Abstract
Free full text

Effect of novel Nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats
Abstract
Activation of the NOP receptor by the endogenous ligand nociceptin/orphanin FQ (N/OFQ) reduces alcohol consumption in genetically selected alcohol preferring Marchigian Sardinian (msP) rats. The present study evaluated the effect of three newly synthesized peptidergic and one brain-penetrating heterocyclic NOP receptor agonists on alcohol drinking in the two bottle choice paradigm. MsP rats were intracerebroventricularly (ICV) injected with the NOP receptor agonists OS-462 (0.5 and 1.0 μg), UFP-102 (0.25 and 1.0 μg) or UFP-112 (0.01 and 0.05 μg), or with Ro 64-6198 (0.3 and 1.0 mg/kg) given intraperitoneally (IP), and tested for 10% alcohol consumption. Results showed decreased alcohol consumption after treatment with all three peptidergic NOP receptor agonists (OS-462, UFP-102 and UFP-112). OS-462 (at the 1.0 μg dose) and UFP-102 (at the 0.25 μg dose) induced a significant increase in food intake as well. Surprisingly, Ro 64-6198 was ineffective at the 0.3 mg/kg dose whereas it increased ethanol and food consumption at the 1.0 mg/kg dose. Interestingly, pre-treatment with the selective μ-receptor antagonist naloxone (0.5 mg/kg, IP) reduced these effects of 1.0 mg/kg of Ro 64-6198. These findings confirm that activation of brain NOP receptors reduces alcohol drinking in msP rats and demonstrate that OS-462, UFP-102 and UFP-112 act as potent NOP receptor agonists. On the other hand, Ro 64-6198 was not effective nor did it increase alcohol drinking, an effect probably induced by a residual agonist activity of this compound at μ-opioid receptors. Overall, the results indicate that OS-462, UFP-102 and UFP-112 may represent valuable pharmacological tools to investigate the functional role of the brain N/OFQ system.
INTRODUCTION
Nociceptin/orphanin FQ (N/OFQ), the endogenous ligand for the NOP opioid receptor, previously referred to as ORL-1 or OP4 receptor [31,44], is known to be structurally related to the opioid peptide dynorphin A [32,33,45,46]. However, N/OFQ does not bind to MOP, DOP or KOP opioid receptors, nor do opioid peptides activate the NOP receptor [45,46]. In contrast, N/OFQ activates with high selectivity the NOP receptor [45], eliciting intracellular responses with the same intracellular mechanisms as classic opioid receptors [45]. Interestingly, however, N/OFQ has been found to act in the brain as a functional antiopioid peptide by blocking opioid-induced supraspinal analgesia or morphine induced conditioned place preference [6,34,35,38,39].
Brain mapping studies have revealed a neuroanatomical distribution of N/OFQ and the NOP receptor different from that of other opioid peptides and receptors [1,8,16,21,37,40,47]. A wide distribution of this peptidergic system has been found in various corticomesolimbic structures, including the amygdala, the bed nucleus of the stria terminalis and various fronto-cortical areas. Interestingly, such brain areas are known to be involved in the regulation of the motivational properties of drugs of abuse [14,28,50] and an important involvement of the N/OFQ-NOP receptor system in the control of reward and drug abuse processes has been now well established [5]. For instance, pre-treatment with N/OFQ blocks ethanol-, morphine- and cocaine-induced conditioned place preference [4,6,29,39], and reduces ethanol intake in genetically selected Marchigian Sardinian alcohol-preferring (msP) rats both in the two bottle free-choice and operant self-administration paradigms [4,7]. Finally, microdialysis studies demonstrated that central administration of this peptide significantly attenuates the increase of extracellular dopamine (DA) induced in the nucleus accumbens (Nacc) after administration of cocaine or morphine [12,30].
Because of the well documented role of the N/OFQ-NOP receptor system in drug and alcohol reward as well as the ability of this opioid peptide to exert its effects without activating classic opioid receptors (MOP, DOP and KOP), great interest is presently devoted to the development of novel NOP receptor agonists with favorable pharmacodynamic and pharmacokinetic properties such as brain permeability following peripheral administration. Recently, several peptidergic NOP receptor agonists as well as one non-peptidergic brain-penetrating NOP receptor agonist, Ro 64-6198, have been synthesized [49].
The purpose of this study was to evaluate the effect of three newly synthesized peptidergic NOP receptor agonists (OS-462, UFP-102 and UFP-112) and of the brain penetrating agent Ro 64-6198 on home-cage voluntary ethanol drinking in msP rats.
