Abstract
Free full text

Discrepancies between disk diffusion and broth susceptibility studies of the activity of ticarcillin plus clavulanic acid against ticarcillin-resistant Pseudomonas aeruginosa.
Abstract
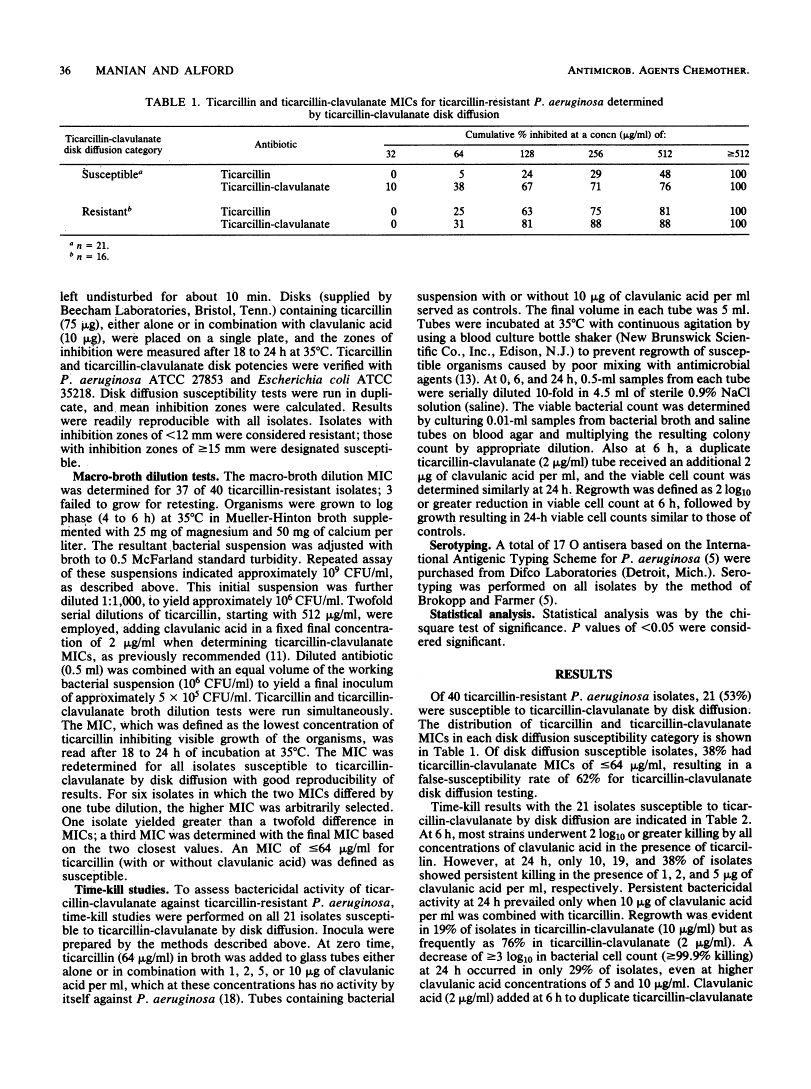
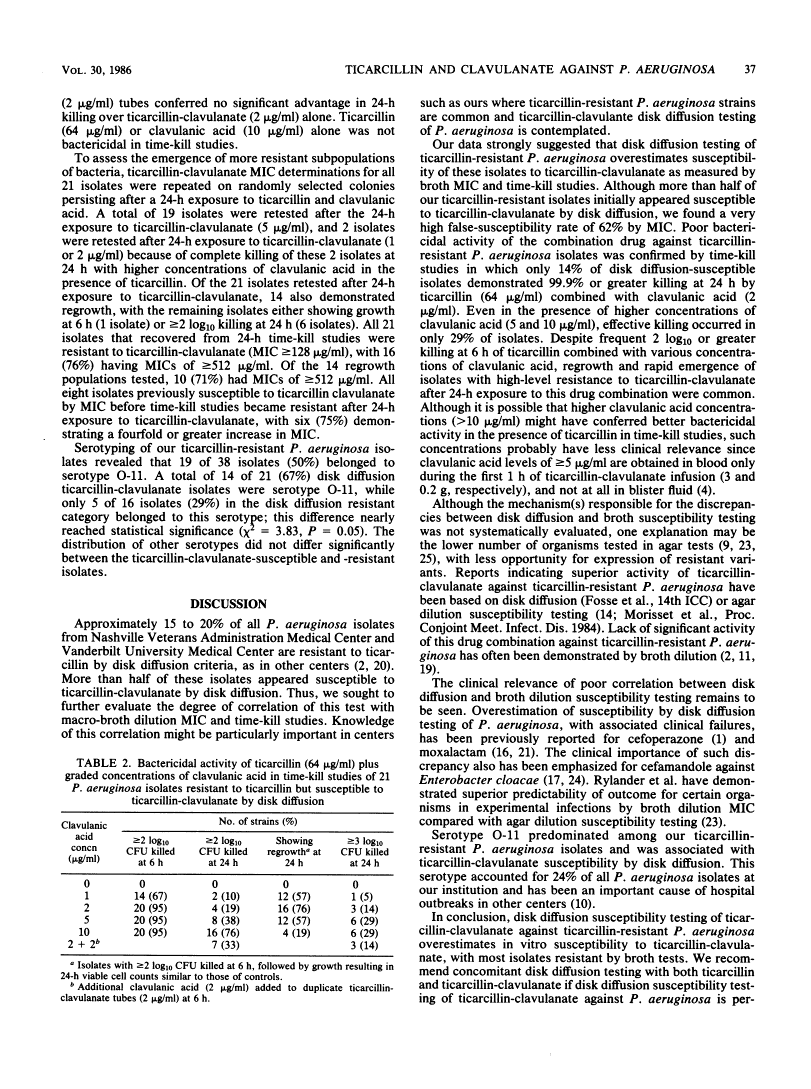
Ticarcillin and clavulanic acid in combination were tested against 40 Pseudomonas aeruginosa isolates resistant to ticarcillin by disk diffusion. A total of 21 isolates (53%) were susceptible to ticarcillin-clavulanate by disk diffusion, under currently recommended criteria for ticarcillin susceptibility. Macro-broth dilution tests (ticarcillin plus clavulanic acid, 2 micrograms/ml) confirmed susceptibility (MIC less than or equal to 64 micrograms/ml) of only 8 (38%) of 21 isolates. Time-kill studies of disk diffusion susceptible isolates indicated 2 log10 or greater killing of most isolates at 6 h in broth containing ticarcillin (64 micrograms/ml) combined with clavulanic acid (1, 2, 5, or 10 micrograms/ml). After 6 h, regrowth was common in all concentrations of clavulanic acid except 10 micrograms/ml. Regrowth populations were resistant to ticarcillin-clavulanate by MIC determination. Poor bactericidal activity of ticarcillin-clavulanate against ticarcillin-resistant P. aeruginosa was confirmed, as most isolates did not undergo 99.9% or greater killing at 24 h in all concentrations of clavulanic acid. Serotype O-11 was our most common serotype and was associated with disk diffusion "pseudosusceptibility." Concomitant disk diffusion testing of ticarcillin-clavulanate and ticarcillin is recommended for testing the susceptibility of P. aeruginosa to ticarcillin-clavulanate by disk diffusion. P. aeruginosa isolates resistant to ticarcillin should as a rule be considered also resistant to ticarcillin-clavulanate, despite apparent susceptibility by disk diffusion.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (875K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey RR, Peddie B, Blake E, Bishop V, Reddy J. Cefoperazone in the treatment of severe or complicated infections. Drugs. 1981;22 (Suppl 1):76–86. [Abstract] [Google Scholar]
- Barry AL, Ayers LW, Gavan TL, Gerlach EH, Jones RN. In vitro activity of ticarcillin plus clavulanic acid against bacteria isolated in three centers. Eur J Clin Microbiol. 1984 Jun;3(3):203–206. [Abstract] [Google Scholar]
- Bennett S, Wise R, Weston D, Dent J. Pharmacokinetics and tissue penetration of ticarcillin combined with clavulanic acid. Antimicrob Agents Chemother. 1983 Jun;23(6):831–834. [Europe PMC free article] [Abstract] [Google Scholar]
- Casey P, Glauser M. Susceptibility of gram-negative bacteria and Staphylococcus aureus to combinations of ticarcillin and clavulanic acid. Eur J Clin Microbiol. 1983 Dec;2(6):541–547. [Abstract] [Google Scholar]
