Abstract
Free full text

Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host
Abstract
Proteorhodopsins (PRs) are retinal-containing proteins that catalyze light-activated proton efflux across the cell membrane. These photoproteins are known to be globally distributed in the ocean's photic zone, and they are found in a diverse array of Bacteria and Archaea. Recently, light-enhanced growth rates and yields have been reported in at least one PR-containing marine bacterium, but the physiological basis of light-activated growth stimulation has not yet been determined. To describe more fully PR photosystem genetics and biochemistry, we functionally surveyed a marine picoplankton large-insert genomic library for recombinant clones expressing PR photosystems in vivo. Our screening approach exploited transient increases in vector copy number that significantly enhanced the sensitivity of phenotypic detection. Two genetically distinct recombinants, initially identified by their orange pigmentation, expressed a small cluster of genes encoding a complete PR-based photosystem. Genetic and biochemical analyses of transposon mutants verified the function of gene products in the photopigment and opsin biosynthetic pathways. Heterologous expression of six genes, five encoding photopigment biosynthetic proteins and one encoding a PR, generated a fully functional PR photosystem that enabled photophosphorylation in recombinant Escherichia coli cells exposed to light. Our results demonstrate that a single genetic event can result in the acquisition of phototrophic capabilities in an otherwise chemoorganotrophic microorganism, and they explain in part the ubiquity of PR photosystems among diverse microbial taxa.
Proteorhodopsins (PRs) are retinal-binding membrane proteins belonging to the rhodopsin family. Prokaryotic members of this family include photosensors (sensory rhodopsins), transmembrane proton pumps (bacteriorhodopsins, xanthorohodopsin, and PRs), and transmembrane chloride pumps (halorhodopsins). Originally discovered in Archaea, rhodopsins were later identified in Gammaproteobacteria of the SAR86 group during a cultivation-independent genomic survey. Dubbed proteorhodopsin, this photoprotein functions as a light-activated proton pump when expressed in Escherichia coli in the presence of exogenously added retinal (1). Since then, numerous molecular surveys have demonstrated that PR genes are ubiquitous in bacteria inhabiting the ocean's photic zone (2–9). An estimated 13% of bacteria in marine picoplankton populations, as well as a significant fraction of planktonic Euryarchaeota, contain a PR gene (4, 8). In a number of marine bacteria, retinal biosynthetic genes and PR are genetically linked, and their lateral transfer and retention appear to be relatively common events, indicating that the photosystem confers a significant fitness advantage (3, 4, 7, 10, 11). A recent report of light-stimulated growth in a PR-containing marine flavobacterium supports this hypothesis (11). Despite all of these observations however, the various specific functions and physiological roles of diverse marine microbial PRs remain to be fully described.
To further characterize PR photosystem structure and function, we directly screened large-insert DNA libraries derived from marine picoplankton for visibly detectable PR-expressing phenotypes. In this report, we describe completely intact PR-based photosystems that can be functionally expressed in E. coli, without addition of exogenous photopigment (e.g., retinal or its precursors). Analyses of insertional mutants verified the functional annotation of each gene product in the photosystem biosynthetic pathway. We also show that light-activated, PR-catalyzed proton translocation, by the chemiosmotic potential it generates, activates photophosphorylation in E. coli.
Results
Screening a Fosmid Library for in Vivo PR Photosystem Expression.
When E. coli expresses a PR apoprotein from an inducible promoter on a high-copy number plasmid, the cells acquire a red or orange pigmentation in the presence of exogenous all-trans retinal (1, 12). Retinal addition is required because E. coli lacks the ability to biosynthesize retinal or its precursor, β-carotene. Based on these observations, we screened for PR-containing clones on retinal-containing LB agar plating medium, which we expected would display an orange to red phenotype under these conditions. To enhance assay sensitivity, we used the copy-control system present in our fosmid vector that allowed a controlled transition from one copy per cell to multiple (up to 100) vector copies upon addition of the inducer l-arabinose (13).