2. Methods
2.1. Animals
Male genetically selected alcohol-preferring rats were used. These rats were bred in the Department of Pharmacological Sciences and Experimental Medicine of the University of Camerino (Marche, Italy) for 50 generations from Sardinian alcohol-preferring (sP) rats of the 13th generation, provided by the Department of Neurosciences of the University of Cagliari [15,17]. These animals are referred to as Marchigian Sardinian P (msP) rats. At the time of the experiments their body weight ranged from 400 to 450 g. The rats were housed in a temperature (20–22°C) and humidity (45–55%) controlled vivarium on a reverse 12:12 h light/dark cycle (lights off at 9:00 a.m.). Rats were offered free access to tap water and food pellets (4RF18, Mucedola, Settimo Milanese, Italy). All procedures were conducted in adherence with the European Community Council Directive for Care and Use of Laboratory Animals.
2.2. Surgical Procedures
For intracranial surgery, rats were anaesthetized by intramuscular injection of 100–150 μl of a solution containing tiletamine hydrochloride (58.17 mg/ml) and zolazepam hydrochloride (57.5 mg/ml). A guide cannula for injections into the lateral ventricle was stereotaxically implanted and cemented to the skull. The following coordinates, taken from the atlas of Paxinos and Watson (1986) [42], were used: antero-posterior = -1.0 mm with respect to bregma, lateral = 1.8 mm from the sagittal suture, ventral = 2 mm from skull surface.
For ICV administration, compounds were injected through a stainless-steel injector protruding 2.5 mm beyond the cannula tip. Experiments began one week after surgery. Cannula placement was verified before the experiment by ICV injection of 100 ng of angiotensin II. Only animals that showed a clear dipsogenic response (at least 6 ml of water in 5 min) were used for further testing.
2.3. Drugs
The NOP receptor agonist OS-462 (N-a-6-guanidinohexyl-3,5-dimethyl-ltyrosyl-L-tyrosyl-N-[(R)-1-(2-naphthyl)ethyl]-L-argininamide, MW=823) corresponds to Example 25 of the European Patent [22,42] and was provided by Nippon Shinyaku, Co Ltd, Kyoto, Japan. The NOP receptor agonists UFP-102 ([(pF)Phe4,Arg14,Lys15]N/OFQ-NH2, MW=2725) and UFP-112 ([(pF)Phe4Aib7Arg14Lys15]N/OFQ-NH2, MW=2738) were generously provided by Dr. Remo Guerrini of the Department of Pharmaceutical Sciences, University of Ferrara, Italy. Ro 64-6198 was kindly provided by Hoffmann-La Roche (Basel, Switzerland). Naloxone was purchased from Tokris (Cookson Ltd, UK).
Ro 64-6198 ((1S,3aS)-8-(2,3,3a,4,5,6-Hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one, MW=438.017) was dissolved in a solution containing 10% DMSO, 10% Tween 80, 80% distilled water and administered intraperitoneally (IP) at a volume of 1 ml/kg. Naloxone was dissolved in distilled water and was given IP at a volume of 1ml/kg. All other NOP receptor agonists were dissolved in sterile isotonic saline and injected ICV in a volume of 1 μl.
2.4. Experimental procedure
At 3 months of age, msP rats were selected for ethanol preference by offering them free choice between water and 10% ethanol (w/w) 24-h a day for 15 days. Water and ethanol were available in graduated drinking tubes equipped with metallic drinking spouts and their consumption intake was measured by reading the volume consumed from the graduated burettes. Food intake was measured by weighing the food containers and taking into account spillage. Ethanol, water and food intakes are expressed as g/kg to reduce the influence of differences in body weight.
The rats used in the present experiments all showed 24-h ethanol intake of 6–7 g/kg with ethanol preference [ml of ethanol solution/ml of total fluids (water + 10% ethanol) ingested in 24 h x 100] greater than 90 percent. Rats were given free access to ethanol, water and food for 24-h/day until a stable baseline alcohol intake was established. Subsequently, ethanol availability was restricted to 30 min/day for animals treated with OS-462, UFP-102 or UFP-112, and to 60 min/day for animals treated with Ro 64–6198. Ethanol was made available at the beginning of the rats’ dark phase (9:00 a.m.). All animals were habituated to the experimental procedure by receiving 3–4 IP or ICV injections before initiation of the experiments (pre-treatment period). All experiments were carried out according to a between-subject design, in which each group of rats received a single dose of a single compound.
2.4.1. Effect of subchronic ICV treatment with OS-462 on voluntary ethanol intake in msP rats
Rats with similar baseline ethanol intake during the pre-treatment period (3 days) (n=22) were divided into 3 groups (n=7–8). Animals were then treated ICV for 6 consecutive days with OS-462 (0.0, 0.5 or 1.0 μg/rat). The drug was administered 10 min before access to ethanol using separate groups of rats for each dose. Ethanol, water and food intake was measured for 30 min after access to ethanol. After completion of the drug treatment, ethanol, water and food intake were measured for another 3 days during which time all animals were injected ICV with saline (post-treatment period). The doses employed for this experiment were chosen on the basis of previous data on the effects of this peptide on food intake [13].
