes involved in cholesterol and fatty acid homeostasis, including the genes for ATP-binding cassette transporter A1 (ABCA1) and sterol response element binding protein 1 (SREBP1). Loss of LXR leads to derepression of the ABCA1 gene in macrophages and the intestine, while the SREBP1c gene remains transcriptionally silent. Here we report that high-density-lipoprotein (HDL) cholesterol levels are increased in LXR-deficient mice, suggesting that derepression of ABCA1 and possibly other LXR target genes in selected tissues is sufficient to result in enhanced HDL biogenesis at the whole-body level. We provide several independent lines of evidence indicating that the repressive actions of LXRs are dependent on interactions with the nuclear receptor corepressor (NCoR) and the silencing mediator of retinoic acid and thyroid hormone receptors (SMRT). While dissociation of NCoR and SMRT results in derepression of the ABCA1 gene in macrophages, it is not sufficient for derepression of the SREBP1c gene. These findings reveal differential requirements for corepressors in the regulation of genes involved in cholesterol and fatty acid homeostasis and raise the possibility that these interactions may be exploited to develop synthetic ligands that selectively modulate LXR actions in vivo.
Liver X receptor α (LXRα) and LXRβ are members of the nuclear receptor superfamily of ligand-activated transcription factors that are regulated by oxidized derivatives of cholesterol termed oxysterols (13, 20). Unlike the sterol response element binding proteins (SREBPs) that induce cholesterol biosynthesis when cellular cholesterol levels are low (2), oxysterol-dependent activation of LXRs induces cholesterol catabolism and/or efflux when cellular cholesterol levels are high. In rodent liver, LXRs positively regulate expression of cholesterol 7α-hydroxylase (Cyp7a), the rate-limiting enzyme in the conversion of cholesterol to bile acids (25). LXRs also regulate the mobilization of cholesterol by inducing expression of the ATP binding cassette (ABC) transporters ABCA1, ABCG1, ABCG5, and ABCG8 and apolipoprotein E (ApoE) (6, 17, 27, 29, 40). The ABC transporters promote the transport of free cholesterol across cell membranes and play important roles in regulating cellular cholesterol homeostasis (16, 19, 23). ABCA1, ABCG5, and ABCG8 are thought to decrease dietary cholesterol absorption by reducing the levels of cholesterol absorbed in the intestine and increasing the levels of cholesterol that are secreted from the liver into the bile for excretion (29, 42).
In addition to influencing net cholesterol absorption, ABCA1 is believed to play an important role in reverse cholesterol transport, a mechanism by which cells transfer excess cholesterol to high-density-lipoprotein (HDL) acceptors. Loss of ABCA1 results in Tangier disease, a condition in which patients have extremely low levels of circulating HDL, massive accumulation of cholesterol in macrophages, and an increased risk for developing atherosclerosis (11, 18). In cultured macrophages and in skeletal muscle C2C12 cells, activation of LXR induces ABCA1 expression and cholesterol efflux (5, 24, 34, 39). Thus, the ability of LXR agonists to increase serum HDL levels may result, at least in part, from increased reverse cholesterol transport. Together the actions of LXR in response to elevated cholesterol in peripheral cells, the liver, and the intestine result in an overall net increase in cholesterol mobilization and catabolism, thus making LXR a pharmaceutical target for therapeutic intervention in hypercholesterolemia and atherosclerosis. In support of this finding, LXR agonists have recently been shown to decrease atherosclerotic lesion development in hypercholesterolemic mice (15).
In addition to regulating cholesterol homeostasis, LXR activation has been shown to regulate fatty acid metabolism that leads to increased serum and hepatic triglyceride levels (28, 33). SREBP1c, a transcription factor that regulates expression of many genes encoding enzymes involved in fatty acid synthesis, is a direct target of LXR (28). In addition to SREBP1c, LXR agonists increase hepatic expression of acetyl-CoA carboxylase, fatty acid synthase, and stearoyl CoA desaturase 1 (SCD-1) (28, 33). In the absence of LXRs, the mRNA expression levels of some of these genes in the liver are decreased compared to those in wild-type mice, suggesting that LXR is required to maintain their basal expression levels (28).
The mechanisms by which LXRs regulate programs of gene expression in a ligand-dependent manner remain relatively unexplored. Nuclear receptors activate gene transcription in response to ligands by recruiting coactivator proteins to target gene promoters (8, 22, 30). These coactivators function by altering local chromatin architecture through enzymatic modifications of histone tails (e.g., acetylation) and by recruiting basal transcriptional machinery. In the absence of ligand, many nuclear receptors repress gene transcription by recruiting corepressor proteins, exemplified by the nuclear receptor corepressor (NCoR) (12) and the silencing mediator of retinoic acid and thyroid hormone receptors (SMRT) (4). Corepressors are thought to function by antagonizing actions of coactivators (e.g., through associated histone deacetylase activities) and by recruiting factors that establish more repressive states of chromatin structure.
Here we report that loss of LXR has differential effects on triglyceride and HDL metabolism. Compared to LXR+/+ mice, LXR−/− mice have decreased levels of triglycerides in serum and increased levels of HDL. Analysis of cholesterol efflux and genes involved in reverse cholesterol transport suggests that LXR can function as both an activator and a repressor of reverse cholesterol transport in a gene- and tissue-specific fashion. Several lines of evidence indicate that active repression of LXR target genes is mediated, at least in part, by interactions with the corepressors NCoR and SMRT to repress basal expression of target promoters. The selective derepression of LXR target genes observed in LXR−/− mice is proposed to reflect differential promoter requirements for LXRs as ligand-dependent activators. Together our observations implicate transcriptional repression in the regulation of reverse cholesterol transport and HDL metabolism.
MATERIALS AND METHODS
Animals and lipoprotein analysis.