A fosmid library prepared from ocean surface water picoplankton containing 12,280 clones (≈440 Mb of cloned DNA) (14) was screened by using the above approach. Three orange colonies were identified as potential PR-expressing clones on the LB-retinal-l-arabinose agar plates. All three showed no pigmentation in the absence of the high-copy number inducer. Unexpectedly, these clones also displayed an orange phenotype in the absence of l-retinal when induced to high copy number. The sequence of one clone, HF10_19P19, revealed the presence of a PR gene near the fosmid vector junction (see below). Because the clones exhibited orange pigmentation in the absence of exogenous retinal, we expected that they must also be expressing retinal biosynthetic genes. Two clones, HF10_25F10 and HF10_19P19, were analyzed further for PR photosystem gene expression and function.
Genomic Analyses of Candidate PR Photosystem-Expressing Clones.
The full DNA sequence of the two putative PR photosystem-containing fosmids was obtained by sequencing a collection of transposon-insertion clones. The approach facilitated rapid DNA sequencing while simultaneously providing a set of precisely located insertion mutants for phenotypic analysis of specific gene functions (15).
Both PR photosystem-containing clones were derived from Alphaproteobacteria based on ORF content similarity to homologues in the National Center for Biotechnology Information nonredundant protein database [supporting information (SI) Tables 1 and 2]. The clones exhibited highest identity to other PR-containing BAC clones from Alphaproteobacteria from the Mediterranean and Red Seas (8). This similarity was evident across the entire cloned insert, although some large-scale rearrangements were apparent. The HF10_19P19 PR-inferred protein sequence was most similar to a homologue from another environmental BAC, MedeBAC66A03 (67% identity, 83% similarity). The MedeBAC66A03 PR was previously reported to exhibit fast photocycle kinetics and light-activated proton translocation when expressed in E. coli in the presence of exogenous retinal (8). Clone HF10_25F10 PR was most similar in inferred protein sequence to another BAC clone, RED17H08 PR (93% identity, 97% similarity) and was very similar to MedeBAC66A03 as well (62% identity, 78% similarity). Both of the PR genes analyzed here encoded proteins with a glutamine residue at position 105, a characteristic of blue light-absorbing PRs (5) and consistent with the orange pigmentation observed in clones expressing them.
Adjacent to the PR gene in both clones was a predicted six-gene operon encoding putative enzymes involved in β-carotene and retinal biosynthesis (Fig. 1A). A similar arrangement was reported in MedeBAC66A03 and RED17H08 (8) and more recently in a wide variety of diverse marine bacterial groups (7). The genes encoded on these operons include crtE [putative geranylgeranyl pyrophosphate (GGPP) synthase], crtI (phytoene dehydrogenase), crtB (phytoene synthase), crtY (lycopene cyclase), blh (15,15′-β-carotene dioxygenase), and idi [isopentenyl diphosphate (IPP) δ-isomerase]. The putative role of these proteins in the retinal biosynthetic pathway (for review, see ref. 16) is indicated in Fig. 1C. The first reactions in the pathway are catalyzed by the IPP δ-isomerase and farnesyl diphosphate (FPP) synthase. Both enzymes are part of the isoprenoid and ubiquinone metabolic pathways and are present in E. coli. crtE, crtB, crtI, and crtY appear to encode all of the enzymes necessary to synthesize β-carotene from FPP. The blh gene found in MedeBAC66A03 was previously shown to encode a 15,15′-β-carotene dioxygenase that cleaves β-carotene, producing two molecules of all-trans-retinal (8).
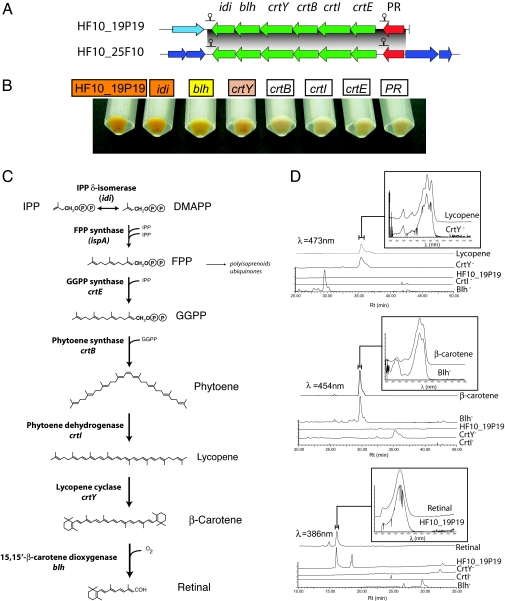
Genetic and phenotypic analysis of PR photosystem transposon mutants. (A) Schematic representation of the PR gene clusters identified in this work. Predicted transcription terminators in the clusters are indicated. (B) Color phenotype of intact cells of transposon-insertion mutants grown in liquid cultures with arabinose. (C) Retinal biosynthesis pathway. Names of genes encoding pathway enzymes are indicated. The genes that are present in E. coli are in parentheses. (D) HPLC profiles of wild-type and transposon mutant extracts. Detection wavelengths are indicated. Absorption spectrum of relevant peaks, including standards used for identification, are shown on the top for each panel.