2.4.2. Effect of subchronic ICV treatment with UFP-102 on voluntary ethanol intake in msP rats
MsP rats (n=23) were divided into 3 groups (n=7–8) with similar baseline ethanol intake during the pre-treatment period (3 days). At this point and for 6 consecutive days animals were ICV treated, 10 min before access to ethanol, with UFP-102 (0.25 or 1.0 μg/rat) or its vehicle (controls), respectively. Ethanol, water and food intake was measured for 30 min after access to ethanol. After the end of the treatment, intakes were measured for another 3 days during which all animals received ICV injection of saline. The doses employed for this experiment were chosen based on our data on the effects of this peptide on food intake (unpublished observations).
2.4.3. Effect of subchronic ICV treatment with UFP-112 on voluntary ethanol intake in msP rats
A group of msP rats (n=21) was divided into 3 sub-groups (n=7) with similar baseline level of ethanol consumption during the pre-treatment period (3 days). At this point and for 6 consecutive days animals were treated, 30 min before access to ethanol, with UFP-112 (0.01 or 0.05 μg/rat, ICV) or its vehicle (controls), respectively. Ethanol, water and food intake was measured for 30 min after access to ethanol. After the end of the treatment, intake was measured for another 3 days during which all animals were injected ICV with saline.
2.4.4. Effect of subchronic IP treatment with Ro 64-6198 on voluntary ethanol intake in msP rats
To evaluate the effect of Ro 64-6198 on voluntary ethanol intake, rats with similar baseline ethanol consumption during the pre-treatment period were divided into 3 groups (n=8–10). At this point, the first group of animals received IP vehicle injection (controls), while the other two groups received 0.3 and 1.0 mg/kg of Ro 64-6198, respectively. Ethanol was given 30 min after injections, and ethanol, water and food intake were recorded for the subsequent 60 min. Upon completion of drug testing, ethanol, water and food intake were monitored for another 3 days (post-treatment), during which all animals received only IP injections of saline.
2.4.5. Effect of subchronic IP treatment with naloxone on the increase of voluntary ethanol intake induced by Ro 64-6198 in msP rats
A group of msP rats (n=27) was divided into 4 subgroups with similar baseline ethanol consumption during the pre-treatment period. At this point and for 6 consecutive days, animals received IP naloxone (0.5 mg/kg) or its vehicle, and after 10 min received an IP injection of 1.0 mg/kg of Ro 64-6198 or its vehicle, respectively. Ethanol was offered to the animals 30 min after Ro 64-6198 administration. Ethanol, water and food intake were recorded at 30 and 60 min. Upon completion of drug testing, ethanol, water and food intake were monitored for another 3 days (post-treatment), during which all animals received only IP injections of vehicles.
Statistical analysis
Effects of OS-462, UFP-102 and UFP-112 were separately analyzed by two-way analysis of variance (ANOVA) with drug doses as between-groups comparisons and days of treatment as within-groups comparisons. Effects of Ro 64-6198 alone or in combination with naloxone were analyzed by three-way ANOVA with 2 within-group comparisons (time and day of treatment) and 1 between-group comparison (doses). Post-hoc comparisons were carried out by Newman-Keuls test.
3. Results
3.1. Effect of subchronic ICV treatment with OS-462 on voluntary ethanol intake in msP rats
The overall ANOVA revealed a significant treatment effect [F(2,19) = 6.96; P < 0.01]. As shown in Fig. 1A, post-hoc comparisons confirmed a significant difference between controls and animals treated with both doses of OS-462 (P < 0.01). More specifically, treatment with the 0.5 μg dose significantly reduced alcohol intake on days 2, 3, 4 and 6, whereas the 1.0 μg dose induced a significant decrease in alcohol consumption on days 2, 3, 5 and 6 of treatment. Alcohol intake returned to baseline levels during post-treatment period (Fig. 1A).
