Abstract
Background & aims
Two advanced cytologic techniques for detecting aneuploidy-digital image analysis (DIA) and fluorescence in situ hybridization (FISH)-have recently been developed to help identify malignant pancreatobiliary strictures. The aim of this study was to assess the clinical utility of cytology, DIA, and FISH for the identification of malignant pancreatobiliary strictures.Methods
Brush cytologic specimens from 233 consecutive patients undergoing endoscopic retrograde cholangiopancreatography for pancreatobiliary strictures were examined by all 3 (cytology, DIA, and FISH) techniques. Strictures were stratified as proximal (n = 33) or distal (n = 114) based on whether they occurred above or below the cystic duct, respectively. Strictures in patients with primary sclerosing cholangitis (n = 86) were analyzed separately.Results
Despite the stratification, the performances of the tests were similar. Conventional cytology has a low sensitivity (4%-20%) but 100% specificity. Because of the high specificity for cytology, we assessed the performance of the other tests when conventional cytology was negative. In this clinical context, FISH had an increased sensitivity (35%-60%) when assessing for chromosomal gains (polysomy) while preserving the specificity of cytology. The sensitivity and specificity of DIA was intermediate as compared with routine cytology and FISH but was additive to FISH values demonstrating only trisomy of chromosome 7 or chromosome 3.Conclusions
These findings suggest that FISH and DIA increase the sensitivity for the diagnosis of malignant pancreatobiliary tract strictures over that obtained by conventional cytology while maintaining an acceptable specificity.Free full text

Advanced Cytologic Techniques for the Detection of Malignant Pancreatobiliary Strictures
Abstract
Background & Aims
Two advanced cytologic techniques for detecting aneuploidy, digital image analysis (DIA) and fluorescence in situ hybridization (FISH) have recently been developed to help identify malignant pancreatobiliary strictures. The aim of this study was to assess the clinical utility of cytology, DIA, and FISH for the identification of malignant pancreatobiliary strictures.
Methods
Brush cytologic specimens from 233 consecutive patients undergoing ERCP for pancreatobiliary strictures were examined by all three techniques. Strictures were stratified as proximal (n=33) or distal (n=114) based on whether they occurred above or below the cystic duct, respectively. Strictures in patients with PSC (n=86) were analyzed separately.
Results
Despite the stratification, the performances of the tests were similar. Routine cytology has a low sensitivity (5–20%) but 100% specificity. Because of the high specificity for cytology, we assessed the performance of the other tests when routine cytology was negative. In this clinical context, FISH had an increased sensitivity (35–60%) when assessing for chromosomal gains (polysomy) while preserving the specificity of cytology. The sensitivity and specificity of DIA was intermediate as compared to routine cytology and FISH, but was additive to FISH values demonstrating only trisomy of chromosome 7 or chromosome 3.
Conclusions
These findings suggest that FISH and DIA increase the sensitivity for the diagnosis of malignant pancreatobiliary tract strictures over that obtained by conventional cytology while maintaining an acceptable specificity.
Pancreatobiliary strictures of the extrahepatic bile ducts are a common occurrence in clinical practice. While many of these strictures are due to malignancies of the biliary tract and pancreas, the strictures may also have a nonmalignant etiopathogenesis. For example, inflammatory conditions such as choledocholithiasis, chronic pancreatitis, surgical trauma, ischemia, and idiopathic processes are all recognized as causes of pancreatobiliary strictures 1–3. The distinction between benign and malignant pancreatobiliary strictures can be problematic for several reasons. A cancer may be present but not identified on cross-sectional imaging studies because these cancers often grow longitudinally along the bile duct rather than radially away from the bile duct. Access to the bile duct is limited for cytologic and tissue acquisition and these cancers are frequently desmoplastic resulting in acellular sampling. Indeed, routine cytology obtained from endoscopic brushings has sensitivities of only 20–40% and when the sample is from bile duct aspirates, the sensitivities can be as low as 6 to 32% 4. The distinction between malignant and inflammatory strictures is further confounded in primary sclerosing cholangitis (an inflammatory, stricturing disease of the bile ducts which predisposes to the development of cholangiocarcinoma), since the inflammation associated with this disorder complicates cytologic assessment. Advanced endoscopic approaches such as intraductal ultrasound and choledochoenteroscopy are being developed to further assess these strictures 5–7, although these techniques have not been well validated nor are they widely available. Serum CA 19-9 determinations which are elevated in cancer can be useful, however, serum CA 19-9 levels can be elevated in patients without malignancy (e.g. patients with bacterial cholangitis). Thus, the accurate diagnosis of pancreatobiliary strictures is often challenging and this compromises management decisions. Additional tools are needed to accurately diagnose pancreatobiliary strictures of the bile duct.
Chromosomal instability is a nearly universal hallmark of the cancer genome that results in aneuploidy (i.e. abnormalities in the number of chromosomes within a cell) and/or structural chromosomal abnormalities (e.g. gene deletion and amplification) 8, 9. The detection of these chromosomal abnormalities has the potential to serve as a sensitive technique for identifying tumor cells in cytologic specimens. Based on these concepts and the observation that approximately 80% of biliary cancers exhibit aneuploidy 10, two advanced cytologic techniques for detecting chromosomal alterations (aneuploidy) have been developed to identify cancer in the biliary tract. Digital Image Analysis (DIA) and Fluorescence in situ Hybridization (FISH) are two cytologic techniques that have been shown to significantly increase the diagnostic sensitivity of biliary tract malignancies over cytology while maintaining the high specificity of cytology. In limited studies, both techniques have shown promise in accurately identifying malignant pancreatobiliary strictures 11, 12.