Apart from the PR and putative β-carotene and retinal biosynthesis operon, no other genes were shared between the two PR-containing fosmids. With the exception of a gene encoding a putative deoxyribodipyrimidine photolyase in HF10_25F10, no other genes flanking the PR photosystem had an obvious, light-related function (SI Tables 1 and 2).
Genetic and Phenotypic Analysis of the PR Photosystem.
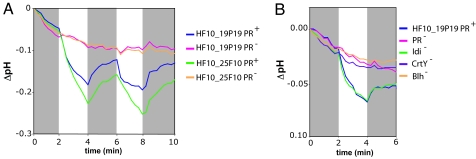
To obtain direct evidence for the functional annotations of putative retinal biosynthesis genes, we analyzed different transposon mutant phenotypes that carried an insertion in predicted PR photosystem genes. The cell pigmentation and HPLC pigment analyses in selected mutants are shown in Fig. 1 B and D, respectively. HF10_19P19 cells carrying the intact vector were orange when grown in the presence of arabinose, consistent with expression of a blue-adapted retinal–PR complex. HPLC analysis revealed the presence of retinal in cell extracts, demonstrating that clone HF10_19P19 contained all genes required for retinal biosynthesis in E. coli. Neither lycopene nor β-carotene was observed in the intact clone extracts, indicating that there was little if any accumulation of pigment intermediates (Fig. 1D). Cells containing transposon insertions in the idi gene were also orange and contained retinal. The lack of phenotype in this mutant can be attributed to the presence of the endogenous idi gene in E. coli (17).
As expected, transposon insertion mutants disrupted in the PR gene itself were devoid of orange pigmentation, and HPLC analysis showed a low but detectable level of retinal in these extracts (data not shown). It is unclear at present whether the low levels of retinal were due to polar effects caused by the transposon insertion in downstream expression or whether they result from pathway inhibition due to product accumulation.
Transposon-insertion mutants in crtE, crtB, and crtI showed no pigmentation, as expected for this biosynthetic pathway if it is interrupted before lycopene formation, the first colored product in the pathway. crtY-insertion mutants, however, were pink, suggesting that they were accumulating lycopene. Pigment analysis verified that crtY-insertion mutant extracts contained lycopene but not retinal or β-carotene (Fig. 1 B and D). Finally, blh-insertion mutants had a yellow phenotype, and HPLC analysis showed that these cells lacked detectable retinal but instead accumulated β-carotene. This finding demonstrates that the blh gene in HF10_19P19 encodes a 15,15′-β-carotene dioxygenase, similar to a homologue recently described by Sabehi et al. (8). Transposon insertions in all other predicted genes outside the PR cluster had no visibly obvious phenotype. Identical pigmentation phenotypes were observed with insertions in the corresponding genes of the other PR photosystem clone, HF10_25F10. Taken together, these results strongly support the functional assignments of PR-associated retinal biosynthetic pathway genes and demonstrate that they are necessary and sufficient to induce retinal biosynthesis in E. coli.
Light-Activated Proton Translocation.
We assayed both HF10_19P19 and HF10_25F10 grown under high-copy number conditions for light-activated proton-translocating activity. Light-dependent decreases in pH were observed in PR+ clones but not in mutants containing a transposon insertion in the PR gene (PR−) (Fig. 2A). In addition, no light-dependent proton-translocating activity was observed in insertion mutants unable to synthesize retinal (CrtY− or Blh−). In contrast, Idi− mutants had normal proton-pumping activity, confirming that this gene was not required under our growth conditions (Fig. 2B). These results demonstrate that both fosmids independently expressed a functional PR with light-activated proton-translocating activity.