LXRαβ+/+ (LXR+/+) and LXRαβ−/− (LXR−/−) mice (mixed background strain A129-C57BL/6) (28) were housed in a controlled environment and given access to feed (Purina 5001) and water ad libitum. Blood was collected from 8-week-old male animals during the middle of a 12-h light cycle. Triglycerides in plasma were measured by using an enzymatic, colorimetric assay (Sigma, St. Louis, Mo.). Plasma HDL levels were measured by an enzymatic assay for total cholesterol (Sigma) following precipitation of non-HDL cholesterol by a heparin-manganese precipitating reagent (Wako Diagnostics, Richmond, Va.). Fourteen wild-type and 11 LXR−/− mice were used for these studies. The P values were 10−6 and 0.0003 for the significance of the triglyceride and HDL tests, respectively.
Macrophage isolation.
Bone marrow-derived macrophages were obtained as described previously (3, 38). Peritoneal macrophages were isolated from mice 4 days after peritoneal injection of thioglycolate broth medium.
RNA isolation, RT PCR analysis, and Northern blot analysis.
Total RNA was isolated by using RNeasy kits (Qiagen Inc., Valencia, Calif.). Real-time (RT) PCR analysis was performed on an ABI PRISM 7700 sequence detection system with target-specific probes and primers designed with Primer Express (PE Biosystems, Foster City, Calif.). Samples were analyzed as described previously (24). For Northern blot studies, the total RNA was isolated by using Trizol reagent (Invitrogen Life Technologies, Carlsbad, Calif.). Total RNA samples (10 μg per lane) were separated in 1.2% agarose gels containing formaldehyde and transferred to GeneScreen nylon membranes (NEN, Boston, Mass.). Probes were labeled with [α-32P]dCTP (ICN, Costa Mesa, Calif.), and hybridization was performed with Quikhyb (Stratagene, La Jolla, Calif.). Membranes were exposed to X-AR films (Kodak, Rochester, N.Y.).
Cholesterol efflux.
Efflux analysis was performed as described by Muscat et al. (24). Briefly, cells were incubated with 14C-labeled cholesterol for 48 h. The medium was then replaced with serum-free medium with or without 10 μg of ApoA1/ml together with ligand or vehicle. After a 24-h incubation, the levels of 14C in the medium and cell lysate were determined.
Transient transfections.
CV-1 cells and mouse embryonic fibroblasts (MEFs) were transfected by using FuGene6 (Roche Applied Science, Indianapolis, Ind.) according to the manufacturer's instructions. Medium containing ligand was added directly to the cells 5 h after transfection. The cells were harvested 18 h later and analyzed for luciferase and β-galactosidase (β-Gal) activities. Luciferase activity was normalized to β-Gal activity. The GAL4-LXR gene constructs used in these assays encode full-length human LXRα and LXRβ. In the two-hybrid analysis, the ligand binding domains of human LXRα (amino acids [aa] 164 to 447) and human LXRβ (aa 155 to 461) were fused to the VP16 activation domain, and the receptor interacting domains (IDs) of human SMRT (ID1 and ID2; aa 2131 to 2352) and human NCoR (ID1 and ID2; aa 794 to 1397) were fused to the GAL4 DNA binding domain.
ChIP analysis.
The chromatin immunoprecipitation (ChIP) assay was conducted as previously described (14, 35). Briefly, bone marrow-derived macrophages were fixed with 1% formaldehyde and treated as described previously (10). Cross-linked adducts were resuspended and sonicated, resulting in DNA fragments of 200 to 1,200 bp. Immunoprecipitation was performed by using the following antibodies: anti-RXRα (Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-NCoR (Affinity Bioreagents, Golden, Colo.), anti-SMRT (Affinity Bioreagents), anti-acetylated histone H3 (anti-acH3; acetylation on lysines 9 and 14) (Upstate Biotechnology, Lake Placid, N.Y.), anti-acH4 (acetylation on lysines 4, 7, 11, and 15) (Upstate Biotechnology), anti-upstream stimulating factor 1 (anti-USF1; H-86) (Santa Cruz Biotechnology), and anti-USF2 (N-18) (Santa Cruz Biotechnology). Rabbit preimmune serum (Jackson ImmunoResearch Laboratories, West Grove, Pa.) was used as a control for nonspecific binding. For each treatment and immunoprecipitation tested, we used 20 × 106 macrophages and 1 μg of specific antibody. Protein-bound, immunoprecipitated DNA was reverse cross-linked at 65°C overnight and then purified by using a PCR purification kit (Qiagen). Four microliters from a 50-μl DNA extraction volume was used for PCR amplification (25 to 30 cycles). The set of primers shown in Table Table11 was used to amplify the regions on the promoter of the genes that were the subjects of this study.
TABLE 1.
Primers used to amplify indicated regions on the promoters of the genes studied
| Promoter | Region amplified | Primers (5′ to 3′) |
|---|---|---|
| ABCA1 | LXRE | GCTTTCTGCTGAGTGACTGAACTAC |
| GAATTACTGCTTTTTGCCGCG | ||
| SREBP-1c | LXRE | GAACCAGCGGTGGGAACACAGAGC |
| GACGGCGGCAGCTCGGGTTTCTC | ||
| RARβ2 | RARE | GTGAGAATCCTGGGAGTTGGT |
| CAAAGAATAGACCCTCCTGGC | ||
| hsf2 | TCTGCCAGCCACAGCCGGTG | |
| GCGGTGAGAGGCGGAGAGAC |
Western blot analysis.
Membrane proteins were isolated by lysing cells in a solution containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitors. The cells were lysed by subjecting them to three cycles of freezing and thawing, followed by 5 min of microcentrifugation to remove nuclei. Extracts were incubated in sodium dodecyl sulfate gel loading buffer containing 5% β-mercaptoethanol at room temperature for 10 min before being loaded on the gel. Blots were incubated with specific antibodies overnight at 4°C. Anti-ABCA1 antibodies were purchased from Novus Biologicals (Littleton, Colo.), while anti-USF1 (C-20 and H-86) and anti-USF2 (C-20 and N-18) antibodies were obtained from Santa Cruz Biotechnology.
EMSA.