DIA is a technique that uses a microscope and camera to quantify the amount of cellular DNA by measuring the intensity of nuclei stained with the Feulgen dye, a cytochemical stain that stoichiometrically binds to nuclear DNA 13. FISH is a technique that utilizes fluorescently labeled DNA probes to detect chromosomal abnormalities in cells and has been shown to detect malignancy in cytologic specimens from different body sites 14–16. Although these techniques have been shown to increase the sensitivity of detecting malignancy in biliary tract specimens over cytology, there is a lack of data assessing the utility of these techniques in tandem and their ability to detect malignancy in tumors from different pancreatobiliary locations in patients with and without a history of PSC. Malignant biliary strictures distal to the cystic duct are often due to pancreatic cancers and have a different biology than cholangiocarcinomas which more frequently involve the bile duct proximal to the cystic duct 1, 17. Likewise, the genetic profile of cholangiocarcinomas arising in the background of PSC may be different from that of cholangiocarcinomas occurring in the absence of hepatobiliary disease.
The overall objective of the current study was to assess the diagnostic utility of cytology, DIA and FISH in assessing pancreatobiliary strictures of the bile duct. Specifically, we sought to determine the sensitivity, specificity and positive and negative predictive value of these assays for the detection of malignancies in proximal and distal pancreatobiliary strictures occurring in patients with or without PSC. This information may help clinicians in the clinical management of patients with biliary tract strictures.
MATERIALS AND METHODS
Patient Population
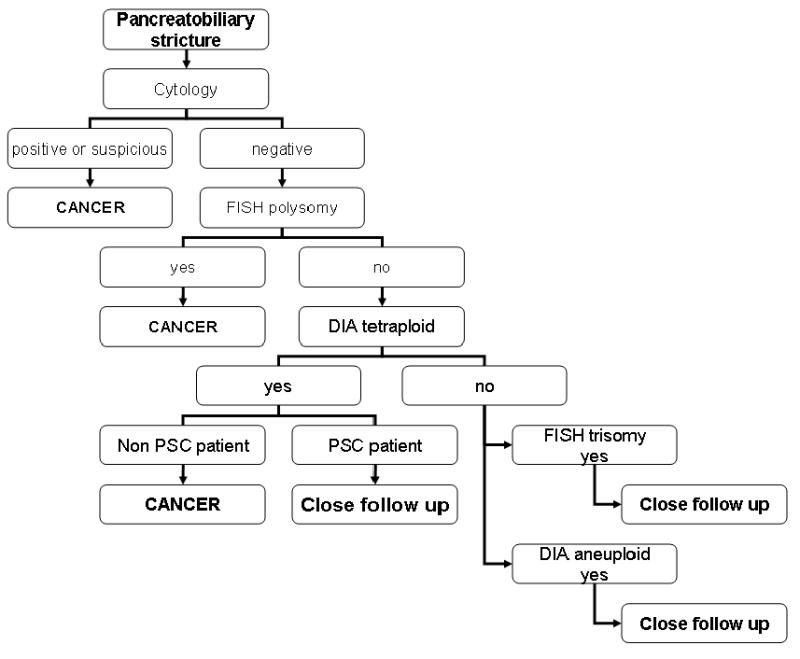
This study was approved by the Mayo Clinic Institutional Review Board and all patients gave written informed consent. Biliary tract brushing specimens for cytology, FISH and DIA were prospectively collected from 277 unique patients undergoing ERCP for possible pancreatobiliary malignancy between October 2003 and August 2004. Twenty-three of the 277 patients declined participation in the study, 9 patients were excluded because therapy (radiation therapy) precluded a definitive diagnosis, 5 patients were excluded because we were missing sufficient follow up information to confidentially make a diagnosis, 3 patients were excluded because a definitive diagnosis was obtained more than 3 months after the screening test was performed, 2 patients where lost to follow up and two patients were not included because their medical records did not reside at our institution. Thus, a total of 233 patients were included in the study. Eligibility criteria for this study were: a) a pancreatobiliary stricture of recent onset (≤ 12 months) diagnosed by endoscopic retrograde cholangiopancreatography (ERCP); b) a definitive diagnosis of the stricture as benign or malignant by either surgery, surgical pathology, or sufficient follow-up (at least 9 months) to assure a benign or malignant course (i.e. obvious progression of a malignant disease with metastases, and/or the presence of a progressive “tumor mass” on cross-sectional imaging studies and/or death from cancer); and c) a cholangiogram available to determine the location of the stricture (proximal or distal) and to assess for the presence or absence of primary sclerosing cholangitis (PSC). The diagnosis of PSC was based on a cholangiogram demonstrating a diffuse biliary stricturing process, an elevated serum alkaline phosphatase, and an absence of secondary causes of biliary tract disease (e.g. choledocholithiasis, prior biliary tract surgery, etc.). The stratification of the patients is depicted in Figure 1.
The medical records were reviewed for patient demographic information and the results of cross sectional imaging studies (CT scan and MRI). Although the cytologic studies (conventional cytology, DIA, and FISH) were obtained by protocol for all patients, there was no protocol for the systematic determination of serum CA 19-9 values or the type of non-invasive imaging tests performed.
Of the 233 patients enrolled in this study, 86 had PSC and 147 did not. There were 132 men and 101 women. Patient age ranged from 9 to 91 years (mean 59.4 years, median 61 years).