PR-Driven Proton Translocation Results in Photophosphorylation in E. coli.
Analogous to earlier studies of haloarchaeal bacteriorhodopsins (18, 19), it was previously postulated that light-activated, PR-induced proton motive force could drive ATP synthesis as protons reenter the cell through the ATP synthase complex (Fig. 3A) (1, 12). This hypothesis was not previously tested, however, in either native or heterologously expressed PR-based photosystems. To this end, we measured light-induced changes in ATP levels in the PR-photosystem-containing clones and PR− mutant derivatives by using a luciferase-based assay. The assay measures total ATP, and so we expected to observe increases in ATP concentration only if PR-driven ATP biosynthesis exceeded endogenous turnover rates, under our experimental conditions. Control pH measurements indicated that PR+ cells used in the ATP assay were indeed capable of light-activated proton translocation (Fig. 3B). ATP measurements performed after 5 min of illumination showed significant light-induced increases in cellular ATP levels in the PR+ clone but not in a PR− mutant (Fig. 3C). The 0.3-pmol increase in ATP observed in the PR+ cells exposed to light (Fig. 3C) represents a 29% increase over identical cell preparations maintained in the dark, which corresponds to a net gain after 5 min of illumination of ≈2.2 × 105 molecules of ATP per colony-forming unit (or viable cell) assayed. For comparison, oxidative phosphorylation, measured by ATP increases observed 5 min after the addition of 0.2% succinate to PR+ cells in the dark, resulted in a net gain of 9 × 105 ATP molecules per live cell (data not shown).
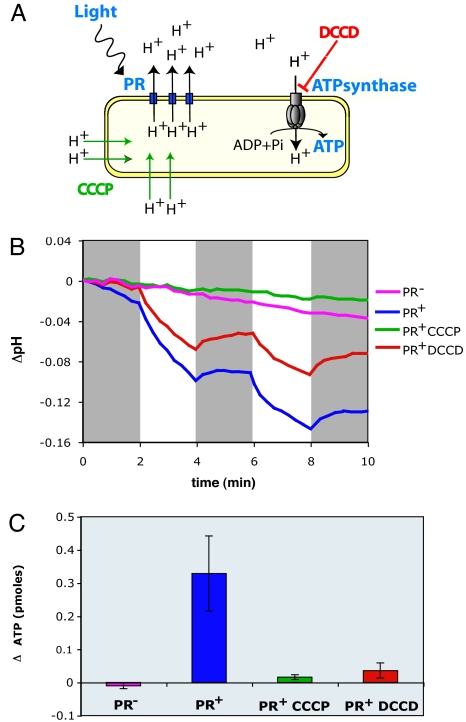
Light-activated, PR-enabled photophosphorylation in E. coli. (A) Diagram of the proposed mechanism of PR-dependent ATP synthesis. The effects of the inhibitors used are indicated. (B) Proton-pumping assays with HF10_19P19 cells. pH measurements are expressed as the pH change respect to the pH at time 0 for each sample. CCCP, 25 μM; DCCD, 1 mM. Gray boxes indicate dark periods. (C) ATP assays with HF10_19P19 cells. Results are expressed as the difference between the ATP level in the light and the ATP level in the dark, ΔATP, for each treatment.
Similar light-activated, PR-catalyzed photophosphorylation was also observed in cells containing the HF10_25F10 fosmid. Although the PR photosystem of this clone is similar to that of HF10_19P19, all of the genes flanking the two different photosystem gene suites are completely different and derive from different chromosomal contexts. Because the PR and retinal biosynthetic genes are the only shared genes on both clones, the results strongly suggest that these specific PR photosystem genes are both necessary and sufficient to drive photophosphorylation in E. coli cells.
To characterize more fully the light-driven photophosphorylation observed in PR+ E. coli cells, we tested the effects of carbonylcyanide m-chlorophenylhydrazone (CCCP), an uncoupler, and N,N′-dicyclohexylcarbodiimide (DCCD), a covalent inhibitor of H+-ATP synthase, on light-driven ATP synthesis (Fig. 3). We used concentrations that inhibited aerobic growth of E. coli on succinate, an oxidative phosphorylation process requiring both proton-motive force and ATP synthase activity (20). Addition of CCCP, which permeabilizes the cell membrane to H+, completely abolished the light-driven decrease in pH and subsequently photophosphorylation. This result demonstrates that both processes depend on the establishment of an H+ electrochemical gradient. In contrast, addition of the H+-ATP synthase inhibitor DCCD did not affect external pH changes resulting from PR-catalyzed proton translocation, but it completely abolished photophosphorylation. This result indicates that H+-ATP synthase is indeed responsible for the light-activated ATP increases we observed in PR+ cells.