Nuclear extracts were obtained as previously described (32). Electrophoretic mobility shift assays (EMSAs) were run as previously described (41). Briefly, nuclear extracts (2 μg) were incubated with EMSA buffer [20 mM Tris (pH 7.9), 60 mM KCl, 0.2 mM EDTA, 0.5 mM diothiothreitol, 1.3 mM MgCl2, 10% glycerol, 3% Ficoll, 2 μg of poly(dI-dC)] for 15 min at 4°C. For supershift experiments, 0.6 μg of specific antibody (Santa Cruz Biotechnology) was incubated with the mixture on ice for 30 min. A probe was prepared by labeling a double-strand oligonucleotide with Klenow enzyme in the presence of [γ-32P]dCTP. The sequence for the oligonucleotide used was the following: 5′GGCGGGCCATGTCTCCACGTGCTTTCTGCT3′. The nuclear extracts were then incubated with the radiolabeled probe (2 × 105 cpm) at room temperature for 20 min. After the incubation process, the samples were subjected to electrophoretic separation with 6% retardation gels (Invitrogen) in 0.25× Tris-borate-EDTA buffer. The gel was dried and autoradiographed.
RESULTS
ABCA1 expression is up-regulated in a tissue-specific manner in LXR−/− mice.
Analysis of serum lipoproteins from LXR+/+ mice and LXR−/− mice (lacking both LXRα and LXRβ) maintained on a normal chow diet containing 0.02% cholesterol confirmed that LXR−/− mice have significantly lower serum triglyceride levels than LXR+/+ mice (Fig. (Fig.1a)1a) (33). Previous studies demonstrated a decreased level of expression of hepatic SREBP1c, FAS, and SCD-1 in the absence of LXR, suggesting that LXR is required for basal expression of genes in the fatty acid synthesis pathway (25, 28). Therefore, the decrease in triglycerides in serum is most likely a result of decreased fatty acid synthesis. Conversely, serum HDL levels were increased in the absence of LXR (Fig. (Fig.1a).1a). LXR agonists have been shown to increase serum HDL levels (33). Therefore, the increased levels of HDL observed in the absence of LXR activity were paradoxical and suggested that LXR may negatively regulate HDL levels in the absence of agonists.
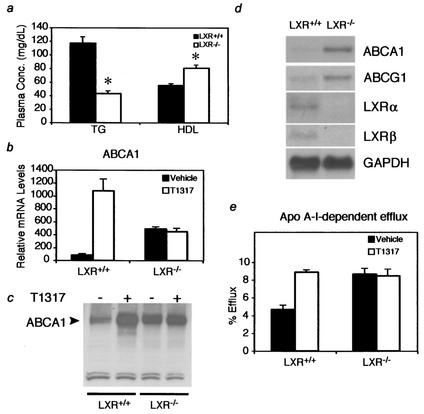
LXRs function as activators and inhibitors of ABCA1 expression and cholesterol efflux. (a) Effect of LXR expression on plasma lipids. Plasma samples from LXR+/+ and LXR−/− mice were analyzed for HDL and triglyceride content. An asterisk indicates that the LXR−/− value is significantly different (P < 0.001) from that for LXR+/+. Plasma Conc., concentration in plasma; TG, triglycerides. (b and c) LXR functions as an activator and inhibitor of ABCA1 mRNA and protein expression in peritoneal macrophages. Peritoneal macrophages were isolated from LXR+/+ and LXR−/− mice as indicated in the text. Cells were treated in culture with 1 μM T1317 or vehicle for 18 h before analysis of ABCA1 mRNA by RT PCR (b) and ABCA1 protein by Western blotting (c). ABCA1 mRNA levels determined by RT PCR in panel b are normalized to cyclophilin levels. (d) Unliganded LXRs function as inhibitors of ABCA1 and ABCG1 expression in bone marrow-derived macrophages. The cells were treated as described for panel b prior to isolation of total RNA and analysis by Northern blotting with the indicated probes. (e) Unliganded LXRs repress cholesterol efflux in peritoneal macrophages. The cells were treated as described for panel b prior to measurement of ApoAI-dependent cholesterol efflux.
Maturation of nascent HDL particles involves uptake of free cholesterol and its esterification by lecithin-cholesterol acyl transferase to build the cholesterol ester core (36). This mechanism is thought to represent a major pathway for reverse cholesterol transport, the process by which excess cholesterol in peripheral cells is delivered to the liver for biliary excretion. The ABCA1 and ABCG1 genes, known LXR targets, have been shown to mediate cholesterol efflux from cells to nascent HDL particles (16, 18). Since loss of ABCA1 significantly decreases cholesterol efflux and HDL levels, we examined the levels of ABCA1 and ABCG1 in macrophages isolated from LXR+/+ and LXR−/− mice. Peritoneal macrophages were isolated and treated in culture with vehicle or the LXR agonist T0901317 (T1317) (29) for 18 h and examined for ABCA1 mRNA and protein levels. As expected, treatment with T1317 increased ABCA1 mRNA and protein levels in the LXR+/+ macrophages but had no effect on the LXR−/− macrophages (Fig. 1b and c). ABCA1 mRNA and protein levels were also increased in the vehicle-treated LXR−/− macrophages in comparison to those in the LXR+/+ macrophages, suggesting that in the absence of ligands LXRs function to repress ABCA1 expression. A similar analysis performed on bone marrow-derived macrophages revealed that both ABCA1 and ABCG1 are increased in the absence of LXR (Fig. (Fig.1d1d).
Since ABCA1 functions in reverse cholesterol transport, we examined the effects of LXR on cholesterol efflux. In correlation with the ABCA1 mRNA and protein levels, cholesterol efflux was increased by LXR agonist treatment in the LXR+/+ macrophages (Fig. (Fig.1e).1e). Comparison of the basal efflux levels of LXR+/+ and LXR−/− macrophages revealed that, as with ABCA1 and ABCG1 expression, basal efflux was increased in the LXR−/− macrophages. These results are consistent with those of previous studies (5, 29, 40) and suggest that ABCA1 expression and cholesterol efflux are increased by LXR in the presence of ligand and repressed by LXR in the absence of ligand.