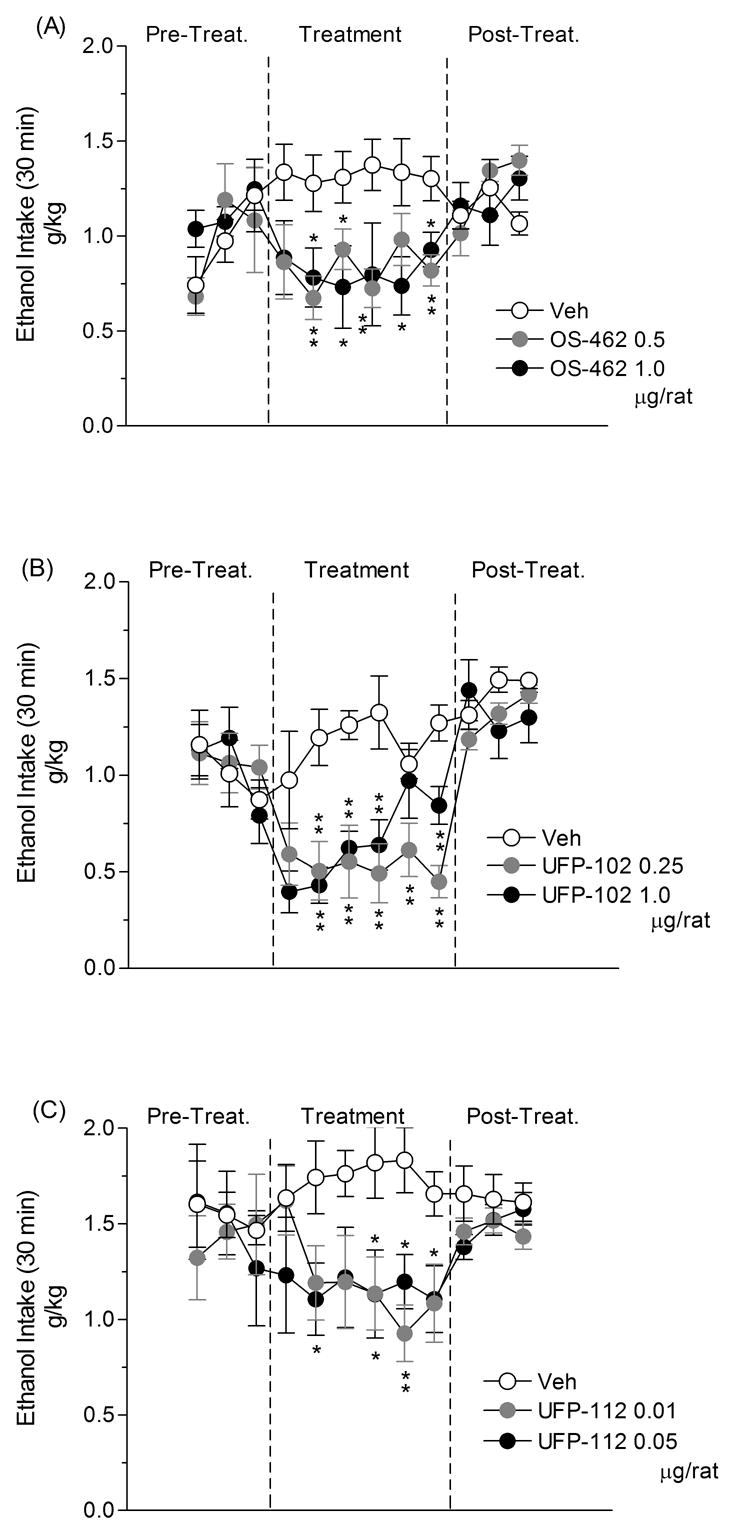
Voluntary 10% alcohol intake (g/kg) following subchronic (6 days) ICV treatment with: (A) OS-462 (0.0, 0.5 and 1.0 μg/rat), (B) UFP-102 (0.0, 0.25 and 1.0 μg/rat) and, (C) UFP-112 (0.0, 0.01 and 0.05 μg/rat) in msP rats. OS-462 and UFP-102 were given ICV 10 min before access to ethanol. UFP-112 was ICV injected 30 min before access to ethanol. Data represent the means ± S.E.M. *P < 0.05, **P < 0.01 vs. controls.
Overall ANOVA also revealed a significant treatment effect on food intake [F(2,19) = 4.8; P < 0.05]. More specifically, treatment with 1.0 μg of OS-462 significantly increased food intake on days 1, 2 and 5. Water intake was never modified by OS-462 (Table 1).