- Chattopadhyay B, Hall I. In vitro activity of ticarcillin plus clavulanic acid (BRL 28500, 'Timentin') against ticarcillin-resistant gram-negative rods. Curr Med Res Opin. 1984;9(3):157–160. [Abstract] [Google Scholar]
- Clarke AM, Zemcov SJ. Clavulanic acid in combination with ticarcillin: an in-vitro comparison with other beta-lactams. J Antimicrob Chemother. 1984 Feb;13(2):121–128. [Abstract] [Google Scholar]
- Ericsson HM, Sherris JC. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [Abstract] [Google Scholar]
- Farmer JJ, 3rd, Weinstein RA, Zierdt CH, Brokopp CD. Hospital outbreaks caused by Pseudomonas aeruginosa: importance of serogroup O11. J Clin Microbiol. 1982 Aug;16(2):266–270. [Europe PMC free article] [Abstract] [Google Scholar]
- Fuchs PC, Barry AL, Thornsberry C, Jones RN. In vitro activity of ticarcillin plus clavulanic acid against 632 clinical isolates. Antimicrob Agents Chemother. 1984 Mar;25(3):392–394. [Europe PMC free article] [Abstract] [Google Scholar]
- Fuchs PC, Jones RN, Barry AL, Thornsberry C. Disk diffusion susceptibility testing of ticarcillin plus clavulanic acid. J Clin Microbiol. 1984 Apr;19(4):555–557. [Europe PMC free article] [Abstract] [Google Scholar]
- Gwynn MN, Webb TL, Rolinson GN. Regrowth of Pseudomonas aeruginosa and other bacteria after the bactericidal action of carbenicillin and other beta-lactam antibiotics. J Infect Dis. 1981 Sep;144(3):263–269. [Abstract] [Google Scholar]
- Henry D, Skidmore AG, Ngui-Yen J, Smith A, Smith JA. In vitro activities of enoxacin, ticarcillin plus clavulanic acid, aztreonam, piperacillin, and imipenem and comparison with commonly used antimicrobial agents. Antimicrob Agents Chemother. 1985 Aug;28(2):259–264. [Europe PMC free article] [Abstract] [Google Scholar]
- Hunter PA, Coleman K, Fisher J, Taylor D. In vitro synergistic properties of clavulanic acid, with ampicillin, amoxycillin and ticarcillin. J Antimicrob Chemother. 1980 Jul;6(4):455–470. [Abstract] [Google Scholar]
- Mathisen GE, Meyer RD, Thompson JM, Finegold SM. Clinical evaluation of moxalactam. Antimicrob Agents Chemother. 1982 May;21(5):780–786. [Europe PMC free article] [Abstract] [Google Scholar]
- Murray PR, Granich GG, Krogstad DJ, Niles AC. In vivo selection of resistance to multiple cephalosporins by enterobacter cloacae. J Infect Dis. 1983 Mar;147(3):590–590. [Abstract] [Google Scholar]
- Neu HC, Fu KP. Clavulanic acid, a novel inhibitor of beta-lactamases. Antimicrob Agents Chemother. 1978 Nov;14(5):650–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Paisley JW, Washington JA., 2nd Combined activitiy of clavulanic acid and ticarcillin against ticarcillin-resistant, gram-negative bacilli. Antimicrob Agents Chemother. 1978 Aug;14(2):224–227. [Europe PMC free article] [Abstract] [Google Scholar]
- Parry MG, Neu HC. Ticarcillin for treatment of serious infections with gram-negative bacteria. J Infect Dis. 1976 Nov;134(5):476–485. [Abstract] [Google Scholar]