Discussion
The results presented here demonstrate the utility of functionally screening large-insert DNA “metagenomic” libraries for new phenotypes and activities directly and without subcloning, an approach pioneered by soil microbiologists (15, 21, 22). Although large-insert libraries increase the probability of capturing complete metabolic pathways in a single clone, their low copy number decreases the sensitivity of detecting heterologous gene expression. We show here that increasing fosmid copy number (13) can significantly enhance detectable levels of recombinant gene expression and therefore increases the detection rate of desired phenotypes in metagenomic libraries. The PR photosystem recombinants we characterized could be detected visually by pigment production and exhibited light-dependent proton translocation and subsequent photophosphorylation, only when the fosmid vector was induced to high copy number. The approach was not completely effective in detecting all targeted genotypes however. Even under “copy-up” conditions, we were unable to detect all PR-containing clones known to exist in our library (4, 7). Despite the limitations, this approach for functional screening of microbial community genomic libraries is useful for identifying specific activities or phenotypes, even in the absence of sequence information. Additionally, this approach provides useful material for downstream genetic and biochemical characterization and for testing hypotheses derived from bioinformatic analyses.
Genetic and biochemical characterization of PR photosystem-containing clones reported here provided direct evidence that only six genes are required to enable light-activated proton translocation and photophosphorylation fully in a heterologous host. Sabehi et al. (8) demonstrated previously that coexpression of marine bacterial blh with PR, in the presence of the β-carotene biosynthetic genes from Erwinia herbicola, led to β-carotene cleavage and subsequent formation of retinal-bound PR. We show here that a set of six genetically linked genes known to be found in a wide variety of different marine bacterial taxa (7, 8, 11) are both necessary and sufficient for the complete synthesis and assembly of a fully functional PR photoprotein in E. coli. These heterologously expressed marine bacterial photosystems exhibited light-dependent proton translocation activity in the absence of exogenously added retinal or β-carotene. One gene in the PR photosystem cluster was dispensable under our conditions: the idi gene that encodes IPP δ-isomerase, an activity already present in E. coli (17), as is the FPP synthase, catalyzing the next two steps in the pathway (23). The presence of the idi gene in the cluster likely enables retinal production in the native organism because isomerization of IPP to dimethylallyl pyrophosphate can be a rate-limiting step in β-carotene biosynthesis (24, 25).
It was previously postulated that light-activated proton translocation catalyzed by PR elevates the proton-motive force, thereby driving ATP synthesis as protons reenter the cell through the H+-ATP synthase complex (1, 12). Although this capability has been demonstrated for haloarchaeal bacteriorhodopsins (18, 19, 26, 27), PR-based photophosphorylation has not been demonstrated previously. Our data demonstrate that illumination of cells expressing a native marine bacterial PR photosystem generates a proton-motive force that does indeed drive cellular ATP synthesis. Under our experimental conditions, 5 min of illumination resulted in a net gain of 2.2 × 105 ATP molecules per cell. It should be quite possible to utilize the light to biochemical energy conversion enabled by the PR photosystem, for biosynthetic purposes.
The PR photosystem-catalyzed photophosphorylation described here is consistent with a proposed role for PR in marine microbial photoheterotrophy. A few previous studies were unable to detect light-enhanced growth rates or yields in PR-containing isolates grown in seawater or natural seawater incubations (28, 29). In one recent report, light stimulation of growth rate or yield in Pelagibacter ubique, a ubiquitous PR-containing marine planktonic bacterium, could not be detected. These negative results are somewhat difficult to interpret because natural seawater incubations are by necessity chemically undefined, and preferred growth substrates or other limiting nutrients in these experiments were unknown. In contrast, a significant enhancement of both growth rate and yield was recently reported in PR-expressing marine flavobacterium (11) albeit a direct link between PR and the light-induced growth stimulation was not conclusively demonstrated. Our direct observation of an intact PR photosystem gene expression and subsequent photophosphorylation, the recently reported light-enhanced growth rates and yields of PR-containing Flavobacteria (11), and the general ubiquity of PR photosystem genes in diverse microbial taxa of the ocean's photic zone (2–9) all strongly support a significant role for PR-based phototrophy in planktonic marine microorganisms.
