Abstract
Free full text

Gadolinium chloride suppresses hepatic oval cell proliferation in rats with biliary obstruction.
Abstract
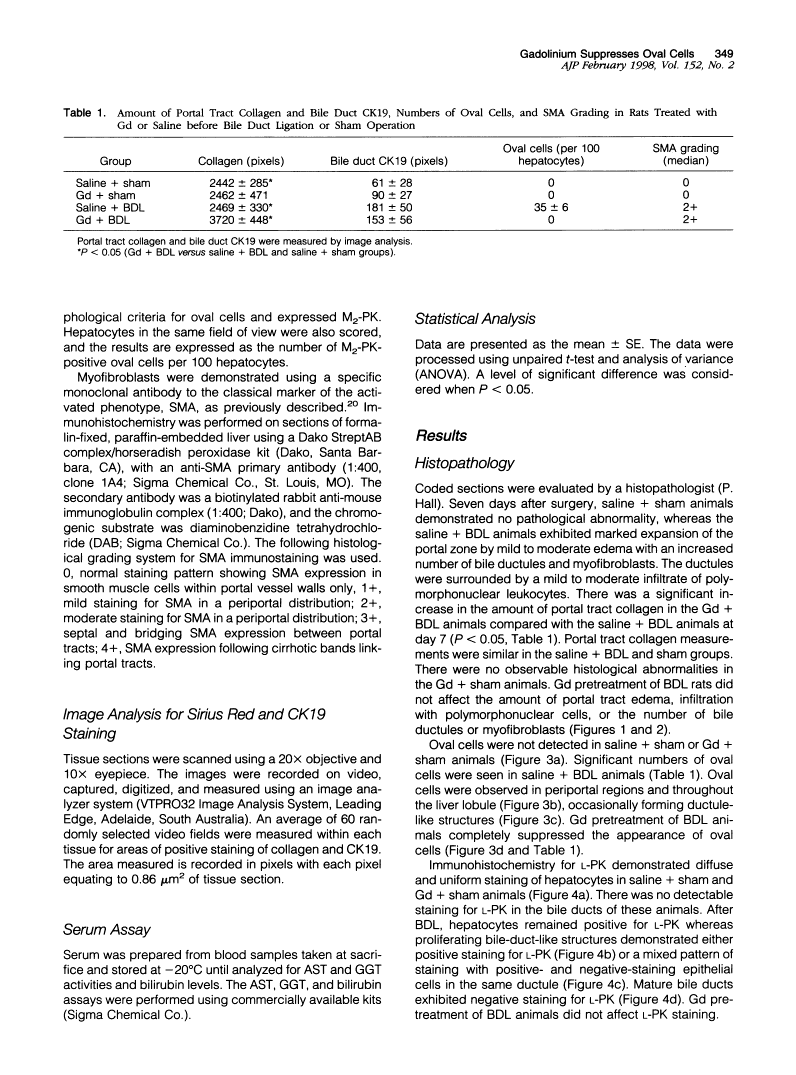
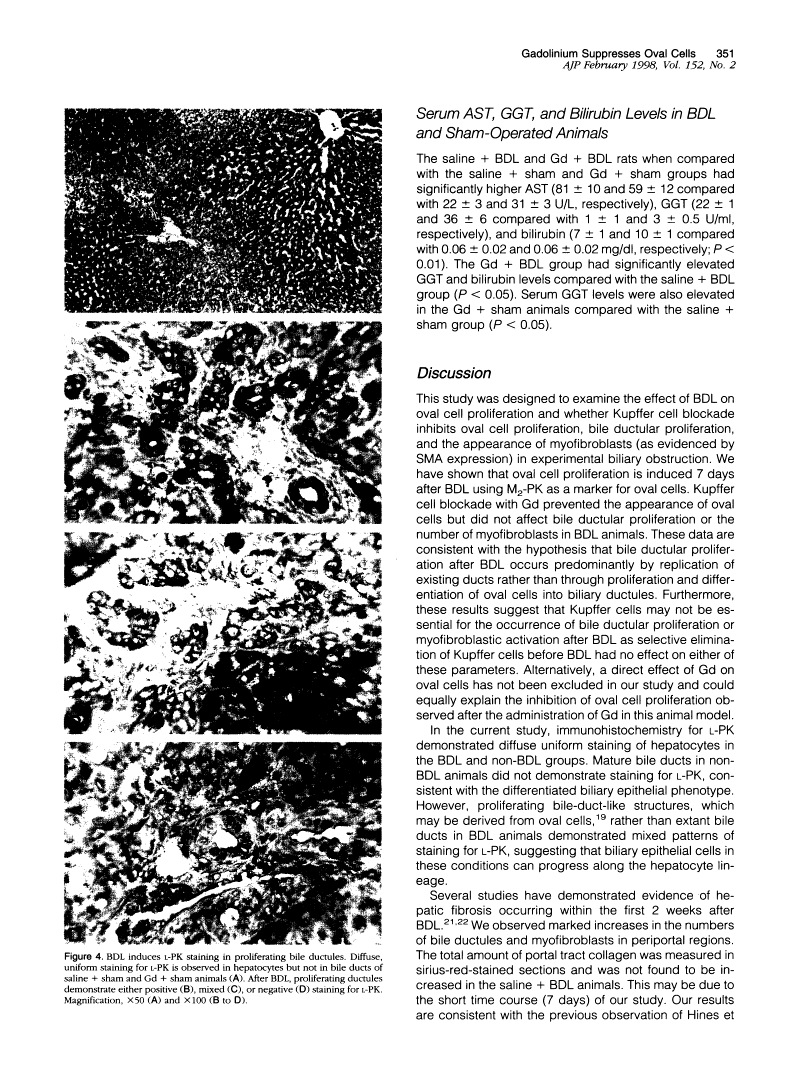
Liver injury due to bile duct ligation (BDL) is histologically characterized by cholestasis, bile ductular proliferation, hepatocellular damage, portal fibrosis, and ultimately biliary cirrhosis. Stem cells within the liver may act as progenitor cells for small epithelial cells termed oval cells that can differentiate into bile duct cells or hepatocytes, whereas myofibroblasts are the principal source of collagen production in fibrosis. The aims of this study were to determine 1) whether BDL induces oval cell proliferation and 2) whether blockade of Kupffer cells affects oval cell proliferation, bile duct proliferation, and myofibroblast transformation in experimental biliary obstruction. Male Sprague-Dawley rats were divided into two groups to receive either a single dose of gadolinium chloride (a selective Kupffer cell blocking agent) or vehicle. One day later, the gadolinium- and vehicle-treated groups were further subdivided to receive either BDL or sham operation. The rats were sacrificed on day 7 postoperatively. Serum was collected for measurement of aspartate aminotransferase, gamma-glutamyl transpeptidase, and bilirubin levels. Liver tissue was taken for evaluation of fibrosis, bile ductular cells, oval cells, and myofibroblasts. BDL resulted in elevated aspartate aminotransferase, gamma-glutamyl transpeptidase, and bilirubin in serum, and gadolinium pretreatment did not modify these effects. BDL induced marked oval cell proliferation, which was completely prevented by gadolinium pretreatment. Gadolinium did not affect the induction of bile duct expansion or myofibroblasts after BDL. We conclude that experimental biliary obstruction induces oval cell proliferation, which can be prevented by gadolinium pretreatment. This suggests that bile ductular proliferation and myofibroblast transformation are not mediated by Kupffer cells and that ductular proliferation can proceed in the absence of oval cells. Alternatively, gadolinium may directly affect oval cell proliferation after BDL.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Slott PA, Liu MH, Tavoloni N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology. 1990 Aug;99(2):466–477. [Abstract] [Google Scholar]