To determine if LXR represses other target genes in the absence of ligand, we examined the mRNA levels of SREBP1c, SCD-1, lipoprotein lipase (LPL), and ApoE in the same macrophages used to examine ABCA1 expression. As observed with the ABCA1 gene, all of the target genes were increased in LXR+/+ macrophages treated with T1317 (Fig. (Fig.2a).2a). However, SREBP1c, SCD-1, LPL, and ApoE mRNA levels were not derepressed by loss of LXR, suggesting that LXR-mediated repression is gene specific.
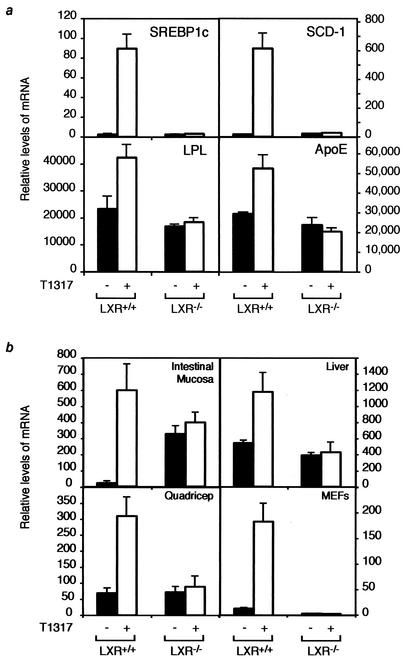
LXR-mediated repression is gene and tissue specific. (a) LXR target gene expression in LXR+/+ and LXR−/− peritoneal macrophages treated with vehicle or 1 μM T091317. SREBP1c, SCD-1, LPL, and ApoE levels were analyzed by RT PCR analysis and normalized to that of cyclophilin. (b) LXR represses ABCA1 in a tissue-specific manner. LXR+/+ and LXR−/− mice were dosed daily for 7 days by oral gavage with vehicle or 10 mg of T1317/kg of body weight. Four mice per group were used in this study. MEFs were isolated and treated in culture with vehicle or 1 μM T1317. RT PCR analysis was used to determine the levels of ABCA1 mRNA relative to those of cyclophilin in the intestinal mucosa, liver, quadriceps, and MEFs.
While macrophages are highly dependent on reverse cholesterol transport to maintain appropriate levels of cholesterol, recent reports suggest that macrophages account for only a small percentage of total HDL production (9). Importantly, ABCA1 is expressed in a number of other tissues, including liver, intestine, and skeletal muscle. Therefore, to determine if LXR activates or represses ABCA1 expression in other tissues, mRNA levels were measured in LXR+/+ and LXR−/− mice treated with either vehicle or T1317. As shown in Fig. Fig.2b,2b, LXR agonist treatment increased expression of ABCA1 mRNA in the intestine, liver, muscle, and MEFs in an LXR-dependent manner. Comparison of ABCA1 mRNA levels in the vehicle-treated tissues from the LXR+/+ and LXR−/− mice, however, indicated that intestinal mucosa was the only other tissue examined in which deletion of LXR increased the basal expression of ABCA1, a finding consistent with previous studies (29). Similar analysis of brown and white adipose tissue indicated that basal ABCA1 mRNA levels were the same in LXR+/+ and LXR−/− mice (data not shown). These results suggest that LXR represses basal expression of ABCA1 in a tissue-specific manner, occurring in macrophages and intestinal mucosa but not in several other tissues.
LXRs contain a ligand-reversible repressive function.
To further explore transcriptional repression by LXR, we examined the ability of GAL4-LXR fusions to repress a synthetic GAL4 reporter. As shown in Fig. Fig.3a,3a, recruitment of LXR to a synthetic thymidine kinase promoter containing four copies of a GAL4 response element in CV-1 cells results in repression of basal transcription in the absence of ligand. Upon the addition of LXR agonist, both LXRα and LXRβ activated transcription as expected (Fig. (Fig.3a).3a). Nuclear receptors have been shown to repress basal transcription through the recruitment of corepressor proteins such as NCoR and SMRT (4, 12). Therefore, we analyzed the ability of LXR to interact with the corepressors NCoR and SMRT by a mammalian two-hybrid analysis, using receptor IDs from NCoR and SMRT and VP-16 fusions of LXRα and LXRβ ligand binding domains. In the absence of ligand, both LXRα and LXRβ interacted with the corepressor IDs (Fig. (Fig.3b).3b). As expected, this interaction was inhibited in the presence of the LXR agonist. To further analyze the role of corepressors in LXR-dependent transcriptional repression, we measured the repressive activity of GAL4-LXR fusions in MEFs isolated from NCoR+/+ and NCoR−/− embryos (14). As was observed with CV-1 cells, unliganded GAL4-LXRα and GAL4-LXRβ repressed basal expression of the GAL4-luciferase reporter in the NCoR+/+ MEFs (Fig. (Fig.3c).3c). In contrast, LXR-dependent repression was significantly reduced in NCoR−/− MEFs, suggesting that association with the corepressor is required for LXR-mediated repression. The addition of an agonist induced the transcriptional activation of the luciferase reporter by both the GAL4-LXRα and GAL4-LXRβ systems (Fig. (Fig.3d).3d). Interestingly, the NCoR−/− MEFs showed higher levels of reporter activity upon treatment with ligand than the NCoR+/+ MEFs did, a finding which is consistent with the role of NCoR as a repressor of nuclear receptor-mediated transcription. Moreover, we performed rescue experiments by reconstituting NCoR expression in NCoR−/− MEFs. As shown in Fig. Fig.3e,3e, overexpression of full-length NCoR restored the capability of GAL4-LXRs to repress basal transcription in NCoR−/− cells.