Sample Acquisition and Preparation
Two separate samples were collected from the biliary stricture using standard DLB-35-1.5 or DLB-35-3.5 brushes (Wilson-Cook, Winston-Salem, NC). The brush was advanced through the stricture with at least 5 to 8 to-and-fro movements. In order to optimize the cellular yield, the brush was pushed from the end of the sheath, as opposed to pulling the brush from the sheath, and the cut brush was placed in a vial containing 20 mL of PreservCyt solution (Cytyc Corporation, Marlborough, MA). The specimens were transferred to the Mayo Clinic Cytopathology Department on the same day where a cytotechnologist equally divided the specimen with half of the total sample designated for RC analysis. The other half of the sample was submitted for DIA and FISH analysis, which resulted in 25% of the total sample designated for DIA analysis and 25% for FISH analysis. Of note, a second GI nurse assistant was available at the time of brushing to assist in the process in order to minimize processing time and avoid air-drying. In addition, direct brushing and immediate Pap smear staining for cytology was not performed because this technique involves air drying of the specimen that has been shown to minimize diagnostic sensitivity. Care was taken to avoid sampling of non-stricture regions to avoid filling the brush fibers with normal mucosa, which can also reduce diagnostic accuracy. Given the varied data and uncertain benefit of pre-sampling stricture dilatation, we do not routinely dilate stricture unless absolutely necessary to gain access.
Cytology, DIA and FISH Testing
Cytology, DIA, and FISH analyses were performed in the Department of Laboratory of Medicine and Pathology, Mayo Clinic, Rochester, MN by cytotechnologists who had no knowledge of the other test results or patient’s clinical history. Cytologic diagnoses were classified as malignant, suspicious for malignancy, atypical, or benign using accepted criteria 18. DIA was performed as previously described 11, and the results were categorized as diploid (DNA index between 0.95 and 1.10), aneuploid (DNA index between 1.11 and 1.89), or tetraploid (DNA index between 1.90 to 2.10). Aneuploid and tetraploid results were considered positive for malignancy.
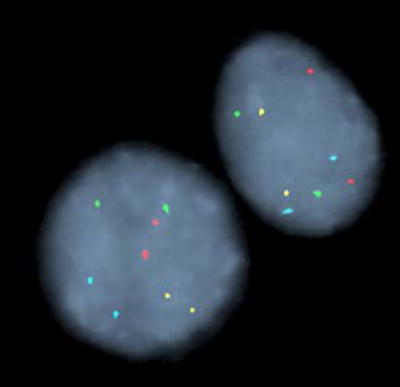
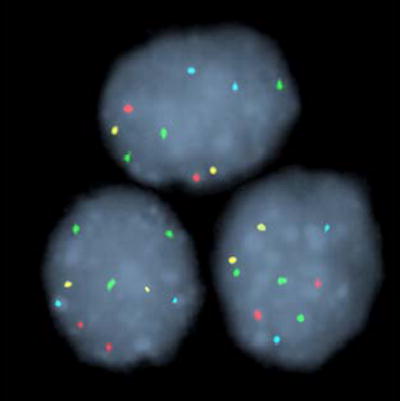
FISH was performed as previously described using the UroVysion probe set (Abbott Molecular Inc., Des Plaines, IL) 12. This probe set consists of directly labeled DNA probes to the peri-centromeric regions of chromosomes 3 (CEP® 3), 7 (CEP® 7), and 17 (CEP® 17), and to chromosomal band 9p21 (LSI® 9p21). Following probe hybridization, slides were assessed as previously described 12. Two general types of chromosomal abnormality were observed by FISH in this study, polysomy and trisomy of chromosome 7 or 3. Representative examples of polysomy, trisomy 7 and trisomy 3 are shown in Figure 2. A patient’s specimen was positive for malignancy if ≥5 cells showed gains of two or more of the four probes (polysomy) or if ≥10 cells showed three copies of chromosome 7 (or 3) and two or fewer copies of the other three probes. We have a higher cutoff for trisomy 7 or trisomy 3 because signal splitting can lead to false positive trisomic signals being observed at low numbers even in normal specimens.
Statistical analysis
Nominal variables were expressed as proportions. Sensitivity, specificity, and positive and negative predictive values together with their exact 95% confidence intervals were obtained based upon the binomial distribution. For each of the three methods we made three evaluations based upon the different possible outcomes indicative of most likely and probable for malignancy. In particular, for cytology we considered the tests based upon cytology positive or suspicious, then cytology positive and finally cytology suspicious. For DIA we considered the tests based upon a finding of either aneuploid or tetraploid, then aneuploid (irrespective of tetraploid results), and finally tetraploid and not aneuploid, and for FISH we first considered the tests based upon a finding of either polysomic or trisomy, then polysomic (irrespective of trisomy results) and finally only trisomy (trisomy 7 or trisomy 3) and not polysomic. Not all specimens obtained were useable, inadequate samples (n= 1 for cytology, n= 17 for DIA and n= 11 for FISH) were excluded from the analysis. Statistical significance was inferred for P < 0.05.
RESULTS
Proximal strictures in non-PSC patients
Description
Baseline characteristics are described in Table 1. Among non PSC-patients with proximal strictures, twenty two (67%) of the 33 proximal strictures were malignant and 11 were benign (Figure 1). The diagnosis of malignancy was based on surgical pathology for 18 (82%) of the patients and by the presence of metastases and/or tumor progression in 4 patients (18%) (Table 2). The etiology of benign and malignant strictures is shown in Table 3.