In different physiological, ecological, phylogenetic, and genomic contexts, PR activity may benefit cells in a variety of ways, some not directly related to enhanced growth rates or yields. In some bacteria, the H+-ATP synthase functions as an ATPase under low respiratory conditions, hydrolyzing ATP and driving proton efflux to maintain the proton-motive force (30). In the light, PR activity could offset this effect and reverse conditions from ATP consumption to ATP production. PR contributions to cellular energy metabolism are likely to be particularly important in starved or substrate-limited cells. Similar to the situation for some Haloarchaea, which use bacteriorhodopsin under oxygen-limiting conditions (18, 19, 26, 27), low respiratory rates may trigger PR expression or activity in marine bacteria, as well. The PR-generated proton-motive force can also be directly coupled to other energy-requiring cellular activities, including flagellar motility or active transport of solutes into or out of the cell (32–34). This phenomenon was recently demonstrated by the coupling of PR activity to flagellar rotation in E. coli (31). Although a sensory function for some PR variants is also a possibility (10, 35), the PR photosystems described here, and the vast majority of others observed to date (1, 3, 4, 7, 8, 11), are not genetically linked to sensory transducers, the hallmark of all known sensory rhodopsins. Most all PRs characterized so far therefore appear to function as light-activated ion pumps.
The marine PR family is remarkably diverse, has a widespread phylogenetic distribution, and is functional in both ether-linked phytanyl and ester-linked fatty acid membrane systems of Archaea and Bacteria (4, 7, 10). A recent survey found that one-third of PR clones examined were colocalized with photopigment biosynthetic operons (7). Operon arrangement and distribution, as well as phylogenetic relationships, suggest that lateral transfer of PR photosystem genes is relatively common among diverse marine microbes (4, 7, 10). The observations reported here demonstrate that acquisition of just a few genes can lead to functional PR photosystem expression and photophosphorylation. The ability of a single lateral transfer event to confer phototrophic capabilities likely explains the ecological and phylogenetic prevalence of these photosystems in nature. In principle, any microorganism capable of synthesizing FPP (a widespread intermediate in isoprenoid biosynthesis) could readily acquire this capability, as we have observed in E. coli.
Apparently, many otherwise chemoorganotrophic microbes in the ocean's photic zone have acquired the ability to use light energy to supplement cellular energy metabolism. The broad array of PR-containing microbes reflects the photosystem's fundamental contribution to cellular bioenergetics, a simplicity and compactness that favors PR photosystem lateral mobility, and a remarkable plasticity that enables photoprotein assembly and function in a diversity of phylogenetic groups and cell membrane types. From a genetic, physiological, and ecological perspective, the transition from heterotrophy to PR-enabled photoheterotrophy seems to represent a relatively small evolutionary step for contemporary microorganisms.
Materials and Methods
Fosmid Library.
The HOT_10m fosmid library screened in this work has been described previously (14). It contains DNA from a planktonic sample collected 10 m below the surface at the ALOHA station (22°45′ N, 158°W) of the Hawaii Ocean Time series (HOT) cloned into the copy-controlled pCC1FOS fosmid vector (Epicentre Biotechnologies, Madison, WI). The library host, E. coli EPI300 (Epicentre Biotechnologies), supports the copy-control option of pCC1FOS.
Screening for PR Expression.
High-density colony macroarrays [12,280 clones of the HOT_10m library (ref. 14)] were prepared on a Performa II filter (Genetix Ltd., Boston, MA) by using a Q-PixII robot (Genetix Ltd.). The filter was carefully laid over a 22-cm plate containing 250 ml of LB agar supplemented with l-arabinose (0.02%), the copy-up inducer, and all-trans-retinal (20 μM), and the plate was incubated at 37°C for 24 h. The filters were used to facilitate the visual detection of color against the white background. Colonies were inspected visually for the appearance of orange or red color. Fosmid DNA from positive clones was retransformed into fresh E. coli EPI300 and rescreened as above to verify that the color was conferred by the fosmid. The end DNA sequence of the positive clones was obtained by using primers T7 and EpiFos5R as described previously (14).