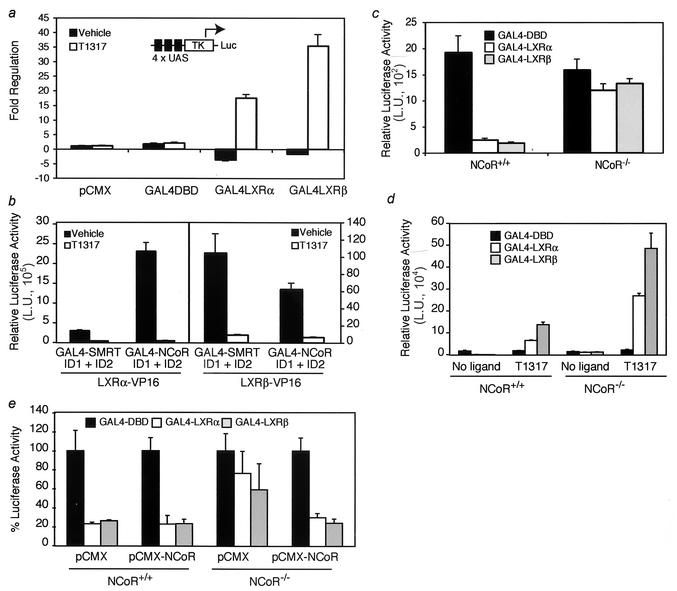
LXR represses basal transcription and interacts with corepressors. (a) CV-1 cells were transiently cotransfected with a luciferase reporter under the control of four copies of a GAL4 response element (4 × UAS) and either empty pCMX vector, a plasmid containing the GAL4 DNA binding domain (DBD) alone, or a construct with the GAL4 DBD fused to full-length LXRα or LXRβ. The cells were then treated with vehicle or 1 μM T1317 overnight. TK, thymidine kinase minimal promoter; Luc, luciferase. (b) LXRα and LXRβ interact with the nuclear receptor IDs of NCoR and SMRT. A mammalian two-hybrid system was established by transiently cotransfecting CV-1 cells with VP16 fusions of LXRα or LXRβ ligand binding domains together with GAL4 fusions of the receptor ID1 and ID2 of NCoR and SMRT and a luciferase reporter under the control of four copies of a GAL4 response element. The cells were treated with agonist overnight prior to being assayed for reporter activity. L.U., luciferase units. (c) Repression by LXR requires NCoR expression. MEFs isolated from NCoR+/+ and NCoR−/− embryos were transiently cotransfected with the GAL4 response element-luciferase reporter and the full-length LXRα- or LXRβ-GAL4 fusion constructs described for panel a. After an overnight incubation in the absence of ligand, the cells were lysed and analyzed for promoter activity. (d) The effect of a ligand was analyzed with MEFs transfected with the GAL4 one-hybrid system. NCoR+/+ and NCoR−/− MEFs were transfected with the same constructs as described for panel c, followed by addition of vehicle or T1317 (1 μM). (e) Overexpression of exogenous NCoR rescues repression of basal transcription in NCoR−/− MEFs. The cells were cotransfected with the constructs described for panel c together with empty vector (pCMX) or full-length NCoR (pCMX NCoR). For panels a through e, all cells were cotransfected with a cytomegalovirus-β-Gal expression vector and luciferase values were normalized to those of β-Gal activity. In panel e, the luciferase activity for each control sample (cells transfected with GAL4-DBD) was assigned a value of 100%. The luciferase activities of cells transfected with GAL4-LXRs are represented as percentages of the respective control activity.
LXRs mediate recruitment of NCoR and SMRT to target gene promoters.
Analysis of GAL4-LXR fusions and two-hybrid experiments suggest that LXR can interact with the corepressors NCoR and SMRT and repress basal transcription. Therefore, to determine if NCoR and SMRT are recruited by LXR to target gene promoters, we performed ChIP analysis on extracts isolated from LXR+/+ and LXR−/− macrophages. ChIP analysis was performed in the regions of the ABCA1 and SREBP1c promoters that have previously been shown to contain LXR response elements (LXREs) (26, 28, 31). Antibodies suitable for detecting LXRα or LXRβ in ChIP assays are not currently available. Therefore, we used an antibody to the retinoid X receptor alpha (RXRα), the heterodimeric partner of LXR, as a marker for the RXR/LXR heterodimer. As shown in Fig. Fig.4,4, RXRα occupies the ABCA1 and SREBP1c promoters in the LXR+/+ macrophages in the presence and absence of ligand. In the LXR−/− macrophages, we did not detect RXRα above background levels, confirming that the LXR/RXR heterodimer binds to the regions of the ABCA1 and SREBP1c promoter amplified in this assay. As a control, we also analyzed the recruitment of RXRα to the retinoic acid receptor (RAR) response element at the RARβ2 promoter and observed no effect of LXR ligand or loss of LXR expression on the recruitment of RXRα to the RARβ2 promoter (Fig. (Fig.4).4). Moreover, in other experiments we did not observe recruitment of RXRα to the ABCA1 or SREBP1c promoters in LXR−/− cells stimulated with T1317 (data not shown), which suggests that this compound does not promote the recruitment of a different RXR heterodimeric partner to these sites.
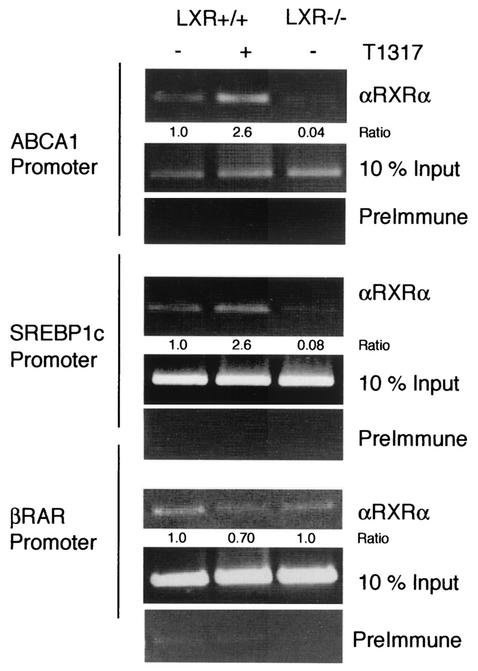
RXR is recruited to the ABCA1 and SREBP1c promoters in an LXR-dependent manner. Bone marrow-derived macrophages were obtained from LXR+/+ and LXR−/− mice. Control macrophages from each genotype were treated with vehicle. The effect of an LXR ligand was assessed in LXR+/+ cells by stimulating them with T1317 (1 μM) for 90 min. ChIP analysis was performed with antibodies specific to RXRα and control rabbit preimmune serum. Primers specific to the LXRE-containing regions of the ABCA1 and SREBP1c promoters and the control RAR-response element-containing region of the RARβ2 promoter were used for PCR analysis. Quantitation of the bands was performed by densitometry. Intensity values obtained for the immunoprecipitated products were first normalized to their respective input controls. The indicated ratios represent the quotient derived from each normalized value and the value obtained for unstimulated wild-type cells.
