Abstract
Free full text

Immunobiology
Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7
Associated Data
Abstract
Idiopathic CD4+ T lymphocytopenia (ICL) is a rare heterogeneous disorder defined by CD4+ T-cell counts below 300 cells/μL in the absence of human immunodeficiency virus (HIV) infection or other known immune deficiency disorders. Here, we report the expansion of immature/transitional B cells in patients with ICL, which is associated with elevated serum levels of IL-7. Both the percentage of immature/transitional B cells and levels of IL-7 were inversely correlated with levels of CD4+ T-cell counts and directly correlated to each other. Further analyses of B cells indicated that, in contrast to the activating effects of HIV disease on mature B cells, the expansion of immature/transitional B cells in patients with ICL occurred at the expense of memory B cells. These findings extend previous reports on primary immunodeficiencies as well as HIV disease by suggesting that CD4+ T-cell lymphopenia has an impact on human B-cell development either directly or indirectly via the associated elevation of IL-7 levels.
Introduction
Idiopathic CD4+ T lymphocytopenia (ICL) was initially described in individuals whose low CD4+ T-cell counts and opportunistic infections suggested human immunodeficiency virus (HIV) disease but in whom no evidence of viral infection could be established.1,2 ICL remains a disorder of unknown etiology with variable clinical manifestations and heterogeneous immunologic profiles.3,4 The working definition of ICL is a reproducible CD4+ T-cell count of less than 300 cells per microliter in the absence of HIV infection or other known causes of immunodeficiency.5,6 CD4+ T lymphocytopenia may be accompanied by lymphocytopenia of CD8+ T, B, or natural killer (NK) cells,2,7,8 such that individuals with ICL may have also been diagnosed with other immunodeficiencies.9
Hallmarks of HIV disease progression include CD4+ T lymphocytopenia, ongoing HIV replication, and perturbations of other immune cell compartments. We recently described the expansion of immature/transitional B cells in the blood of HIV-infected individuals with advancing disease that was associated with increased serum levels of IL-7.10 Immature/transitional B cells, defined as CD10-expressing recent bone marrow emigrants distinguishable from germinal center founders by the absence of CD27,11–13 have recently been characterized in human peripheral blood in association with various B-cell immunodeficiencies.14,15 In addition, serum levels of IL-7 have been correlated inversely with CD4+ T-cell counts and directly with HIV plasma viremia.10,16,17 While homeostatic compensation associated with loss of CD4+ T cells has been proposed to explain the high serum levels of IL-7 observed in HIV disease and other deficiencies,17,18 the correlations suggest that HIV viremia may also contribute to the high serum levels of IL-7 and increases in immature/transitional B cells. In order to extend previous findings,10,16 gain further insight into primary and HIV-induced immunodeficiencies, and help segregate the effects of ongoing HIV replication from those associated with lymphocytopenia, we evaluated the association between B-cell subpopulations, CD4+ T cells, and serum levels of IL-7 in a cohort of patients with ICL.
Patients, materials, and methods
Study subjects included 25 patients who were evaluated for ICL, as previously described,10,16 and 19 healthy individuals. Patients with ICL with severe B-cell lymphocytopenia (< 50 cells/μL) were excluded from the current study. All subjects provided informed consent, in accordance with the Declaration of Helsinki and Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Serum and peripheral blood mononuclear cells (PBMCs) were isolated beforehand from blood products and cryopreserved until analysis. The PBMCs were stained with antibodies from BD Biosciences, San Jose, CA (CD10, CD19, CD27) and Beckman Coulter, Fullerton, CA (CD21), and analyzed on a FACSCalibur (BD Biosciences) with FlowJo software (TreeStar, Ashland, OR). Serum levels of IL-7 were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Spearman rank correlation was used to quantify associations between variables, 2-tailed Mann-Whitney U tests were used to compare independent groups, and all P values were adjusted for multiple testing by the Bonferroni method. Multiple regression was used to determine the independent effects of CD4 and IL-7 on immature/transitional B cells.