In Vitro Transposition and Full Fosmid Sequencing.
Fosmid clones to be characterized were submitted to random in vitro transposition by using the EZ-Tn5<kan-2> insertion kit (Epicentre Biotechnologies) according to the manufacturer's instructions. The transposition reaction was transformed by electroporation into EPI300 cells, and clones containing fosmids with Tn5 insertions were selected in LB chloramphenicol, kanamycin (12 μg/ml and 25 μg/ml, respectively). The color phenotype of individual Tn5-insertion clones was analyzed on LB plates containing chloramphenicol, kanamycin, and 0.02% l-arabinose as above. DNA sequencing off the Tn5 ends was performed by using KAN-2 FP-1 and KAN-2 RP-1 primers, a BigDye version 3.1 cycle sequencing kit, and ABI Prism 3700 DNA analyzer (Applied Biosystems, Forest City, CA). The complete DNA sequence was assembled by using Sequencher version 4.5 (Gene Codes Corporation, Ann Arbor, MI) and annotated with FGENESB (Softberry, Mount Kisco, NY) and Artemis version 6 (The Wellcome Trust Sanger Institute, Cambridge, U.K.).
Carotenoid Extraction.
Overnight cultures of the appropriate clones were diluted 1:100 into 50 ml of LB chloramphenicol (12 μg/ml) and incubated for 3 h at 37°C with shaking (200 rpm). At that point, l-arabinose was added to a 0.02% final concentration, and cultures were incubated for 16 h. Cells were harvested by centrifugation and rinsed twice in salt solution. Cell pellets were kept frozen (−20°C) in the dark. Frozen cells were extracted by sonication (5 min) in a cold 4:1 (vol/vol) mixture of acetone/methanol (OmniSolv; EMD Chemicals, Gibbstown, NJ) with 0.1 mM butylated hydroxytoluene added as an antioxidant. Cells were pelleted by centrifugation, and the supernatant was removed and filtered through ashed silica gel (230–400 mesh; EMD Chemicals). Extracts were then concentrated by evaporation under dry N2. All extraction steps were performed in darkness or low light to minimize carotenoid photooxidation.
HPLC Analysis.
Chromatographic separation and analysis of carotenoids by high-performance liquid chromatography (HPLC) adapted a reverse-phase method from Barua and Olson (36). A 5-μm Zorbax-ODS C18 column (150 × 4.6 mm) (Agilent Technologies, Palo Alto, CA) was used at 30°C in a column oven with a Waters (Milford, MA) 2795 separations module operated with MassLynx 4.0 software. Separation was achieved with a linear gradient at a flow rate of 0.8 ml/min: 100% solvent A to 100% B over 20 min followed by isocratic elution with B for an additional 20 min, where A = methanol/water (3:1 vol/vol) and B = methanol/dichloromethane (4:1 vol/vol). The column was equilibrated after each run with solvent A for 10 min. The detector was a Waters 996 photodiode array detector scanning wavelengths from 190 to 800 nm with a resolution of 1.2 nm and sampling rate of one spectrum per s. Carotenoids were identified by comparing absorbance spectra and retention times with authentic standards.
Proton-Pumping Experiments.
Clones to be analyzed for proton-pumping activity were streaked on 15-cm LB agar plates containing 12 μg/ml chloramphenicol and 0.001% l-arabinose and incubated at 37°C for 48 h. Cells were resuspended in 20 ml of salt solution (10 mM NaCl/10 mM MgCl2/100 μM CaCl2, pH7.0), rinsed twice, and adjusted to an A600 of 0.5–0.7. Two milliliters of cell suspension was placed in an RPC-100 photosynthetic chamber (i-Works, Dover, NH) connected to a 22°C circulating water bath. pH was measured by using a Beckman (Fullerton, CA) Φ360 pH meter equipped with a Futura microelectrode. Light was provided by a 160-watt halogen lamp placed 4 cm from the chamber. Irradiance within the chamber was 500–650 μmol Q m−2 s−1.
ATP Measurements.