Table 1
Patient characteristics
| Characteristic | Non PSC | PSC |
|---|---|---|
| Total patients | 147 | 86 |
| Mean Age (range) | 65 (9–91) | 50 (21–84) |
| Male: female | 84: 63 | 48:38 |
| Caucasian : Non Caucasian | 123:4 | 73:2 |
Table 2
Diagnosis of cancer in PSC and non PSC patients
| Subgroups of patients with the diagnosis of cancer | N (%) |
|---|---|
| Proximal Strictures | N=22 |
| Metastases and/or tumor progression | 4 (18%) |
| Biopsy | 18 (82%) |
| Distal Strictures | N=66 |
| Metastases and/or tumor progression | 20 (30%) |
| Biopsy | 46 (70%) |
| Primary Sclerosing Cholangitis | N=17 |
| Metastases and/or tumor progression | 6 (35%) |
| Biopsy | 11 (65%) |
Table 3
Diagnosis of strictures in non PSC patients
| Strictures Etiology | N (%) |
|---|---|
| Proximal Strictures | N=33 |
| Malignant Strictures | |
 Cholangiocarcinoma Cholangiocarcinoma | 19 (86%) |
 Gallbladder adenocarcinoma Gallbladder adenocarcinoma | 1 (4%) |
 Other cancers* Other cancers* | 2 (9%) |
| Benign strictures | |
 Postsurgical Postsurgical | 6 (54%) |
 Biliary varices Biliary varices | 2 (18%) |
 Autoimmune pancreatitis Autoimmune pancreatitis | 2 (18%) |
 Polycystic liver disease Polycystic liver disease | 1 (9%) |
| Distal Strictures | N=114 |
| Malignant strictures | |
 Pancreatic adenocarcinoma Pancreatic adenocarcinoma | 35 (53%) |
 Cholangiocarcinoma Cholangiocarcinoma | 19 (29%) |
 Metastatic cancer to the bile duct Metastatic cancer to the bile duct | 5 (7%) |
 Ampullary adenocarcinoma Ampullary adenocarcinoma | 4 (6%) |
 Gallbladder adenocarcinoma Gallbladder adenocarcinoma | 1 (1%) |
 Other cancers § Other cancers § | 2 (3%) |
| Benign strictures | |
 Acute/chronic pancreatitis Acute/chronic pancreatitis | 21 (44%) |
 Choledocholithiasis Choledocholithiasis | 8 (17%) |
 Post surgical Post surgical | 6 (12%) |
 Idiopathic Idiopathic | 5 (10%) |
 Ampullary stenosis Ampullary stenosis | 4 (8%) |
 Cholangiopathy and biliary cast Syndrome Cholangiopathy and biliary cast Syndrome | 1 (2%) |
 Cavernous transformation of the portal vein Cavernous transformation of the portal vein | 1 (2%) |
 Biliary varices Biliary varices | 1 (2%) |
 Serous cystadenoma Serous cystadenoma | 1 (2%) |
Cytologic techniques results
The sensitivity, specificity, positive and negative predictive value for the detection of malignancy in patients with proximal strictures are shown in Table 4. As expected, cytology had a very low sensitivity if only positive cases were considered positive for malignancy. Sensitivity increased if both positive and suspicious cases were considered positive. Cytology results interpreted as suspicious for adenocarcinoma must be recognized as almost equivalent to cases interpreted as positive for cancer since the specificity of cytology was also high when only suspicious specimens were categorized as positive (Table 4). In the DIA aneuploid patients there was one false positive DIA result, but no patient with a tetraploid DIA had a false positive result. DIA aneuploid and tetraploid had a higher sensitivity than that of cytology, even when suspicious samples were considered as positive for the cytology analysis. The DIA sensitivity and specificity are intermediate between cytology and FISH. There were no false positive polysomy FISH results in the group of patients with proximal strictures and consequently the specificity was 100%.
Table 4
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of Cytology, DIA, and FISH for the Detection of Malignancy by Stricture Classification
| NON PSC PATIENTS | SENSITIVITY (95% CI) | SPECIFICITY (95% CI) | PPV* (95% CI) | NPV≠ (95% CI) |
|---|---|---|---|---|
| PROXIMAL | ||||
| Cytology (positive or suspicious) | 9% (0.01, 0.30) | 100% (0.71, 1) | 100% (0.16, 1) | 37% (0.20, 0.56) |
 Positive Positive | 4% (0.001, 0.24) | 100% (0.71, 1) | 100% (-) | 35% (0.19, 0.55) |
 Suspicious Suspicious | 4% (0.001, 0.24) | 100% (0.71, 1) | 100% (-) | 35% (0.19, 0.55) |
| DIA (aneuploid or tetraploid) | 30% (0.12, 0.54) | 90% (0.55, 1) | 86% (0.42, 1) | 39% (0.20, 0.61) |
 Tetraploid Tetraploid | 5% (0.001, 0.25) | 100% (0.69, 1) | 100% (-) | 34% (0.18, 0.54) |
 Aneuploid Aneuploid | 25% (0.09, 0.49) | 90% (0.55, 1) | 83% (0.36, 0.99) | 37% (0.19, 0.59) |
| FISH (polysomy or trisomy) | 63% (0.38, 0.84) | 100% (0.66, 1) | 100% (0.73, 1) | 56% (0.30, 0.80) |
 FISH polysomy FISH polysomy | 31% (0.12, 0.56) | 100% (0.66, 1) | 100% (0.54, 1) | 41% (0.21, 0.64) |
 FISH trisomy FISH trisomy | 31% (0.12, 0.56) | 100% (0.66, 1) | 100% (0.54, 1) | 41% (0.21, 0.64) |
| DISTAL | ||||
| Cytology (positive or suspicious) | 41% (0.29, 0.54) | 96% (0.86, 0.99) | 93% (0.77, 0.99) | 54% (0.43, 0.65) |