Table 1
Summary of the effects of OS-462, UFP-112, UFP-102 and Ro 64-6198 alone or in combination with naloxone on food and water intake in msP rats
| Food Intake | ||||||
|---|---|---|---|---|---|---|
| Treatment | d1 | d2 | d3 | d4 | d5 | d6 |
| OS-462 (μg/rat, ICV) | ||||||
| Veh | 5.8 ± 1.3 | 4.5 ± 1.5 | 6.9 ± 1.4 | 10.7 ± 2.0 | 5.9 ± 0.8 | 4.9 ± 0.9 |
| OS-462 0.5 | 5.3 ± 1.4 | 3.3 ± 1.7 | 6.2 ± 1.4 | 8.9 ± 1.3 | 5.0 ± 1.0. | 6.0 ± 1.2 |
| OS-462 1.0 | 13.6 ± 2.5** | 9.2 ± 1.2* | 6.6 ± 1.6 | 8.3 ± 2.8 | 9.6 ± 1.1* | 7.3 ± 0.9 |
| UFP-102 (μg/rat, ICV) | ||||||
| Veh | 1.6 ± 1.3 | 1.9 ± 0.9 | 0.7 ± 0.5 | 5.6 ± 1.2 | 1.1 ± 0.4 | 2.0 ± 0.8 |
| UFP-102 0.25 | 2.9 ± 1.2 | 7.2 ± 1.2** | 7.8 ± 1.5** | 6.6 ± 1.3 | 8.0 ± 1.1** | 5.9 ± 0.8** |
| UFP-102 1.0 | 0.6 ± 0.4 | 1.4 ± 0.7 | 2.5 ± 1.1 | 2.9 ± 0.9 | 2.4 ± 1.2 | 2.8 ± 0.9 |
| UFP-112 (μg/rat, ICV) | ||||||
| Veh | 6.9 ± 1.9 | 7.6 ± 1.1 | 9.4 ± 1.4 | 5.7 ± 1.5 | 6.8 ± 1.1 | 4.3 ± 1.3 |
| UFP-112 0.01 | 9.3 ± 1.9 | 6.9 ± 1.7 | 7.6 ± 1.8 | 6.6 ± 1.4 | 10.6 ± 0.9 | 7.4 ± 1.8 |
| UFP-112 0.05 | 8.0 ± 1.4 | 7.3 ± 1.7 | 9.1 ± 1.9 | 6.5 ± 2.1 | 8.3 ± 1.9 | 8.5 ± 2.5 |
| Ro 64-6198 (mg/kg, IP) | ||||||
| Veh | 11.8 ± 1.0 | 7.9 ± 0.8 | 11.2 ± 1.1 | 7.5 ± 0.6 | 9.6 ± 0.9 | 8.4 ± 1.3 |
| Ro 0.3 | 8.8 ± 1.9 | 9.2 ± 1.9 | 8.6 ± 1.9 | 7.8 ± 1.9 | 9.2 ± 1.9 | 7.0 ± 1.0 |
| Ro 1.0 | 10.8 ± 1.0 | 11.2 ± 1.2* | 14.0 ± 1.9 | 13.3 ± 1.7** | 16.4 ± 1.8** | 15.0 ± 2.6* |
| Naloxone (mg/kg, IP) + Ro 64-6198 (mg/kg, IP) | ||||||
| Veh +Veh | 13.6 ± 2.0 | 11.3 ± 1.3 | 11.0 ± 1.6 | 9.0 ± 1.6 | 9.6 ± 0.9 | 11.5 ± 1.0 |
| Veh + Ro 1.0 | 15.0 ± 4.0 | 17.1 ± 3.1 | 16.5 ± 2.7 | 16.9 ± 2.2* | 14.8 ± 1.5* | 20.3 ± 3.3* |
| Nlx 0.5 + Ro 1.0 | 14.0 ± 3.1 | 9.4 ± 1.4 | 11.1 ± 1.8 | 12.0 ± 2.1 | 12.0 ± 2.5 | 8.9 ± 1.7 |
| Nlx 0.5 + Veh | 13.0 ± 1.0 | 11.8 ± 0.6 | 13.9 ± 1.4 | 12.6 ± 1.7 | 12.4 ± 3.3 | 13.1 ± 1.8 |
|
| ||||||
| Water Intake | ||||||
| OS-462 (μg/rat, ICV) | ||||||
| Veh | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| OS-462 0.5 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.8 ± 0.8 | 0.2 ± 0.1 | 0.3 ± 0.3 |
| OS-462 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| UFP-102 (μg/rat, ICV) | ||||||
| Veh | 0.6 ± 0.6 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| UFP-102 0.25 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.0 ± 0.1 |
| UFP-102 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.5 ± 0.0 | 0.0 ± 0.5 |
| UFP-112 (μg/rat, ICV) | ||||||
| Veh | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| UFP-112 0.01 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 |
| UFP-112 0.05 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 |
| Ro 64-6198 (mg/kg, IP) | ||||||
| Veh | 1.1 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 1.3 ± 1.1 |
| Ro 0.3 | 0.6 ± 0.6 | 0.2 ± 0.2 | 0.4 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Ro 1.0 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 4.8 ± 2.2 |
| Naloxone (mg/kg, IP) + Ro 64-6198 (mg/kg, IP) | ||||||
| Veh +Veh | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.0 ± 0.0 |
| Veh + Ro 1.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Nlx 0.5 + Ro 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Nlx 0.5 + Veh | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Thirty min. food and water intake in msP rats treated with UFP-102, UFP-112 and OS-462 injected intracerebroventricularly (ICV) or Ro 64-6198 (Ro) and naloxone (Nlx) given intraperitoneally (IP). Each value represents the mean (± SEM) of intake corrected for body weight (g/kg). Data represent the means ± S.E.M.
3.2. Effect of subchronic ICV treatment with UFP-102 on voluntary ethanol intake in msP rats
The overall ANOVA revealed a significant treatment effect [F(2,20) = 11.45; P < 0.01]. As shown in Fig. 1B, post-hoc comparisons confirmed significant differences between controls and animals treated with both doses of UFP-102 (P < 0.01). More specifically, the 0.25 μg dose significantly reduced alcohol intake on days 2, 3, 4, 5 and 6, whereas the 1.0 μg dose induced a significant decrease in alcohol consumption on days 2, 3, 4 and 6 of drug treatment. ANOVA also revealed a significant treatment effect on food intake [F(2,20) = 13.72; P < 0.01]. More specifically, treatment with 0.25 μg of UFP-102 significantly increased (P < 0.01) food intake on days 2, 3, 5 and 6 of treatment. Water intake was not altered by UFP-102 (Table 1).