To determine if the LXR/RXR heterodimer recruits corepressors to the ABCA1 promoter, ChIP analysis was performed with antibodies specific for NCoR. As shown in Fig. Fig.5a,5a, NCoR associated with the ABCA1 promoter in LXR+/+ macrophages in the absence of ligand (lane 1). Addition of agonist significantly reduced the association of NCoR with the promoter. In contrast, significantly lower levels of NCoR were detected on the ABCA1 promoter in LXR−/− macrophages under basal conditions, indicating that the unliganded LXR/RXR heterodimer is required for their effective recruitment of NCoR to this region of the promoter. The addition of ligand had no effect on NCoR recruitment to the ABCA1 promoter in LXR-deficient cells. Similar results were obtained for SMRT, although the signals observed were weaker than those for NCoR (data not shown). Importantly, loss of LXR expression or treatment with LXR ligand does not affect NCoR or SMRT recruitment to the RARβ2 promoter (Fig. (Fig.5a),5a), supporting the specificity and LXR dependence of the findings observed with the ABCA1 promoter.
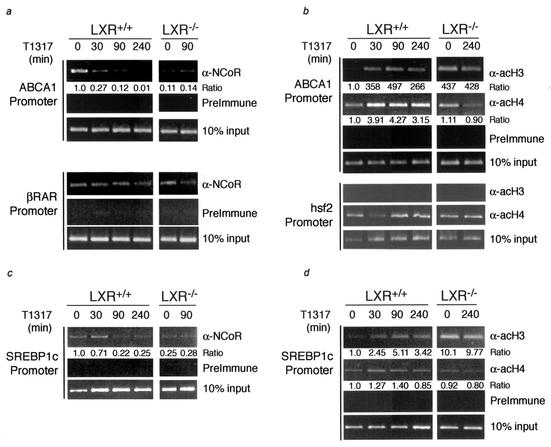
ChIP analysis of the ABCA1 and SREBP1c promoters. Bone marrow-derived macrophages from LXR+/+ and LXR−/− mice were treated with vehicle or 1 μM T1317 for various lengths of time (0 to 240 min) prior to ChIP analysis. Rabbit preimmune serum or antibodies specific to NCoR (a and c) or acH3 and acH4 (b and d) were used to immunoprecipitate the ABCA1, SREBP1c, hsf2, and RARβ2 promoters as indicated in the text. Quantitation of the bands was performed by densitometry. Intensity values obtained for the immunoprecipitated products were first normalized to those of their respective input controls. The indicated ratios represent the quotient derived from each normalized value and the value obtained for unstimulated wild-type cells.
Since NCoR and SMRT are thought to repress transcription by recruiting histone deacetylases to target promoters, we extended the characterization of the ABCA1 promoter by using ChIP analysis to examine the acetylation of histone H3 and H4. In macrophages from LXR+/+ mice, there is minimal histone acetylation at the ABCA1 promoter in the absence of ligand (Fig. (Fig.5b).5b). Increases in H3 and H4 acetylation were observed within 30 min of ligand addition, and this increased state of acetylation remained for up to 4 h. Similar analysis of the LXR−/− macrophages revealed that histone H3 at the ABCA1 promoter is hyperacetylated in the absence of LXR (Fig. (Fig.5b).5b). The increased acetylation observed at the ABCA1 promoter with either treatment with LXR agonist or loss of LXR expression is specific, as no effect on histone acetylation was observed at the hsf2 promoter (Fig. (Fig.5b).5b). ChIP analysis of the ABCA1 promoter suggests that the recruitment of corepressors is largely mediated by LXR and that agonist binding or genetic deletion of LXR induces corepressor dissociation from the promoter, leading to histone acetylation and relief of transcriptional repression.
Dissociation of NCoR/SMRT does not account for differential regulation of LXR target genes.
We next investigated whether differential recruitment of NCoR and SMRT could account for the lack of derepression of the SREBP1c gene in macrophages. ChIP experiments indicated that NCoR associated with the SREBP1c promoter under basal conditions in LXR+/+ macrophages, with lower levels of interaction observed in LXR−/− macrophages (Fig. (Fig.5c).5c). The interaction of NCoR with the SREBP1c promoter was decreased upon ligand activation of LXR. Similar results were obtained for SMRT, although signal strength was lower than that observed for NCoR, as was the case for the ABCA1 promoter (data not shown). Agonist treatment of LXR+/+ macrophages also resulted in an increase in histone acetylation at the SREBP1c promoter, with a time course that was similar to that observed for ABCA1 (Fig. (Fig.5d).5d). Interestingly, as is the case for the ABCA1 promoter, the acetylation of histone 3 at the SREBP1c promoter was greatly increased in the LXR−/− macrophages in comparison to that in the LXR+/+ macrophages (Fig. (Fig.5d).5d). Thus, LXR-dependent recruitment of NCoR, its ligand-dependent dissociation, and hyperacetylation in the absence of LXR all occur on the SREBP1c promoter in a manner that is indistinguishable from that in the ABCA1 promoter.