- Factor VM, Radaeva SA, Thorgeirsson SS. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994 Aug;145(2):409–422. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith PG, Yeoh GC. Chronic iron overload in rats induces oval cells in the liver. Am J Pathol. 1996 Aug;149(2):389–398. [Europe PMC free article] [Abstract] [Google Scholar]
- Omori M, Evarts RP, Omori N, Hu Z, Marsden ER, Thorgeirsson SS. Expression of alpha-fetoprotein and stem cell factor/c-kit system in bile duct ligated young rats. Hepatology. 1997 May;25(5):1115–1122. [Abstract] [Google Scholar]
- Hepatic stellate cell nomenclature. Hepatology. 1996 Jan;23(1):193–193. [Abstract] [Google Scholar]
- Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. [Europe PMC free article] [Abstract] [Google Scholar]
- Hines JE, Johnson SJ, Burt AD. In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am J Pathol. 1993 Feb;142(2):511–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Miyazaki H, Van Eyken P, Roskams T, De Vos R, Desmet VJ. Transient expression of tenascin in experimentally induced cholestatic fibrosis in rat liver: an immunohistochemical study. J Hepatol. 1993 Nov;19(3):353–366. [Abstract] [Google Scholar]
- Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [Abstract] [Google Scholar]
- Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994 Apr;14(2):76–82. [Abstract] [Google Scholar]
- Tuchweber B, Desmoulière A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996 Jan;74(1):265–278. [Abstract] [Google Scholar]
- Hines JE, Johnson SJ, Burt AD. In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am J Pathol. 1993 Feb;142(2):511–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994 Aug;20(2):453–460. [Abstract] [Google Scholar]
- Hardonk MJ, Dijkhuis FW, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992 Sep;52(3):296–302. [Abstract] [Google Scholar]
- Iimuro Y, Yamamoto M, Kohno H, Itakura J, Fujii H, Matsumoto Y. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats--analysis of mechanisms of lethality in endotoxemia. J Leukoc Biol. 1994 Jun;55(6):723–728. [Abstract] [Google Scholar]
- Tian YW, Smith PG, Yeoh GC. The oval-shaped cell as a candidate for a liver stem cell in embryonic, neonatal and precancerous liver: identification based on morphology and immunohistochemical staining for albumin and pyruvate kinase isoenzyme expression. Histochem Cell Biol. 1997 Mar;107(3):243–250. [Abstract] [Google Scholar]
- Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW. Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol. 1997 Mar;26(3):584–592. [Abstract] [Google Scholar]