3.3. Effect of subchronic ICV treatment with UFP-112 on voluntary ethanol intake in msP rats
The overall ANOVA revealed a significant treatment effect [F(2,18) = 4.23; P < 0.05]. As shown in Fig. 1C, post-hoc comparisons demonstrated a significant difference between controls and animals treated with both doses of UFP-112 (P < 0.05). More specifically, treatment with 0.01 μg significantly reduced alcohol intake on days 4 and 5, whereas treatment with 0.05 μg of the peptide induced a significant decrease in alcohol consumption on days 2, 4, 5 and 6. Neither food nor water intake were modified by UFP-112 (Table 1).
3.4. Effect of subchronic IP treatment with Ro 64-6198 on voluntary ethanol intake in msP rats
ANOVA revealed an overall effect of treatment [F(2,24) = 6.98; P < 0.01]. Post-hoc comparisons showed a significant increase of ethanol intake following administration of 1.0 mg/kg of Ro 64-6198 (P < 0.01), whereas the 0.3 mg/kg dose did not modify alcohol consumption. In particular, as shown in Fig. 2, administration of 1.0 mg/kg of Ro 64-6198 significantly increased ethanol intake during the first 30 min of drinking on days 5 and 6 (Fig. 2A), and at 60 min on days 5, 6 and 7 of drug treatment (Fig. 2B).
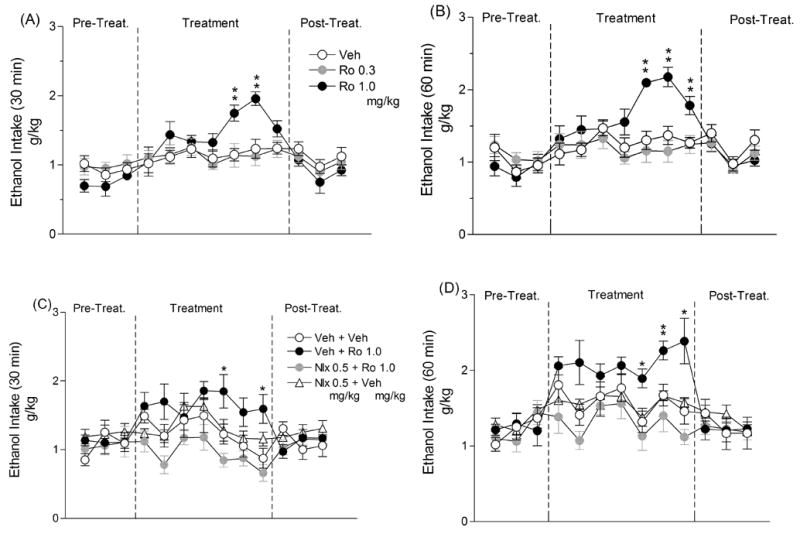
Voluntary 10% alcohol intake (g/kg) following subchronic (7 days) treatment with Ro 64-6198, ((1S,3aS)-8-(2,3,3a,4,5,6-Hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5] decan-4-one) (0.0, 0.03 and 1.0 mg/kg, IP) in msP rats. The compound was given alone intraperitoneally 30 min before access to ethanol and data were recorded at A) 30 min and B) 60 min. Alternatively, rats were pretreated with 0.5 mg/kg of naloxone (Nlx) or its vehicle followed by 1.0 mg/kg of Ro 64-6198 (Ro) or its vehicle, respectively. Also in this case data were recorded at C) 30 min and D) 60 min. Data represent the means ± S.E.M. *P < 0.05, **P < 0.01 vs. controls.
Ro 64-6198 elicited a significant increase in food intake [F(2,24) = 11.58; P < 0.05]. Post-hoc comparisons showed a significant increase of food intake (P < 0.01) on days 2, 4, 5 and 6 of drug treatment at the highest (1.0 mg/kg) dose (Table 1). Water intake was not modified by Ro 64-6198 (Table 1).