Cell suspensions were prepared as above. Three milliliters of cell suspension was placed in 5-ml screw-cap glass vials. The vials for the dark samples were wrapped in foil. Ten centimeters of water was used to minimize heat transfer to the samples. Irradiance under these conditions was 650 μmol Q m−2 s−1. ATP was measured by using a luciferase-based assay (BactTiter Glo, Promega, Madison, WI) as follows. At each time point, 5 aliquots (20 μl each) of every sample were dispensed into white 96-well assay plates [CoStar (Bethesda, MD) 3917]. One hundred microliters of BactTiterGlo reagent was added per well, and luminescence was measured after 10 min using a Victor3 plate reader (PerkinElmer, Waltham, MA) with a 1-s integration time. An ATP standard curve was used to calculate the concentration of ATP in the samples. For inhibitor experiments, cell suspensions were incubated in the dark for 20 h in the presence of 1 mM DCCD or for 2 h in the presence of 25 μM CCCP or the ethanol vehicle. Succinate was added to a 0.2% final concentration to measure ATP synthesis from respiration in the dark.
Acknowledgments
We thank Drew Endy for the use of the luminometer, and Jay McCarren for comments on the manuscript. This work was supported by a grant from the Gordon and Betty Moore Foundation (to E.F.D.), National Science Foundation (NSF) Microbial Observatory award (MCB-0348001), and NSF Science and Technology Center Award EF0424599 (to E.F.D.).
Abbreviations
| CCCP | carbonylcyanide m-chlorophenylhydrazone |
| DCCD | N,N′-dicyclohexylcarbodiimide |
| FPP | farnesyl diphosphate |
| GGPP | geranylgeranyl pyrophosphate |
| IPP | isopentenyl diphosphate |
| PR | proteorhodopsin. |
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. EF100190 (HF10_19P19) and EF100191 (HF10_25F10)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0611470104/DC1.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.0611470104
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1838496?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101963053
Article citations
Effects of Light and Dark Conditions on the Transcriptome of Aging Cultures of Candidatus Puniceispirillum marinum IMCC1322.
J Microbiol, 62(4):297-314, 25 Apr 2024
Cited by: 0 articles | PMID: 38662311
Cyanorhodopsin-II represents a yellow-absorbing proton-pumping rhodopsin clade within cyanobacteria.
ISME J, 18(1):wrae175, 01 Jan 2024
Cited by: 0 articles | PMID: 39485071 | PMCID: PMC11528372
A comparative study reveals the relative importance of prokaryotic and eukaryotic proton pump rhodopsins in a subtropical marginal sea.
ISME Commun, 3(1):79, 18 Aug 2023
Cited by: 4 articles | PMID: 37596487 | PMCID: PMC10439184
Rhodopsin-mediated nutrient uptake by cultivated photoheterotrophic Verrucomicrobiota.
ISME J, 17(7):1063-1073, 29 Apr 2023
Cited by: 0 articles | PMID: 37120702 | PMCID: PMC10284914
Metabolic Versatility of the Family Halieaceae Revealed by the Genomics of Novel Cultured Isolates.
Microbiol Spectr, e0387922, 14 Mar 2023
Cited by: 2 articles | PMID: 36916946 | PMCID: PMC10100682
Go to all (101) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (2)
- (1 citation) ENA - EF100191
- (1 citation) ENA - EF100190
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Proteorhodopsin lateral gene transfer between marine planktonic Bacteria and Archaea.
Nature, 439(7078):847-850, 01 Feb 2006
Cited by: 167 articles | PMID: 16482157
Proteorhodopsin photosystem gene clusters exhibit co-evolutionary trends and shared ancestry among diverse marine microbial phyla.
Environ Microbiol, 9(4):846-858, 01 Apr 2007
Cited by: 67 articles | PMID: 17359257
Function and regulation of Vibrio campbellii proteorhodopsin: acquired phototrophy in a classical organoheterotroph.
PLoS One, 7(6):e38749, 07 Jun 2012
Cited by: 30 articles | PMID: 22741028 | PMCID: PMC3380642
Marine Bacterial and Archaeal Ion-Pumping Rhodopsins: Genetic Diversity, Physiology, and Ecology.
Microbiol Mol Biol Rev, 80(4):929-954, 14 Sep 2016
Cited by: 82 articles | PMID: 27630250 | PMCID: PMC5116876
Review Free full text in Europe PMC