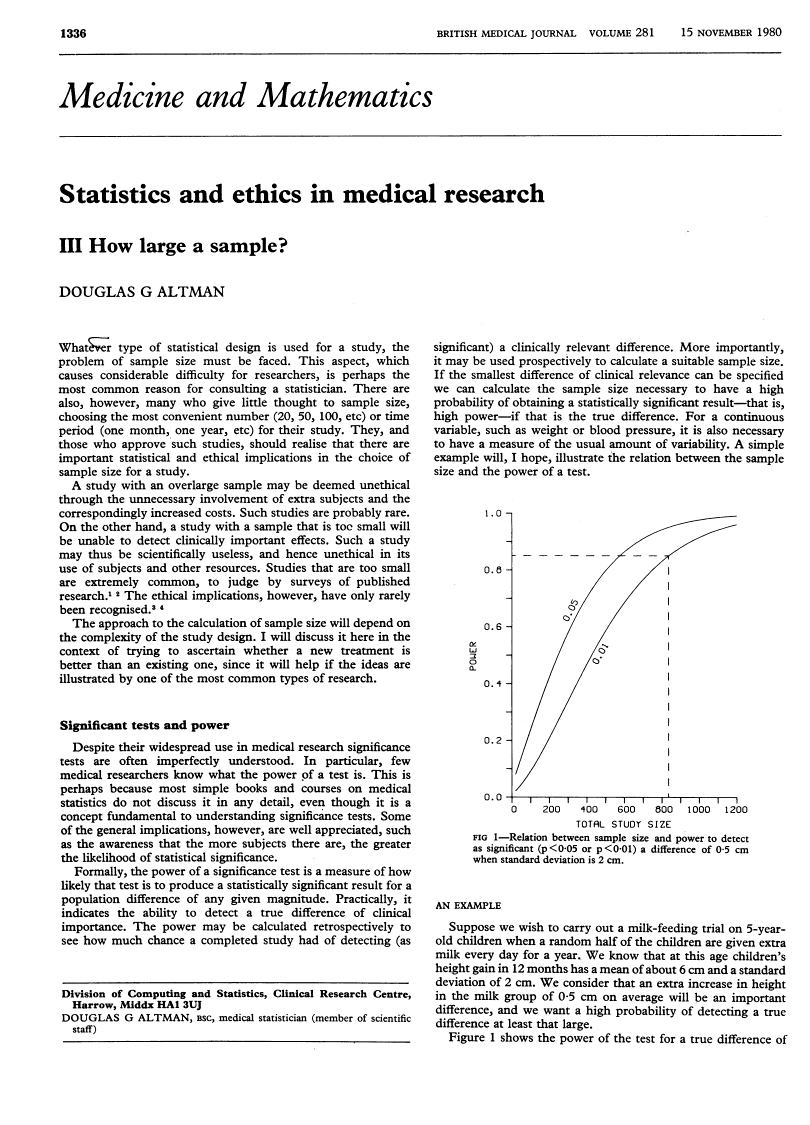
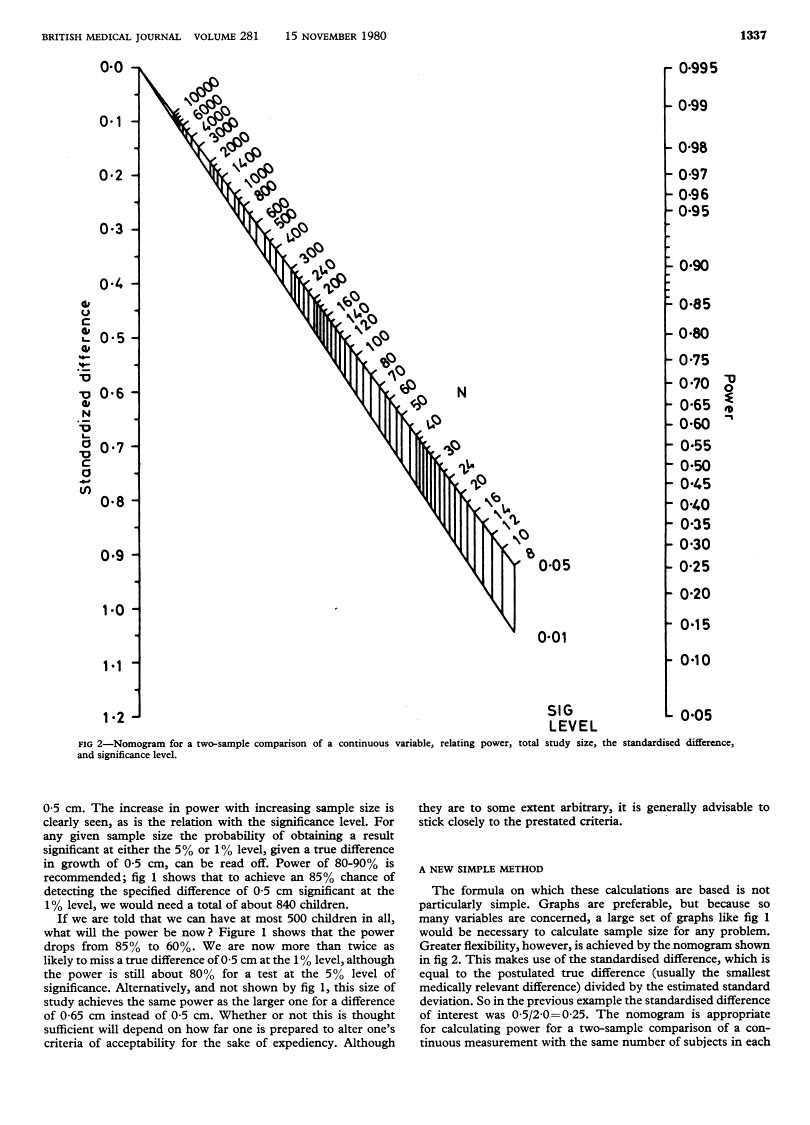
Abstract
Free full text

Statistics and ethics in medical research: III How large a sample?
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (510K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freiman JA, Chalmers TC, Smith H, Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. N Engl J Med. 1978 Sep 28;299(13):690–694. [Abstract] [Google Scholar]
- Newell DJ. Type II errors and ethics. Br Med J. 1978 Dec 23;2(6154):a1789–1789. [Europe PMC free article] [Google Scholar]
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. [Europe PMC free article] [Abstract] [Google Scholar]
- Boag JW, Haybittle JL, Fowler JF, Emery EW. The number of patients required in a clinical trial. Br J Radiol. 1971 Feb;44(518):122–125. [Abstract] [Google Scholar]
- Mould RF. Clinical trial design in cancer. Clin Radiol. 1979 Jul;30(4):371–381. [Abstract] [Google Scholar]
Associated Data
Articles from British Medical Journal are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/bmj.281.6251.1336
Read article for free, from open access legal sources, via Unpaywall:
https://www.bmj.com/content/bmj/281/6251/1336.full.pdf
Citations & impact
Impact metrics
Article citations
Screening for Risk of Fall-Related Inpatient Trauma in a US Acute Care Setting.
Cureus, 16(6):e63199, 26 Jun 2024
Cited by: 0 articles | PMID: 38933346 | PMCID: PMC11203275