- Preheim LC, Penn RG, Sanders CC, Goering RV, Giger DK. Emergence of resistance to beta-lactam and aminoglycoside antibiotics during moxalactam therapy of Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 1982 Dec;22(6):1037–1041. [Europe PMC free article] [Abstract] [Google Scholar]
- Reading C, Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. [Europe PMC free article] [Abstract] [Google Scholar]
- Rylander M, Brorson JE, Johnsson J, Norrby R. Comparison between agar and broth minimum inhibitory concentrations of cefamandole, Cefoxitin, and cefuroxime. Antimicrob Agents Chemother. 1979 Apr;15(4):572–579. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanders CC, Moellering RC, Jr, Martin RR, Perkins RL, Strike DG, Gootz TD, Sanders WE., Jr Resistance to cefamandole: a collaborative study of emerging clinical problems. J Infect Dis. 1982 Jan;145(1):118–125. [Abstract] [Google Scholar]
- Stratton CW. Susceptibility testing revisited. Prog Clin Pathol. 1984;9:65–100. [Abstract] [Google Scholar]
Associated Data
Articles from Antimicrobial Agents and Chemotherapy are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aac.30.1.35
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc176430?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
In vitro susceptibilities of feline and canine Escherichia coli and Pseudomonas spp. isolates to ticarcillin and ticarcillin-clavulanic acid.
Aust Vet J, 91(5):171-178, 01 May 2013
Cited by: 0 articles | PMID: 23614511
Stenotrophomonas maltophilia: emergence of multidrug-resistant strains during therapy and in an in vitro pharmacodynamic chamber model.
Antimicrob Agents Chemother, 40(12):2859-2864, 01 Dec 1996
Cited by: 54 articles | PMID: 9124855 | PMCID: PMC163636
Ticarcillin/clavulanic acid combination. Treatment of bacterial infections in hospitalized children.
Clin Pediatr (Phila), 28(11):521-524, 01 Nov 1989
Cited by: 3 articles | PMID: 2805557
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparative disk and broth dilution susceptibility test results with ticarcillin and timentin against Pseudomonas aeruginosa and Pseudomonas maltophilia.
Chemotherapy, 33(5):340-346, 01 Jan 1987
Cited by: 4 articles | PMID: 3117502
Disk diffusion susceptibility testing of ticarcillin plus clavulanic acid.
J Clin Microbiol, 19(4):555-557, 01 Apr 1984
Cited by: 3 articles | PMID: 6715524 | PMCID: PMC271119
Resistance to ticarcillin-potassium clavulanate among clinical isolates of the family Enterobacteriaceae: role of PSE-1 beta-lactamase and high levels of TEM-1 and SHV-1 and problems with false susceptibility in disk diffusion tests.
Antimicrob Agents Chemother, 32(9):1365-1369, 01 Sep 1988
Cited by: 49 articles | PMID: 3143303 | PMCID: PMC175869
Beta-lactamase inhibitor combinations with extended-spectrum penicillins: factors influencing antibacterial activity against enterobacteriaceae and Pseudomonas aeruginosa.
Pharmacotherapy, 20(9 pt 2):213S-218S; discussion 224S-228S, 01 Sep 2000
Cited by: 11 articles | PMID: 11001328
Review