Results and discussion
Within the group of 25 patients who were evaluated for ICL, 17 patients met the Centers for Disease Control (CDC) definition of ICL, namely a CD4+ T-cell count below 300 cells/μL,5,6 for a minimal duration of 1 year. Other lymphocytopenias were noted in several of the patients investigated (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The percentage and absolute count of immature/transitional (CD10+/CD27−) B cells were significantly higher in patients with ICL compared with healthy donors (Figure 1A-B), although B-cell lymphopenia was associated with low CD10+/CD27− B-cell counts (Table S1). The increased percentage of immature/transitional B cells is consistent with our previous report on HIV-infected individuals,10 as well as reports on other non-HIV immunodeficiencies and disorders associated with B-cell abnormalities.14,15 Furthermore, serum levels of IL-7 were significantly higher in patients with ICL compared with healthy donors (Figure 1C), consistent with our previous report on HIV-infected individuals10 and a recent report on individuals with common variable immunodeficiency disease,19 a primary immunodeficiency disease affecting B cells also shown to be associated with an expansion of immature/transitional B cells.15
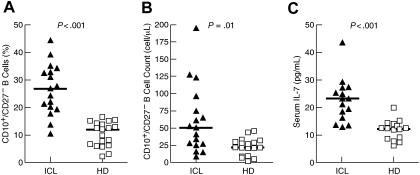
Expansion of immature/transitional B cells and elevated serum levels of IL-7 in patients with ICL. Percentage (A) and absolute count (B) of immature/transitional (CD10+/CD27−) B cells, and (C) serum levels of IL-7 in patients with ICL and healthy donors (HD). Horizontal bars indicate median values for each group.
Longitudinal data collected over several years on patients 3 and 9, whose status remained relatively stable, and on patient 11, who recovered from ICL, suggested a close association between CD4+ T-cell counts, serum levels of IL-7, and percentage of immature/transitional B cells (data not shown). Further insight into these associations and possible regulation between lymphocyte compartments were sought by considering all patients who were evaluated for ICL (Table S1; referred to as the ICL cohort). Homeostasis within the peripheral T-cell compartment has been well documented and illustrated by the cross-regulation between CD8+ and CD4+ T cells in HIV-infected individuals representing a wide spectrum of disease activity.20 In the ICL cohort, a strong direct correlation was observed between CD4+ T-cell count and CD4/CD8 ratio (Figure 2A), consistent with compensatory effects that help maintain homeostasis in the T-cell compartment.21 Although no correlation existed between total number of B cells and CD4+ T cells (data not shown), a strong inverse correlation was observed between the percentage of immature/transitional B cells and CD4+ T-cell count (Figure 2B). Furthermore, and consistent with previous observations in HIV disease,10,16,17 serum levels of IL-7 were inversely correlated to CD4+ T-cell count (Figure 2C) and directly correlated to percentage of immature/transitional B cells (Figure 2D). Multivariate analyses performed to determine which factors influenced the increase in immature/transitional B cells revealed that the influence of CD4+ T-cell count was statistically significant whereas that of IL-7 was not (data not shown). However, this conclusion was reversed if the elder outlying patient identified in Figure 2C-D was excluded, indicating the limits of performing such analyses on this heterogeneous and relatively small cohort. Nonetheless, these data suggest that either CD4+ T cells or IL-7 or both have an impact on the maturation of B cells, and illustrate the cascade of homeostatic effects that occur in the setting of both primary (shown here) and HIV-induced10 immunodeficiency.
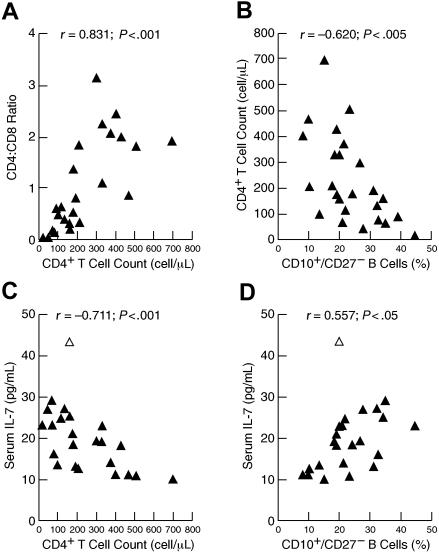
Associations between lymphocyte populations in the peripheral blood and serum levels of IL-7 in patients evaluated for ICL. Correlation between CD4+ T-cell counts and (A) CD4/CD8 ratio or (B) percentage of immature/transitional (CD10+/CD27−) B cells. Correlation between serum levels of IL-7 and (C) CD4+ T-cell counts or (D) percentage of immature/transitional (CD10+/CD27−) B cells. The open triangle in panels C and D identify patient 13 in Table S1, who was 85 years old.