 Positive Positive | 20% (0.11, 0.31) | 100% (0.93, 1) | 100% (0.75, 1) | 47% (0.37, 0.58) |
 Suspicious Suspicious | 21% (0.12, 0.33) | 96% (0.86, 0.99) | 87% (0.62, 0.98) | 47% (0.37, 0.57) |
| DIA (aneuploid or tetraploid) | 49% (0.36, 0.62) | 98% (0.88, 1) | 97% (0.84, 1) | 57% (0.45, 0.69) |
 Tetraploid Tetraploid | 16% (0.08, 0.27) | 100% (0.92, 1) | 100% (0.69, 1) | 45% (0.35, 0.56) |
 Aneuploid Aneuploid | 33% (0.22, 0.46) | 98% (0.88, 1) | 95% (0.77, 1) | 50% (0.39, 0.62) |
| FISH (polysomy or trisomy) | 59% (0.46, 0.71) | 92% (0.80, 0.98) | 90% (0.77, 0.97) | 63% (0.50, 0.74) |
 FISH polysomy FISH polysomy | 48% (0.35, 0.60) | 100% (0.93, 1) | 100% (0.88, 1) | 59% (0.48, 0.70) |
 FISH trisomy FISH trisomy | 11% (0.04, 0.21) | 92% (0.80, 0.98) | 64% (0.31, 0.89) | 44% (0.34, 0.54) |
| PSC PATIENTS | ||||
| Cytology (positive or suspicious) | 41% (0.18, 0.67) | 97% (0.90, 1) | 78% (0.40, 0.97) | 87% (0.77, 0.93) |
 Positive Positive | 18% (0.04, 0.43) | 100% (0.95, 1) | 100% (0.29, 1) | 83% (0.73, 0.90) |
 Suspicious Suspicious | 23% (0.07, 0.50) | 97% (0.90, 1) | 67% (0.22, 0.96) | 83% (0.73, 0.91) |
| DIA (aneuploid or tetraploid) | 43% (0.18, 0.71) | 87% (0.76, 0.94) | 43% (0.18, 0.71) | 87% (0.76, 0.94) |
 Tetraploid Tetraploid | 14% (0.02, 0.43) | 95% (0.86, 0.99) | 40% (0.05, 0.85) | 83% (0.72, 0.91) |
 Aneuploid Aneuploid | 28% (0.08, 0.58) | 92% (0.82, 0.97) | 44% (0.14, 0.79) | 85% (0.74, 0.92) |
| FISH (polysomy or trisomy 7or 3) | 70% (0.44, 0.90) | 86% (0.75, 0.93) | 57% (0.34, 0.78) | 92% (0.82, 0.97) |
 FISH polysomy FISH polysomy | 47% (0.23, 0.72) | 100% (0.94, 1) | 100% (0.63, 1) | 88% (0.78, 0.94) |
 FISH trisomy FISH trisomy | 23% (0.07, 0.50) | 86% (0.75, 0.93) | 31% (0.09, 0.61) | 81% (0.69, 0.89) |
CI denotes 95% confidence interval
(-) = insufficient number of patients to calculate 95% CI
In clinical practice the physician can be presented with a negative cytology and a positive FISH and/or DIA. Therefore, we also calculated the sensitivity, specificity, positive predictive value and negative predictive value when FISH and/or DIA were positive and cytology was neither positive nor suspicious. These results are described in Table 5. FISH either polysomic or trisomy as positive had a high sensitivity and specificity. DIA aneuploid had an intermediate sensitivity with a good specificity; when only tetraploid results were considered as positive there was loss in sensitivity but the specificity was 100%. Most importantly, when combining FISH and DIA, the sensitivity was more than two fold that of cytology with a specificity of 100%. (Table 5)
Table 5
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of Cytology, DIA, and FISH for the Detection of Malignancy by Stricture Classification when Cytology is neither positive nor suspicious
| NON PSC PATIENTS | SENSITIVITY (95% CI) | SPECIFICITY (95% CI) | PPV* (95% CI) | NPV≠ (95% CI) |
|---|---|---|---|---|
| PROXIMAL | ||||
| DIA (tetraploid or aneuploid) | 28% (0.1, 0.53) | 90% (0.55, 1) | 83% (0.36, 0.99) | 41% (0.21, 0.64) |
 Tetraploid Tetraploid | 5% (0.001, 0.27) | 100% (0.69, 1) | 100% (-) | 37% (0.19, 0.58) |
 Aneuploid Aneuploid | 22% (0.06, 0.48) | 90% (0.55, 1) | 80% (0.28, 0.99) | 39% (0.20, 0.61) |
| FISH (polysomy or trisomy 7 or 3) | 59% (0.33, 0.81) | 100% (0.66, 1) | 100% (0.69, 1) | 56% (0.30, 0.80) |
 FISH polysomy FISH polysomy | 23% (0.07, 0.50) | 100% (0.66, 1) | 100% (0.40, 1) | 41%(0.21, 0.64) |
 FISH trisomy (7 or 3) FISH trisomy (7 or 3) | 35% (0.14, 0.62) | 100% (0.66, 1) | 100% (0.54, 1) | 45% (0.23, 0.68) |
| FISH (polysomy or trisomy) and DIA (aneuploid or tetraploid) | 23% (0.07, 0.50) | 100% (0.66, 1) | 100% (0.40, 1) | 41% (0.21, 0.64) |
| FISH (polysomy or trisomy) or DIA (aneuploid or tetraploid) | 65% (0.38, 0.86) | 89% (0.52, 1) | 92% (0.61, 1) | 57% (0.29, 0.82) |
| DISTAL | ||||
| DIA (aneuploid or tetraploid) | 25% (0.12, 0.42) | 98% (0.87, 0.99) | 90% (0.55, 1) | 60% (0.48, 0.72) |
 Tetraploid Tetraploid | 5% (0.007, 0.19) | 100% (0.91, 1) | 100% (0.16, 1) | 55% (0.43, 0.67) |
 Aneuploid Aneuploid | 19% (0.08, 0.36) | 98% (0.87, 0.99) | 87% (0.47, 1) | 58% (0.46, 0.70) |
| FISH (polysomy or trisomy 7 or 3) | 35% (0.20, 0.52) | 93% (0.82, 0.99) | 81% (0.54, 0.96) | 64% (0.51, 0.75) |
 FISH polysomy FISH polysomy | 22% (0.1, 0.38) | 100% (0.92, 1) | 100% (0.63, 1) | 61% (0.49, 0.72) |
 FISH trisomy (7 or 3) FISH trisomy (7 or 3) | 13% (0.04, 0.29) | 93% (0.82, 0.99) | 62% (0.24, 0.91) | 57% (0.45, 0.69) |
| FISH (polysomy or trisomy) and DIA (aneuploid or tetraploid) | 15% (0.05, 0.31) | 98% (0.87, 1) | 83% (0.36, 0.99) | 58% (0.46, 0.70) |