- Aldana PR, Goerke ME, Carr SC, Tracy TF., Jr The expression of regenerative growth factors in chronic liver injury and repair. J Surg Res. 1994 Dec;57(6):711–717. [Abstract] [Google Scholar]
- Tracy TF, Jr, Goerke ME, Bailey PV, Sotelo-Avila C, Weber TR. Growth-related gene expression in early cholestatic liver injury. Surgery. 1993 Sep;114(3):532–537. [Abstract] [Google Scholar]
- Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol. 1996 Jun;270(6 Pt 1):G909–G918. [Abstract] [Google Scholar]
Associated Data
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/152/2/347
Citations & impact
Impact metrics
Citations of article over time
Article citations
Bile Duct Ligation Impairs Function and Expression of Mrp1 at Rat Blood-Retinal Barrier via Bilirubin-Induced P38 MAPK Pathway Activations.
Int J Mol Sci, 23(14):7666, 11 Jul 2022
Cited by: 5 articles | PMID: 35887010 | PMCID: PMC9318728
Biliary Epithelial Senescence in Liver Disease: There Will Be SASP.
Front Mol Biosci, 8:803098, 21 Dec 2021
Cited by: 15 articles | PMID: 34993234 | PMCID: PMC8724525
Review Free full text in Europe PMC
Cholestasis Differentially Affects Liver Connexins.
Int J Mol Sci, 21(18):E6534, 07 Sep 2020
Cited by: 8 articles | PMID: 32906817 | PMCID: PMC7116118
AM-1241 CB2 Receptor Agonist Attenuates Inflammation, Apoptosis and Stimulate Progenitor Cells in Bile Duct Ligated Rats.
Open Access Maced J Med Sci, 7(6):925-936, 29 Mar 2019
Cited by: 5 articles | PMID: 30976335 | PMCID: PMC6454175
Molecular signature of active fibrogenesis prevails in biliary atresia after successful portoenterostomy.
Surgery, 162(3):548-556, 24 Jun 2017
Cited by: 14 articles | PMID: 28655415
Go to all (37) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Gadolinium chloride, a Kupffer cell inhibitor, attenuates hepatic injury in a rat model of chronic cholestasis.
Hum Exp Toxicol, 30(11):1804-1810, 21 Feb 2011
Cited by: 9 articles | PMID: 21339256
Effects of the nitric oxide donor molsidomine on the early stages of liver damage in rats with bile duct ligation: a biochemical and immunohistochemical approach.
Eur Surg Res, 34(4):285-290, 01 Jul 2002
Cited by: 12 articles | PMID: 12145554
Protective effect of Urtica dioica on liver damage induced by biliary obstruction in rats.
Toxicol Ind Health, 29(9):838-845, 14 May 2012
Cited by: 6 articles | PMID: 22585933
Biliary proliferation and adaptation in furan-induced rat liver injury and carcinogenesis.
Toxicol Pathol, 24(1):90-99, 01 Jan 1996
Cited by: 29 articles | PMID: 8839286
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (2)
Grant ID: DK 46831
Grant ID: DK 41816