Finally, we recently reported on the loss of memory B-cell function in a large cohort of HIV-infected individuals representing a wide spectrum of disease activity.22 However, this loss of memory B-cell function was not accompanied by a loss in the frequency of mature B cells expressing the memory marker CD27 (A.M., S.M., A.S.F., unpublished data, January 2005). We speculated that the HIV-induced immune activation contributed to high levels of CD27 expression10 and that it was difficult to investigate population dynamics in a disease that induces both immune activation and lymphopenia. In patients with ICL, the percentage and absolute count of memory CD27+ B cells were decreased compared with healthy donors, whereas those of naive B cells were not (Figure S1). However, these analyses also underscored the heterogeneity of the patients with ICL, as evidenced by distinctly low memory and naive B-cell groups (Figure S1) that were only explained in part by total B-cell lymphopenia (data not shown). Nonetheless, these data indicate that CD27 expression due to immune activation is a distinguishing feature between HIV disease and ICL, whereas increases in immature/transitional B cells and IL-7 due to CD4+ T-cell lymphopenia are common to both diseases. Finally, the possibility of a direct association between IL-7 and the expansion of immature/transitional B cells, although suggested here and indirectly from other studies on B-cell immunodeficiencies,15,19 needs further investigation.
Acknowledgments
We thank Dr Michael Proschan for assistance in the statistical analyses and Sara Stallings for assistance with sample collection. This work was supported by the Intramural Research Program of NIAID, NIH, Bethesda, MD.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M. performed the research and wrote the paper with S.M. and A.S.F.; D.G.C. and C.A.R. recruited patients, and obtained and stored patient samples; A.M., S.M., S.K., J.F., and A.S.F. contributed to the study design and analysis of the data; and J.F. established the patient cohort and oversaw patient visits.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
S.M. and A.M. contributed equally to this work.
Correspondence: Susan Moir, Laboratory of Immunoregulation, NIAID, NIH, Bldg 10/6A02, 10 Center Dr, Bethesda, MD 20892; e-mail: vog.hin.diain@rioms.
References
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2006-06-031385
Read article for free, from open access legal sources, via Unpaywall:
https://ashpublications.org/blood/article-pdf/109/5/2086/1479151/zh800507002086.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1182/blood-2006-06-031385
Article citations
B cells and atherosclerosis: A HIV perspective.
J Cell Physiol, 239(6):e31270, 23 Apr 2024
Cited by: 1 article | PMID: 38651687
Review
Defective Monocyte Enzymatic Function and an Inhibitory Immune Phenotype in Human Immunodeficiency Virus-Exposed Uninfected African Infants in the Era of Antiretroviral Therapy.
J Infect Dis, 226(7):1243-1255, 01 Sep 2022
Cited by: 7 articles | PMID: 35403683
HIV infection and the implication for COVID-19 vaccination.
Public Health Chall, 1(3):e14, 29 Jul 2022
Cited by: 3 articles | PMID: 37521727 | PMCID: PMC9353425
Review Free full text in Europe PMC
Transient viral exposure drives functionally-coordinated humoral immune responses in HIV-1 post-treatment controllers.
Nat Commun, 13(1):1944, 11 Apr 2022
Cited by: 9 articles | PMID: 35410989 | PMCID: PMC9001681
Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis.
Front Immunol, 12:747324, 02 Dec 2021
Cited by: 71 articles | PMID: 34925323 | PMCID: PMC8674869
Review Free full text in Europe PMC
Go to all (75) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Decreased interleukin 7 responsiveness of T lymphocytes in patients with idiopathic CD4 lymphopenia.
J Infect Dis, 205(9):1382-1390, 26 Mar 2012
Cited by: 20 articles | PMID: 22454463 | PMCID: PMC3324404
Complete Multilineage CD4 Expression Defect Associated With Warts Due to an Inherited Homozygous CD4 Gene Mutation.
Front Immunol, 10:2502, 08 Nov 2019
Cited by: 12 articles | PMID: 31781092 | PMCID: PMC6856949
Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia.
Blood, 127(8):977-988, 16 Dec 2015
Cited by: 45 articles | PMID: 26675348 | PMCID: PMC4768432
Idiopathic lymphocytopenia.
Curr Opin Hematol, 22(1):46-52, 01 Jan 2015
Cited by: 12 articles | PMID: 25463685
Review
Funding
Funders who supported this work.
 1
1 