| FISH (polysomy or trisomy) or DIA (aneuploid or tetraploid) | 48% (0.31, 0.66) | 93% (0.80, 0.98) | 85% (0.62, 0.97) | 68% (0.55, 0.80) |
| PSC PATIENTS | ||||
| DIA (aneuploid or tetraploid) | 14% (0.004, 0.58) | 88% (0.77, 0.95) | 12% (0.003, 0.53) | 90% (0.79, 0.96) |
 Tetraploid Tetraploid | 0 (0, 0.41) | 97% (0.88, 0.99) | 0 (0, 0.84) | 89% (0.79, 0.95) |
 Aneuploid Aneuploid | 14% (0.004, 0.58) | 91% (0.81, 0.97) | 17% (0.004, 0.64) | 90% (0.79, 0.96) |
| FISH (polysomy or trisomy 7 or 3) | 60% (0.26, 0.88) | 87% (0.76, 0.94) | 43% (0.18, 0.71) | 93% (0.83, 0.98) |
 FISH polysomy FISH polysomy | 20% (0.02, 0.56) | 100% (0.94, 1) | 100% (0.16, 1) | 88% (0.79, 0.95) |
 FISH trisomy (7 or 3) FISH trisomy (7 or 3) | 40% (0.12, 0.74) | 87% (0.76, 0.94) | 33% (0.1, 0.65) | 90% (0.79, 0.96) |
| FISH (polysomy or trisomy) and DIA (aneuploid or tetraploid) | 14% (0.004, 0.58) | 98% (0.91, 1) | 50% (0.01, 0.99) | 90% (0.80, 0.96) |
| FISH (polysomy or trisomy) or DIA (aneuploid or tetraploid) | 67% (0.30, 0.92) | 75% (0.62, 0.86) | 30% (0.12, 0.54) | 93% (0.82, 0.99) |
CI denotes 95% confidence interval
(-) = insufficient number of patients to calculate 95% CI
Distal strictures in non-PSC patients
Description
Baseline characteristics are described in Table 1. Of the 114 patients with distal strictures, 66 were malignant and 48 were benign (Figure 1). The etiology of benign and malignant strictures is shown in Table 3. In 46 (70%) of the patients the diagnosis of cancer was confirmed by surgical resection, endoscopic biopsy or percutaneous biopsy, in 20 (30%) of the patients, the diagnosis was based on the presence of a malignant appearing mass, metastasis, and/or tumor progression detected by imaging studies.
Cytologic techniques results
The sensitivity, specificity, positive and negative predictive value are described in Table 4. The same approach in analyzing the findings for proximal strictures was applied in this setting. When both positive and suspicious cytology results were considered as positive for malignancy, the sensitivity of cytology was increased compared to proximal strictures, with a very high specificity. When only positive cytology results were considered as positive, the sensitivity dropped to half, but the specificity increased to 100%. DIA overall (aneuploid plus tetraploid) had an intermediate sensitivity and a very high specificity. When only tetraploid results were analyzed, the sensitivity was lower but with a 100% specificity. For the FISH results, we also analyzed the data in the three different ways as previously described for proximal strictures. When both polysomy and trisomy FISH findings were considered as positive for malignancy, the sensitivity of FISH was 59% and the specificity was 92%. If only polysomic results were considered positive the sensitivity dropped and the specificity increased to 100%. Thus, FISH had the highest sensitivity of the three assays but a slightly lower specificity if both polysomy and trisomy were viewed as positive.
We also calculated the sensitivity, specificity, and positive and negative predictive value when FISH and/or DIA were positive and cytology was neither positive nor suspicious for cancer (Table 5). DIA aneuploid or tetraploid showed an intermediate sensitivity for distal pancreatobiliary strictures with a very high specificity. DIA aneuploid results demonstrated a lower sensitivity with a very high specificity. DIA tetraploid for this analysis had the lowest sensitivity, but a specificity of 100%. FISH either polysomic or trisomic, again demonstrated the best sensitivity between the three tests, with a high specificity. When only FISH polysomic samples were analyzed the specificity was again 100%. FISH trisomy alone showed a low sensitivity with a high specificity. When combining FISH and DIA for the analysis, the sensitivity was lower because most of the positive FISH and DIA patients had positive cytology results and these were excluded for this analysis. The specificity was 98%. (Table 5)
PSC patients
Description
Baseline characteristics are described in Table 1. Fifty seven (65%) patients had inflammatory bowel disease (IBD), and 15 (17%) had a history of colonic dysplasia or colon cancer. Sixty seven (78%) patients had benign strictures, 17 (20%) had malignant strictures, and 2 (2%) had strictures of indeterminate status (Figure 1). The mean follow up time was 6 months (range 1 to 118 months). In 11 (65%) patients with a malignant stricture, the diagnosis of cancer was confirmed pathologically, in 6 (35%) patients, the diagnosis was based on the presence of a malignant appearing mass, metastasis and/or tumor progression detected by imaging studies (Table 2).
