Abstract
Free full text

Tangential flow filtration and preliminary phylogenetic analysis of marine picoplankton.
Abstract
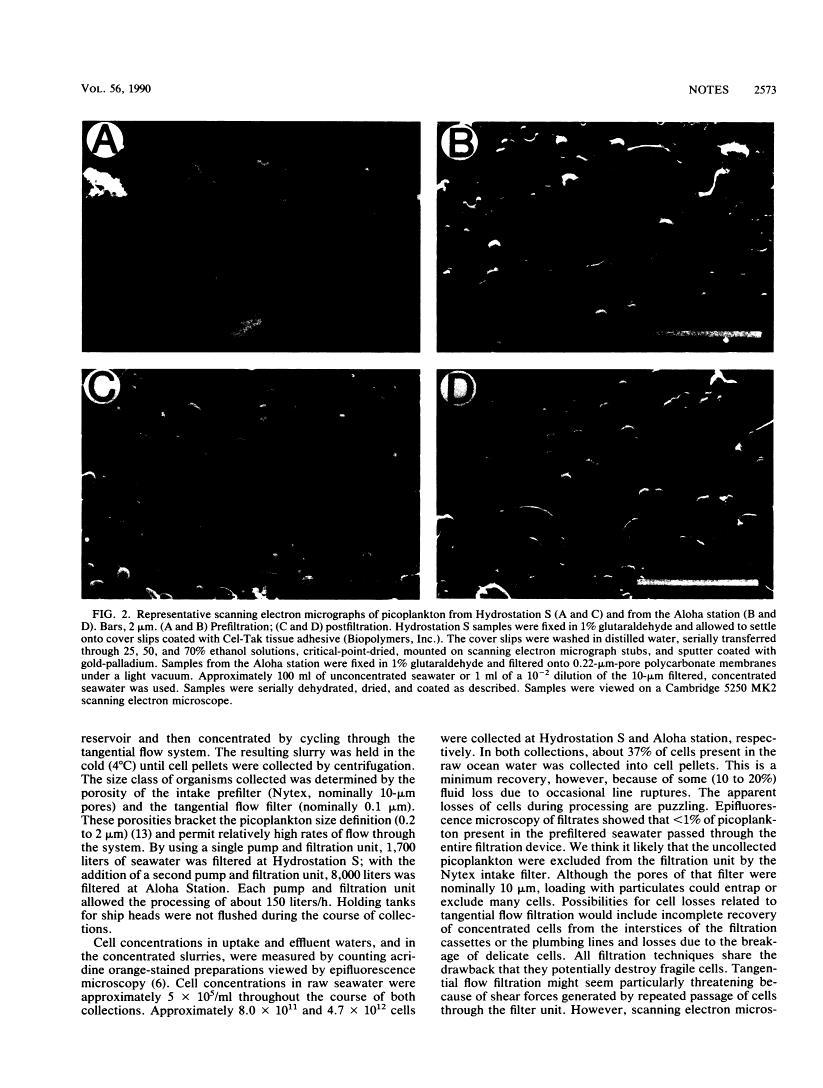
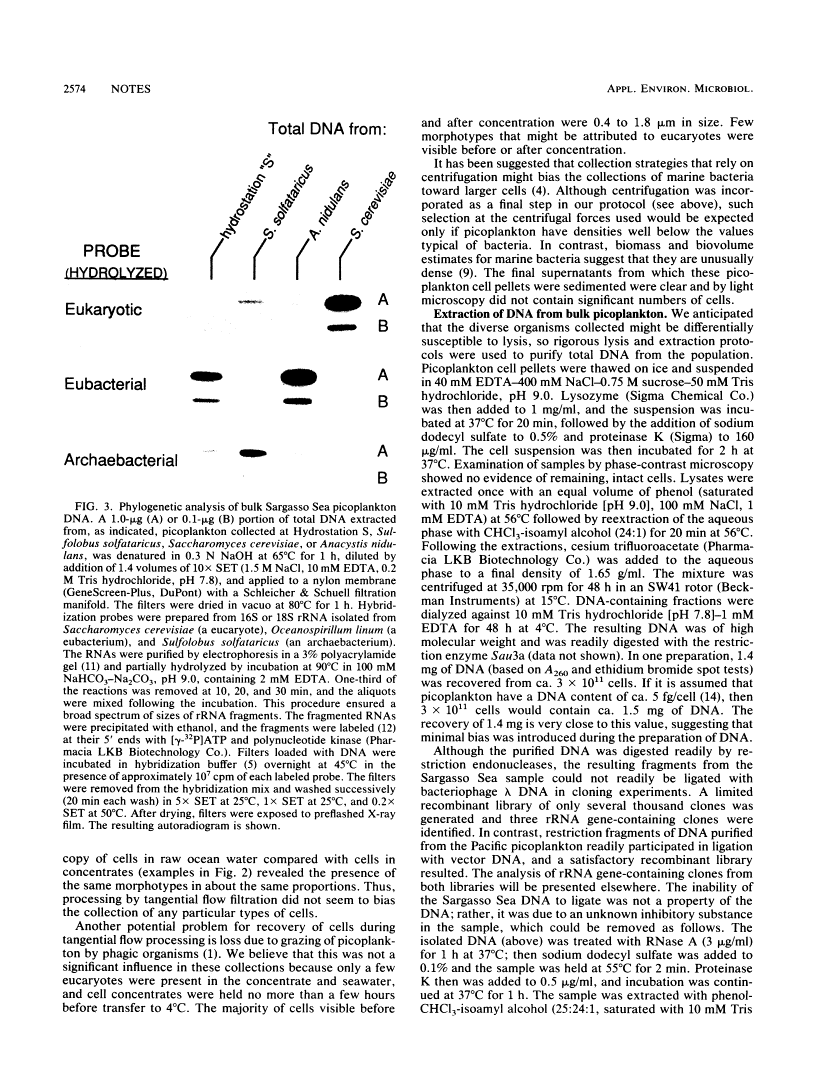
A procedure was developed for harvesting gram quantities of microbial biomass from oligotrophic waters, when mixed populations are present in low abundance. Picoplankton from Atlantic Ocean (Hydrostation S, Sargasso Sea) and Pacific Ocean (Aloha Station) sites were collected in a three-stage process: (i) collection of seawater through an intake covered with 10-microns-pore Nytex; (ii) concentration by a tangential flow filtration device equipped with 10 ft2 (0.929 m2) of 0.1-micron-pore fluorocarbon membrane; (iii) collection of cells from concentrate by centrifugation. The overall efficiency of picoplankton recovery was at least 37%. The cellular morphotypes recovered matched those of the original population. DNA was prepared from frozen cell pellets by enzymatic digestion, solvent extraction, and isopycnic centrifugation. As indicated by the binding of kingdom-specific hybridization probes to the purified DNA, the Sargasso Sea picoplankton in this collection were largely eubacteria.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferguson RL, Buckley EN, Palumbo AV. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. [Europe PMC free article] [Abstract] [Google Scholar]
- Fuhrman JA, Comeau DE, Hagström A, Chan AM. Extraction from natural planktonic microorganisms of DNA suitable for molecular biological studies. Appl Environ Microbiol. 1988 Jun;54(6):1426–1429. [Europe PMC free article] [Abstract] [Google Scholar]
- Giovannoni SJ, DeLong EF, Olsen GJ, Pace NR. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988 Feb;170(2):720–726. [Europe PMC free article] [Abstract] [Google Scholar]
- Hobbie JE, Daley RJ, Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. [Europe PMC free article] [Abstract] [Google Scholar]
- Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. [Abstract] [Google Scholar]
- Lee S, Fuhrman JA. Relationships between Biovolume and Biomass of Naturally Derived Marine Bacterioplankton. Appl Environ Microbiol. 1987 Jun;53(6):1298–1303. [Europe PMC free article] [Abstract] [Google Scholar]
- Olsen GJ, Lane DJ, Giovannoni SJ, Pace NR, Stahl DA. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol. 1986;40:337–365. [Abstract] [Google Scholar]
- Sgaramella V, Khorana HG. CXII. Total synthesis of the structural gene for an alanine transfer RNA from yeast. Enzymic joining of the chemically synthesized polydeoxynucleotides to form the DNA duplex representing nucleotide sequence 1 to 20. J Mol Biol. 1972 Dec 28;72(2):427–444. [Abstract] [Google Scholar]