Metacognitive Management of Attention in Online Learning.
J Intell, 12(4):46, 22 Apr 2024
Cited by: 0 articles | PMID: 38667713 | PMCID: PMC11051084
In reply: Comment on: The fragility index of randomized controlled trials in pediatric anesthesiology.
Can J Anaesth, 71(1):165-166, 21 Nov 2023
Cited by: 1 article | PMID: 37989935
Effects of duration of follow-up and lag in data collection on the performance of adaptive clinical trials.
Pharm Stat, 23(2):138-150, 14 Oct 2023
Cited by: 2 articles | PMID: 37837271
Innovative Technologies in CNS Trials: Promises and Pitfalls for Recruitment, Retention, and Representativeness.
Innov Clin Neurosci, 20(7-9):40-46, 01 Jul 2023
Cited by: 0 articles | PMID: 37817816 | PMCID: PMC10561984
Go to all (286) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Statistics and ethics in medical research. Misuse of statistics is unethical.
Br Med J, 281(6249):1182-1184, 01 Nov 1980
Cited by: 53 articles | PMID: 7427629 | PMCID: PMC1714517
Statistics and ethics in medical research: study design.
Br Med J, 281(6250):1267-1269, 01 Nov 1980
Cited by: 20 articles | PMID: 7000298 | PMCID: PMC1714604
Conference on the scientific basis of respiratory therapy. Controlled clinical trials.
Am Rev Respir Dis, 110(6 pt 2):20-24, 01 Dec 1974
Cited by: 1 article | PMID: 4613228
Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents.
J Natl Cancer Inst, 85(20):1637-1643, 01 Oct 1993
Cited by: 95 articles | PMID: 8411243
Review