Cytologic techniques results
The sensitivity, specificity, and positive and negative predictive values are depicted in Table 4, using the same approach for data analysis described for proximal strictures. In PSC patients, cytology also had a low sensitivity with a specificity of 100% if only unequivocally positive cases were considered positive. The sensitivity of cytology increased if both positive and suspicious samples were considered as positive and the specificity dropped slightly to 97%.
Overall DIA had an intermediate sensitivity and specificity. This was the only subgroup of patients were tetraploid DIA had a lower specificity (95%) emphasizing the different nature of PSC strictures. Among the three tests studied and the three different subgroups of patients, FISH polysomic plus trisomy had the best sensitivity (70%) with a lower specificity. All of the false positive FISH results were in patients who exhibited trisomy cells. Consequently, the specificity of the assay was very high if only polysomy was considered positive for malignancy. We also calculated the sensitivity, specificity, and positive and negative predictive value when FISH and/or DIA were positive and cytology was neither positive nor suspicious. These results are also shown in Table 5. Overall DIA showed a low sensitivity with a good specificity. Interestingly, all tetraploid DIA results had either a positive or a suspicious cytology result; indeed, we were not able to calculate the specificity for this analysis since we did not have any true positive result. Either FISH polysomic or trisomy had the best sensitivity among the tests but with decreased specificity. When only FISH polysomic samples were classified as positive the specificity was very high (100%). FISH trisomy alone showed a better sensitivity compared to polysomy alone (40% vs 20%) with a decrease in specificity (87%). When combining FISH and DIA for the analysis, the sensitivity was low, but the specificity was very high, even higher than cytology (positive or suspicious) results.
ERCP complications
It is possible that the acquisition of multiple samples for cytologic analysis would alter the ERCP complication rate. The post ERCP complication rate in this study was 6%; 7 patients developed pancreatitis, 5 bacterial cholangitis, 2 mild, self-limited pancreatic duct perforations, and 1 patient a bile duct perforation. In a similar patient population, Ong et al reported a 10% complication rate after ERCP.22 Thus, the acquisition of additional cytologic specimens does not appear to increase the ERCP associated complication rate.
DISCUSSION
Cytologic specimens can be interpreted as malignant, suspicious for malignancy, atypical or benign.18 For the purposes of determining the predictive power of the tests, we classified cases interpreted as “atypical” or benign as negative for malignancy and “suspicious” as negative or positive depending on the analysis. Other investigators have considered “suspicious” and “atypical” interpretations as positive in their calculations of sensitivity and specificity. 1, 19, 20 Our results suggest that cytologists may be overly conservative since the specificity of cytology was only slightly lower (96% vs. 100% over entire study group) if both positive and suspicious cases were considered positive but there was a significant increase in the sensitivity of cytology (20% to 41%) if both positive and suspicious cases were considered positive. Perhaps, the current system of categorizing cases as positive and suspicious can be maintained as long as clinicians realize that even suspicious diagnoses are associated with a very high risk of malignancy.
One possible explanation for the lower sensitivity of cytology observed in this study is that the population consisted of referral patients. Such patients are selected for their complex diagnostic and management decisions because the initial studies and assessment elsewhere are often not definitive. These patients frequently have earlier stage disease with smaller, less easily detectable tumors. Another possible explanation is that many other studies have a higher proportion of patients with large tumors that could be easily detected by cytology and other methodologies. Indeed, while the sensitivity of cytology observed in this study is lower than in a number of other studies, our results are comparable to those of De Beelis et al. who used a similar cytologic classification and a referral population of patients 21. Thus, our study suggests that cytology has excellent specificity but poor sensitivity for the detection of malignant pancreatobiliary strictures in a typical referral population. When technically feasible, bile duct biopsies were also taken from all suspicious strictures. From 105 patients with cancer, 95 had a biopsy performed. The biopsy was falsely negative in 13 (14%) of these patients emphasizing the difficulty in establishing a tissue diagnosis in patients with malignant pancreatobiliary strictures.
The sensitivities of DIA and FISH for detecting malignant cells were considerably higher than the sensitivity of cytology. These advanced cytologic techniques identify malignant cells either by detecting aneuploidy (DIA) or aneusomy (FISH). Aneuploidy refers to abnormalities of nuclear DNA content while aneusomy refers to abnormalities (gain or loss) of specific chromosomes or chromosomal loci (specifically chromosomes 3, 7, 17 and the 9p21 band in this study). However, not all cancers are aneuploid/aneusomic, and the percentage of biliary tract cancers displaying aneuploidy has been estimated to be approximately 80% 10. Consequently, DIA and FISH cannot be expected to obtain a sensitivity of 100% for detecting malignant pancreatobiliary strictures. Nonetheless, the sensitivities of FISH and DIA observed in this study represent a significant improvement over the sensitivity obtained by conventional cytology.
Two types of chromosomal alterations were observed by FISH in this study, polysomy and trisomy of a single chromosome. In this study, polysomy FISH results had 100% specificity (i.e. no false positive polysomy results). Given this, the finding of polysomy could possibly be viewed as equivalent to a positive cytology for the diagnosis of cancer. Virtually all of the cases that exhibited trisomy in this study were trisomic for chromosome 7 (28 cases were trisomy 7 and two were trisomy 3). Trisomy 7 has been observed in both neoplastic and non-neoplastic conditions. Studies have shown that synovial fluid cells from patients with rheumatoid arthritis and osteoarthritis frequently exhibit trisomy 7 23, 24. However, trisomy 7 is also observed in neoplasms such as astrocytomas 25, colorectal cancer 26, thyroid cancer 27, kidney cancer 28 and breast cancer 29. Therefore, trisomy FISH results in pancreatobiliary strictures must be interpreted with caution and placed into the clinical context.