3.5. Effect of subchronic IP treatment with naloxone on the increase of voluntary ethanol intake induced by Ro 64-6198 in msP rats
The ANOVA revealed an overall effect of treatment [F(3,23) = 6.80; P < 0.01]. Confirming the results of Exp. 3.4, post-hoc comparisons showed a significant increase of ethanol intake following administration of 1.0 mg/kg of Ro 64-6198 (P < 0.01), whereas pre-treatment with naloxone (0.5 mg/kg, IP) attenuated this effect of Ro 64-6198. In particular, as shown in Fig. 2, treatment with Ro 64-6198 induced a significant increase on ethanol consumption during the first 30 min of drinking on days 5 and 7 (Fig. 2C), and at 60 min on days 5, 6 and 7 of drug treatment (Fig. 2D). This effect of Ro 64-6198 was blocked by naloxone at all time points (Fig. 2C, 2D)
The ANOVA also revealed a significant treatment effect on food intake [F(3,23) = 1.18; P < 0.05]. Post-hoc comparisons showed a significant increase of food intake (P < 0.01) after treatment with Ro 64-6198 (1.0 mg/kg, IP) on days 4 and 5 (Table 1) Also, in this case the effect of Ro 64-6198 was blocked by pre-treatment with 0.5 mg/kg of naloxone (Table 1). Water intake was not modified by drug treatments (Table 1).
DISCUSSION
The results show that subchronic administration of OS-462, UFP-112 and UFP-102 induces a pronounced decrease in voluntary ethanol intake in msP rats. Interestingly, this effect was similar in magnitude to the one reported earlier for N/OFQ [4]. Discontinuation of treatment with these NOP receptor agonists resulted in resumption of ethanol intake that returned to pre-treatment levels; an effect also seen with N/OFQ [4]. Conversely, subchronic treatment with Ro 64-6198 not only failed to decrease ethanol consumption but at the highest dose increased ethanol intake. This effect of Ro 64-6198 was prevented by naloxone, suggesting, a role of μ-opioid receptors for this action of the compound. This result is also linked with data showing a very peculiar pharmacological profile for Ro 64-6198. In fact, while electrophysiological evidence exists that Ro 64-6198 selectively activates a specific subpopulation of NOP receptors [2], binding and drug discrimination studies suggest that this compound has residual agonist activity at μ-opioid receptors [23,44]. This latter finding is particularly relevant and in line with our results with Ro 64-6198. It is known, for example, that activation of μ-opioid receptors by low doses of morphine or the selective μ agonist DAMGO increases ethanol consumption in rats [19,20,51]. In addition, it was recently shown that the partial μ-receptor agonist buprenorphine, which also binds to NOP receptors, increases or reduces ethanol consumption depending on whether its action is predominantly mediated by μ or NOP receptors, respectively [3]. In fact, it was shown that following administration of low doses (0.03 mg/kg) of buprenorphine, ethanol consumption increased in msP rats. This effect was completely reversed by pretreatment with naltrexone. Conversely, high doses of buprenorphine (3.0 mg/kg) reduced ethanol drinking and this effect was selectively blocked by treatment with the NOP antagonist UFP-101 but not with naltrexone [3]. Based on these considerations, activation of μ-receptors by Ro-64-6198 may provide an explanation for the increase in ethanol intake seen in our studies with this compound.
An alternative explanation for the effect of Ro 64-6198 on ethanol intake could also be the marked hyperphagic action of this NOP receptor agonist. In fact, daily ethanol consumption in msP rats is particularly high (7–8 g/kg day), and even under limited access conditions it is rather consistent (1.5–2.0 g/kg in 60 min). The caloric intake originating from consumption of these ethanol doses is high and, therefore, ethanol intake in msP rats may be particularly sensitive to pharmacological manipulation of feeding-related mechanisms. In addition, a classical ingestive behavior phenomenon is the so-called “food-associated drinking,” in which an increase of food intake is often accompanied by a simultaneously increase of water drinking, especially if dry food chow is used as standard meal. If food-associated drinking is a factor here, Ro 64-6198, owing to its potent orexigenic effect, could have caused a significant increase in fluid consumption. In this case, increased alcohol may have been related to the high innate ethanol preference of msP rats and the fact that alcohol solution is the predominant source of fluid in these animals (see Table 1). Nevertheless, above considerations are not in line with the results of our studies obtained with two of the other compounds, OS-462 and UFP-102, which also increased feeding, albeit to a lower extent, while ethanol drinking was simultaneously reduced. On the other hand, we also showed that pre-treatment with naloxone not only blocks the increased ethanol intake induced by Ro 64-6198, but also inhibited the hyperphagic effect of this compound. As it was not possible to dissociate these two effects by giving naltrexone, the possibility that the effect of Ro -64-6198 on ethanol intake is the consequence of a more general regulatory action on feeding behavior cannot be conclusively dismissed.
In addition, NOP-mediated regulation of kidney-function may have influenced the effect of Ro 64-6198 on ethanol intake. In fact, it is known that NOP agonists facilitate diuresis in the rat, and as our animals take substantial amounts of fluids by drinking ethanol it is possible that drug-induced changes in diuresis may also result in epiphenomena such as increase of ethanol drinking [26].