- Somerville CC, Knight IT, Straube WL, Colwell RR. Simple, rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol. 1989 Mar;55(3):548–554. [Europe PMC free article] [Abstract] [Google Scholar]
- Watson SW, Novitsky TJ, Quinby HL, Valois FW. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. [Europe PMC free article] [Abstract] [Google Scholar]
- Woese CR. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Applied and Environmental Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aem.56.8.2572-2575.1990
Read article for free, from open access legal sources, via Unpaywall:
https://aem.asm.org/content/aem/56/8/2572.full.pdf
Free to read at aem.asm.org
http://aem.asm.org/cgi/content/abstract/56/8/2572
Free after 4 months at aem.asm.org
http://aem.asm.org/cgi/reprint/56/8/2572
Citations & impact
Impact metrics
Citations of article over time
Article citations
Long-read powered viral metagenomics in the oligotrophic Sargasso Sea.
Nat Commun, 15(1):4089, 14 May 2024
Cited by: 0 articles | PMID: 38744831 | PMCID: PMC11094077
Suboxic DOM is bioavailable to surface prokaryotes in a simulated overturn of an oxygen minimum zone, Devil's Hole, Bermuda.
Front Microbiol, 14:1287477, 20 Dec 2023
Cited by: 0 articles | PMID: 38179459 | PMCID: PMC10765504
Organic and inorganic nutrients modulate taxonomic diversity and trophic strategies of small eukaryotes in oligotrophic oceans.
FEMS Microbes, 4:xtac029, 07 Dec 2022
Cited by: 1 article | PMID: 37333435 | PMCID: PMC10117809
Capturing marine microbiomes and environmental DNA: A field sampling guide.
Front Microbiol, 13:1026596, 12 Jan 2023
Cited by: 2 articles | PMID: 36713215 | PMCID: PMC9877356
Review Free full text in Europe PMC
Influence of short and long term processes on SAR11 communities in open ocean and coastal systems.
ISME Commun, 2(1):116, 19 Nov 2022
Cited by: 2 articles | PMID: 37938786 | PMCID: PMC9723719
Go to all (97) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing.
J Bacteriol, 173(14):4371-4378, 01 Jul 1991
Cited by: 352 articles | PMID: 2066334 | PMCID: PMC208098
Hydroids (Cnidaria, Hydrozoa) from Mauritanian Coral Mounds.
Zootaxa, 4878(3):zootaxa.4878.3.2, 16 Nov 2020
Cited by: 8 articles | PMID: 33311142
Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel.
Appl Environ Microbiol, 63(1):50-56, 01 Jan 1997
Cited by: 287 articles | PMID: 8979338 | PMCID: PMC168301
Latitudinal distribution of prokaryotic picoplankton populations in the Atlantic Ocean.
Environ Microbiol, 11(8):2078-2093, 30 Apr 2009
Cited by: 125 articles | PMID: 19453607