The fact that SREBP1c expression is not up-regulated in LXR−/− cells indicates that transcriptional activation of SREBP1c requires the presence of an active LXR, while activation of ABCA1 can be achieved by additional tissue-specific factors in the absence of LXR. In extended ChIP assays, we did not detect methylation of histone 3, lysine 9, or arginine 17 on either promoter (data not shown), a finding which argues against silencing mechanisms being involved in permanent repression of the SREBP1c gene. Recent studies have identified two members of the helix-loop-helix transcription factor family, USF1 and USF2, as positive regulators of the human ABCA1 promoter (41). Western blot studies demonstrated that USF1 and USF2 were expressed in bone marrow-derived macrophages (data not shown). Therefore, EMSAs were performed using a radiolabeled oligonucleotide corresponding to the E-box present in the murine proximal ABCA1 promoter. This probe was recognized by a DNA binding activity in nuclear extracts prepared from bone marrow-derived macrophages that was almost completely shifted by antibodies directed against USF1 or USF2 but not against LXRα (Fig. (Fig.6a),6a), a finding that is consistent with binding of USF1/USF2 heterodimers. Similar results were obtained in LXR-deficient macrophages (data not shown). ChIP analysis demonstrated binding of these factors to the murine ABCA1 promoter (Fig. (Fig.6b).6b). Importantly, despite the existence of several E-box elements in the proximal SREBP1c promoter, no binding of USF1 or USF2 was detected on this promoter.
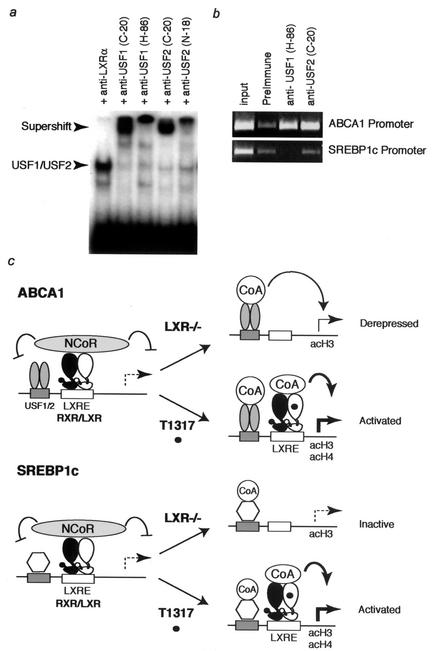
Model for differential derepression of the ABCA1 and SREBP1c genes in LXR−/− macrophages. (a) EMSA detecting a DNA binding activity in nuclear extracts prepared from bone marrow-derived macrophages that recognizes an E-box in the ABCA1 promoter. This activity is supershifted by antibodies directed against USF1 or USF2 but not by an antibody directed against LXRα. (b) ChIP analysis of the ABCA1 and SREBP1c promoters by using antibodies directed against USF1 or USF2. Both antibodies immunoprecipitate the ABCA1 promoter but not the SREBP1c promoter. (c) Derepression of the ABCA1 gene in LXR−/− macrophages is proposed to be due to binding of additional sequence-specific activators, such as USF1 and USF2, that are capable of recruiting HAT-containing complexes. These factors are derepressed in the absence of LXR/NCoR complexes and are sufficient to drive ABCA1 expression. In contrast, HAT-containing coactivators also appear to be recruited to the SREBP1c gene, but these factors are unable to stimulate transcription in the absence of agonist-bound LXRs. CoA, coactivator.
DISCUSSION
Recent findings have identified the LXRs as important regulators of HDL metabolism because of their ability to control the expression of genes involved in reverse cholesterol transport. In this study, we demonstrated that LXRs not only induce reverse cholesterol transport when cholesterol levels are high but also mediate active repression in the unliganded state. These studies therefore link transcriptional repression to regulation of cholesterol homeostasis. The ability of LXR to regulate reverse cholesterol transport is due, at least in part, to regulation of ABCA1. Genetic defects in ABCA1 result in Tangier disease, a condition in which patients have greatly reduced HDL levels (11, 18). An initiating step in atherosclerosis is the formation of macrophage foam cells, which occurs when macrophages in the arterial wall become overloaded with excess cholesterol. Unlike other cells, macrophages take up cholesterol via scavenger receptors and therefore are highly dependent on reverse cholesterol transport to reduce cellular cholesterol levels. The importance of ABCA1 in reverse cholesterol transport is also exemplified in ABCA1 knockout mice. These mice have almost no circulating HDL and show signs of cholesterol accumulation in macrophages similar to that of patients with Tangier disease (23). Additionally, cells overexpressing human ABCA1 show increased cholesterol efflux activity (18). Together these data highlight the importance of ABCA1 in mediating reverse cholesterol transport and suggest that pharmaceuticals that increase this process may be useful for the prevention or treatment of atherosclerosis.
In our analysis of LXR activity, we confirmed earlier studies suggesting that basal ABCA1 levels and cholesterol efflux were higher in LXR−/− macrophages than in LXR+/+ macrophages. We also observed that HDL cholesterol levels were elevated in LXR−/− mice. These results suggest that LXR is capable of both activating and repressing target genes that control cholesterol efflux and HDL biogenesis, such as the ABCA1 gene. Further analysis revealed that LXR-mediated repression of ABCA1 is both gene and tissue specific. Out of six LXR target genes analyzed, only the two ABC transporters, ABCA1 and to a lesser extent ABCG1, were derepressed in the LXR−/− macrophages. Interestingly, LXR-mediated repression of ABCA1 occurred only in macrophages and intestinal mucosa, both of which are routinely exposed to sudden elevations in cellular cholesterol levels. This combination of repression and activation creates an environment in which the induction of ABCA1 expression upon ligand-mediated activation of LXR is greater in the intestinal mucosa and macrophages than in other tissues. Therefore, the combined ability to repress and activate ABCA1 expression allows for a tightly regulated and responsive system to handle sudden pronounced changes in cellular cholesterol levels.
ChIP experiments indicate that NCoR is recruited to the ABCA1 promoter in an LXR-dependent manner and that either addition of ligand or loss of LXR expression similarly results in decreased recruitment to the promoter. Two-hybrid analysis and studies of NCoR−/− cells further support a role for corepressors in transcriptional repression by LXR. Interestingly, the mRNA levels for the SREBP1c gene and several other LXR target genes are not increased by loss of LXR. Nevertheless, genetic deletion of LXR reduces corepressor binding at the SREBP1c promoter, suggesting that loss of corepressor recruitment is not sufficient to induce transcriptional activation. Therefore, while corepressor recruitment may be required for repression of basal transcription by nuclear receptors, corepressor release is clearly not sufficient for transcriptional activation. The results suggest that additional steps or mechanisms must account for the differential expression levels of ABCA1 and SREBP1c that are observed in the LXR−/− macrophages. Examination of the histone acetylation status of the ABCA1 promoter shows a significant increase in histone H3 acetylation upon loss of LXR expression. This result corresponds to the loss of corepressor recruitment and increased gene transcription. However, similar results were obtained with the SREBP1c promoter, indicating that changes in histone acetylation, at least in the vicinity of the nuclear receptor response elements, are not always synonymous with active gene transcription.