There is substantial evidence that N/OFQ acts as a functional anti-opioid agent in the brain. N/OFQ blocks the analgesic effects of morphine [27,34,35,36], prevents the development of morphine-induced conditioned place preference [6,39] and inhibits morphine-induced DA release in the NAcc [12]. In addition, electrophysiological data demonstrate that the N/OFQ system inhibits the firing of β-endorphin cells in the hypothalamic arcuate nucleus [48]. These arcuate neurons project to the ventral tegmental area (VTA) and the NAcc, among other brain regions, where they interact with mesolimbic DA transmission and influence motivated behavior [9,10,11,18,24]. Moreover, it has been shown that 91% of tyrosine hydroxylase-positive cells in the VTA co-express NOP receptors, and that N/OFQ can directly and indirectly (via GABA interneurons) modulate (inhibit) neural activity of VTA DA neurons [31,41,52]. Given this modulatory role of N/OFQ on mesocorticolimbic DA and opioid activity, and taking into consideration the important role of these systems in the regulation of ethanol-related behaviours, one may hypothesize that the N/OFQ-NOP system may reduce the motivational value of alcohol by modulating brain DAergic and opioidergic function.
One of the main challenges for current research on the N/OFQ-NOP receptor system is to generate brain-penetrating molecules, able to selectively bind brain NOP receptors after peripheral administration. Therefore, the ability of Ro 64-6198, a non-peptidergic brain-penetrating NOP receptor agonist [49], to reduce ethanol intake after peripheral injection was evaluated in the present study. However, unlike N/OFQ or peptidergic NOP agonists, Ro 64-6198 failed to decrease voluntary ethanol consumption, possibly because of a residual agonist activity at μ-opioid receptors of this compound [23,44]. Therefore, the results failed to confirm the typical NOP agonist-like effect for Ro 64-6198.
In conclusion, the results demonstrate that, like the endogenous ligand N/OFQ, the synthetic peptides OS-462, UFP-102 and UFP-112 act as potent and selective NOP receptor agonists, as measured by their ability to reduce ethanol intake. In contrast, this effect was not shared by Ro 64-6198. These findings suggest that OS-462, UFP-102 and UFP-112 may represent valuable pharmacological tools to further evaluate the functional role of the N/OFQ-NOP receptor system. Conversely, the pharmacological profile of Ro 64-6198 does not seem to be superimposable with that of N/OFQ or the other peptide agonists tested here. Overall, the present findings suggest that activation of NOP receptors may represent a promising approach for the pharmacotherapeutic treatment of alcohol abuse, but that Ro 64-6198 does not appear to offer promise in this regard.
Acknowledgments
The authors thank Marino Cucculelli for technical assistance and animal care. The study was supported by the NIH/NIAAA grant AA 10531 (to FW) and by grant MIUR 2004 to (MM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.peptides.2006.09.007
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1847604?pdf=render
Citations & impact
Impact metrics
Article citations
Pharmacological blockage of NOP receptors decreases ventral tegmental area dopamine neuronal activity through GABAB receptor-mediated mechanism.
Neuropharmacology, 248:109866, 15 Feb 2024
Cited by: 0 articles | PMID: 38364970
N-acylethanolamine acid amidase (NAAA) inhibition decreases the motivation for alcohol in Marchigian Sardinian alcohol-preferring rats.
Psychopharmacology (Berl), 238(1):249-258, 10 Oct 2020
Cited by: 2 articles | PMID: 33037452 | PMCID: PMC7796956
Andrographis paniculata and Its Main Bioactive Ingredient Andrographolide Decrease Alcohol Drinking and Seeking in Rats Through Activation of Nuclear PPARγ Pathway.
Alcohol Alcohol, 56(2):240-249, 01 Feb 2021
Cited by: 2 articles | PMID: 33401299 | PMCID: PMC7906876
NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area.
Br J Pharmacol, 177(7):1525-1537, 03 Feb 2020
Cited by: 17 articles | PMID: 31713848 | PMCID: PMC7060370
Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys.
Neuropsychopharmacology, 44(8):1476-1484, 10 Apr 2019
Cited by: 8 articles | PMID: 30970376 | PMCID: PMC6784996
Go to all (35) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system.
Biol Psychiatry, 61(1):4-12, 14 Mar 2006
Cited by: 56 articles | PMID: 16533497 | PMCID: PMC3035814
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.
Pharmacol Ther, 141(3):283-299, 01 Nov 2013
Cited by: 109 articles | PMID: 24189487 | PMCID: PMC5098338
Review Free full text in Europe PMC
Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect.
Physiol Behav, 82(1):63-68, 01 Aug 2004
Cited by: 48 articles | PMID: 15234592
Review
Dysregulation of Nociceptin/Orphanin FQ and Dynorphin Systems in the Extended Amygdala of Alcohol Preferring Marchigian Sardinian (msP) Rats.
Int J Mol Sci, 22(5):2448, 28 Feb 2021
Cited by: 8 articles | PMID: 33671048 | PMCID: PMC7957504
Funding
Funders who supported this work.
NIAAA NIH HHS (3)
Grant ID: R01 AA014351
Grant ID: R01 AA010531
Grant ID: AA 10531