There were two different categories of DIA results, aneuploid (DNA index from 1.12 to 1.89) and tetraploid (DNA index greater 1.89). In this study, DIA with aneuploidy had an intermediate sensitivity and specificity as compared to cytology and FISH. Given that DIA is based on populations of cells, its lower sensitivity to FISH is not surprising due to the relatively acellular samples obtained by endoscopic brush cytology. Its lower specificity in PSC patients suggests the inflammation in accompanying biliary tract obstruction results in false positive results with this assay. However, tetraploid DIA results in non PSC patients had a specificity of 100%. This finding can be interpreted as equal to cancer. The difference in DIA results in regards to PSC or non PSC patients supports our stratification of strictures. Therefore, tetraploid DIA results are worrisome for cancer, whereas aneuploid results must be interpreted cautiously taking into account the clinical context. (Figure 3)
Although, the current study is not a cost effective analysis the employment of these techniques into routine practice will be influenced by the costs. The cost of DIA is approximately twice that of cytology and the cost of FISH is about three to four times the cost of cytology. The cost of all three tests is additive. However, the increased sensitivity of DIA and FISH could potentially lead to a reduction in overall health care costs by reducing the need for repetition of tests (e.g., ERCP, CT, MR studies etc) over time or the performance of other expensive diagnostic modalities (e.g., PET scans, etc) which would otherwise be necessary to arrive at the correct diagnosis. In addition, the information afforded by these tests may help guide more complex decisions regarding expensive therapeutic interventions such as the need for surgery. Thus, the cost-effectiveness of these advanced cytologic techniques will need further and careful analysis.
The principal findings of this study relate to the clinical utility of advanced cytologic techniques for the accurate diagnosis of pancreatobiliary strictures. Based on the data, we consider FISH polysomy or DIA tetraploid results (exclusively in non PSC patients) to be equivalent to positive cytology for the diagnosis of malignant pancreatobiliary strictures. The data indicate that in non-PSC patients, FISH has the highest sensitivity of the three techniques while maintaining the high specificity of cytology. In PSC patients, FISH has the highest sensitivity but the specificity is somewhat lower. DIA has good specificity for the diagnosis of malignant pancreatobiliary strictures in PSC and non-PSC groups and increases the sensitivity of pancreatobiliary malignancy detection more than two fold relative to cytology when only positive cytology results are considered clinically appropriate to act on. Based on this extensive experience, we have now incorporated these advanced cytologic techniques into our practice. (Figure 3). However, we note that all samples were obtained from patients with pancreatobiliary strictures suspicious for cancer. This population of patients, although clinically relevant, has a high pre-test probability of cancer. The performance of these tests in screening patient populations (e.g., PSC patients) is unclear and will require additional study.
Acknowledgments
The authors thank John Paul Bida and Linda Stadheim for excellent technical support.
Footnotes
Financial disclosure: Dr. Halling holds a patent on the probe set utilized in this study and receives royalties from the sale of this product.
This work was supported by NIH grants (59427), and the Palumbo and Mayo Foundations.
References
Full text links
Read article at publisher's site: https://doi.org/10.1053/j.gastro.2006.08.021
Read article for free, from open access legal sources, via Unpaywall:
http://www.gastrojournal.org/article/S0016508506017604/pdf
Citations & impact
Impact metrics
Article citations
Still elusive: Developments in the accurate diagnosis of indeterminate biliary strictures.
World J Gastrointest Endosc, 16(6):297-304, 01 Jun 2024
Cited by: 0 articles | PMID: 38946851
Review
Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications.
Cancers (Basel), 16(9):1761, 01 May 2024
Cited by: 1 article | PMID: 38730713 | PMCID: PMC11083053
Review Free full text in Europe PMC
Update on the Screening, Diagnosis, and Management of Cholangiocarcinoma.
Gastroenterol Hepatol (N Y), 20(3):151-158, 01 Mar 2024
Cited by: 2 articles | PMID: 38680168 | PMCID: PMC11047158
Per-oral cholangioscopy in patients with primary sclerosing cholangitis: a 12-month follow-up study.
Endosc Int Open, 12(2):E237-E244, 15 Feb 2024
Cited by: 0 articles | PMID: 38362361 | PMCID: PMC10869209
Update on the optimisation of endoscopic retrograde cholangiography (ERC) in patients with primary sclerosing cholangitis.
Frontline Gastroenterol, 15(1):74-83, 12 Sep 2023
Cited by: 0 articles | PMID: 38487565 | PMCID: PMC10935540
Go to all (173) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures.
Gastroenterology, 136(7):2180-2186, 14 Feb 2009
Cited by: 87 articles | PMID: 19232347
Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures.
Gastrointest Endosc, 75(1):65-73, 10 Nov 2011
Cited by: 37 articles | PMID: 22078103
An Optimized Set of Fluorescence In Situ Hybridization Probes for Detection of Pancreatobiliary Tract Cancer in Cytology Brush Samples.
Gastroenterology, 149(7):1813-1824.e1, 29 Aug 2015
Cited by: 55 articles | PMID: 26327129
FISHing for pancreatobiliary tract malignancy in endoscopic brushings enhances the sensitivity of routine cytology.
Cytopathology, 25(5):288-301, 30 Jul 2014
Cited by: 14 articles | PMID: 25073411
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (1)
Grant ID: R01 DK059427
PHS HHS (1)
Grant ID: NIH 59427