A model for regulation of the ABCA1 and SREBP1c genes suggesting the basis for differential consequences of LXR deletion is illustrated in Fig. Fig.6c.6c. The present study indicates that transcriptional activation of the SREBP1c gene requires agonist-bound LXRs but that activation of the ABCA1 gene does not. These observations imply that the ABCA1 promoter is occupied by additional activators, exemplified by USF1 and USF2, that recruit coactivators that possess histone acetyltransferase (HAT) activity and are also sufficient to drive promoter activity. In the absence of an LXR agonist, recruitment of NCoR is required to maintain the ABCA1 gene in a repressed state. NCoR is not recruited to the ABCA1 promoter in LXR−/− macrophages, and the ABCA1 gene is derepressed. In contrast, while the SREBP1c gene is also likely to be occupied by other activators that recruit HAT-containing coactivators, these factors are not sufficient to activate transcription in the absence of agonist-bound LXRs. Thus, failure of NCoR recruitment to the SREBP1c promoter in LXR−/− macrophages allows it to become hyperacetylated, but this hyperacetylation is not sufficient to promote transcriptional activation (Fig. (Fig.6c6c).
Bone marrow transplant studies reveal that loss of LXR expression in macrophages is proatherogenic (37). Since LXR−/− macrophages have increased levels of ABCA1 and cholesterol efflux activity, these results may appear contradictory. The levels of ABCA1 expression in LXR−/− macrophages, however, are not as great as they are in LXR+/+ macrophages treated with ligand, suggesting the possibility that the levels of ABCA1 needed for antiatherogenic activity in macrophages are not achieved by derepression alone. Additionally, LXR target genes in addition to the ABCA1 gene may be required to mediate the antiatherogenic activities of LXR in macrophages. One possible LXR target gene that may contribute to the antiatherogenic effect of LXR expression is the ApoE gene. Macrophage ApoE expression can significantly reduce lesion development, as revealed by the decreased lesion area observed when ApoE−/− mice receive a bone marrow transplant from ApoE+/+ donors (1, 21).
The increased serum HDL levels and decreased serum triglyceride levels observed in LXR−/− mice are expected to protect against development of atherosclerosis. Because loss of LXR expression results in decreased corepressor recruitment and increased histone acetylation in the LXRE-containing region of target gene promoters, ligands that release corepressors without recruiting coactivators may mimic the effects on HDL observed in the LXR−/− mice. Synthetic ligands with these properties may provide an improved therapeutic index over the first-generation LXR agonists such as T1317, which increase both HDL and triglycerides. Ligands that mediate corepressor release without recruiting coactivators have recently been identified for RARs, suggesting that it may be possible to identify similar ligands for LXRs (7).
Acknowledgments
We thank A. Zulueta for assistance with preparation of the manuscript and C. Bayne for the production of T1317. We also thank Joyce Repa and David Mangelsdorf for making LXR−/− mice available for these studies and for critical reading of the manuscript.
These studies were supported by a Biostar grant from the University of California, an NIH grant to the La Jolla Specialized Center of Research on Atherosclerosis (grant HL56989), and a grant from the Stanford Reynolds Center. A.F.V. was supported in part by a grant from the Ministry of Spain (Programa Formación de Personal Investigador en el Extranjero).
B. L. Wagner and A. F. Valledor contributed equally to the preparation of the manuscript and should be considered joint first authors.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.23.16.5780-5789.2003
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc166346?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Nonlipogenic ABCA1 Inducers (NLAI) for Alzheimer's Disease Validated in a Mouse Model Expressing Human APOE3/APOE4.
J Med Chem, 67(17):15061-15079, 27 Aug 2024
Cited by: 0 articles | PMID: 39191400
Demystifying the Functional Role of Nuclear Receptors in Esophageal Cancer.
Int J Mol Sci, 23(18):10952, 19 Sep 2022
Cited by: 5 articles | PMID: 36142861 | PMCID: PMC9501100
Review Free full text in Europe PMC
Lipid Metabolism: Immune Regulation and Therapeutic Prospectives in Systemic Lupus Erythematosus.
Front Immunol, 13:860586, 18 Mar 2022
Cited by: 17 articles | PMID: 35371016 | PMCID: PMC8971568
Review Free full text in Europe PMC
Remembering your A, B, C's: Alzheimer's disease and ABCA1.
Acta Pharm Sin B, 12(3):995-1018, 24 Jan 2022
Cited by: 19 articles | PMID: 35530134 | PMCID: PMC9072248
Review Free full text in Europe PMC
Development of Agonist-Based PROTACs Targeting Liver X Receptor.
Front Chem, 9:674967, 26 May 2021
Cited by: 1 article | PMID: 34124002 | PMCID: PMC8187946
Go to all (148) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Retinoic acid receptor-mediated induction of ABCA1 in macrophages.
Mol Cell Biol, 23(21):7756-7766, 01 Nov 2003
Cited by: 85 articles | PMID: 14560020 | PMCID: PMC207565
Liver X receptor (LXR)-beta regulation in LXRalpha-deficient mice: implications for therapeutic targeting.
Mol Pharmacol, 70(4):1340-1349, 06 Jul 2006
Cited by: 87 articles | PMID: 16825483
SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR.
Biochem J, 400(3):485-491, 01 Dec 2006
Cited by: 85 articles | PMID: 16901265 | PMCID: PMC1698594
Transcriptional regulatory networks in lipid metabolism control ABCA1 expression.
Biochim Biophys Acta, 1735(1):1-19, 01 Jun 2005
Cited by: 133 articles | PMID: 15922656
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: P50 HL056989
Grant ID: HL56989





