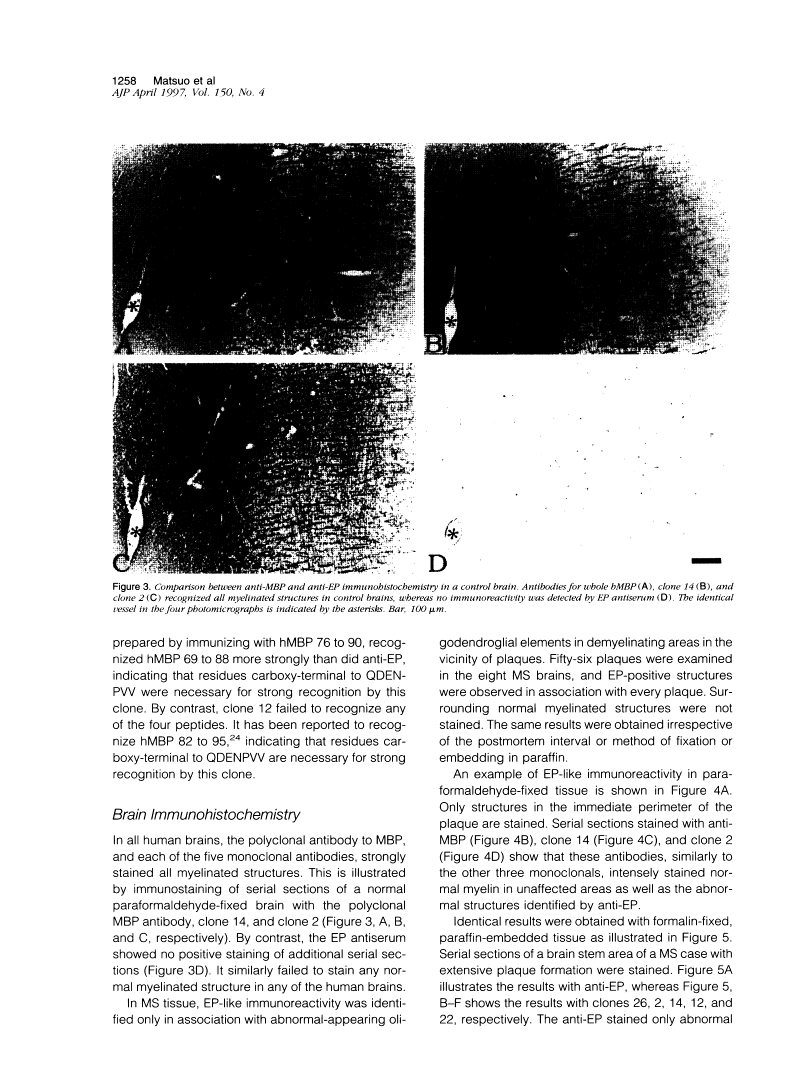
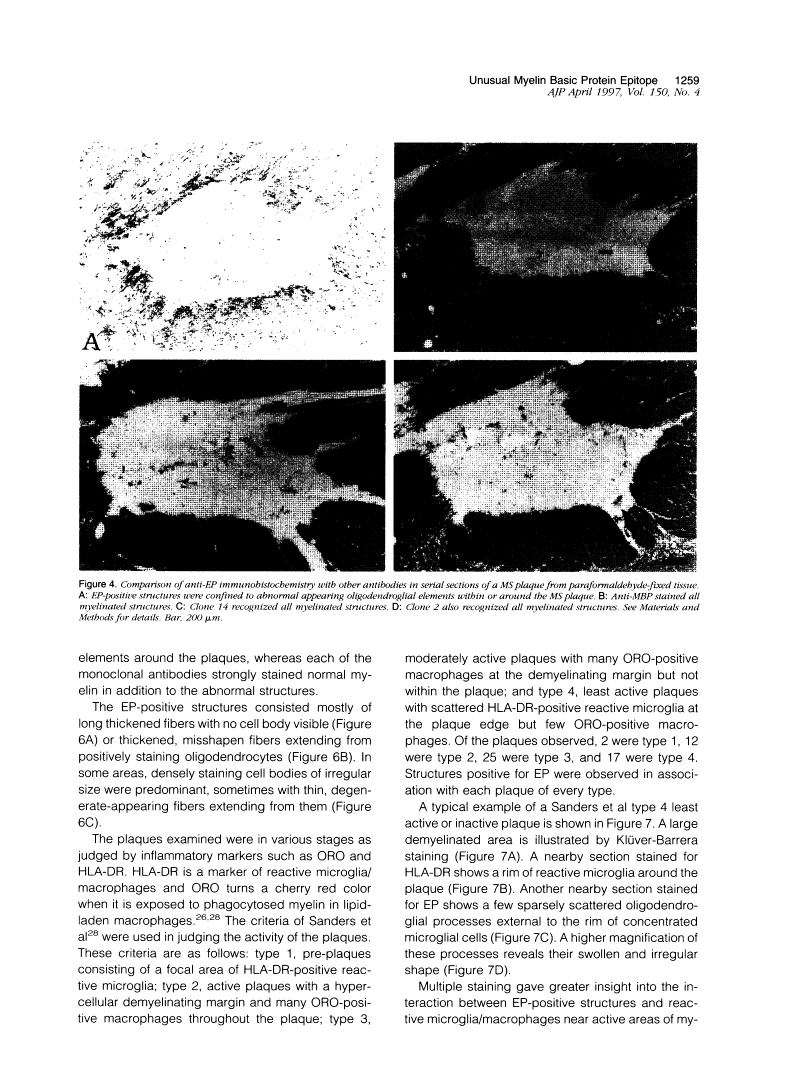
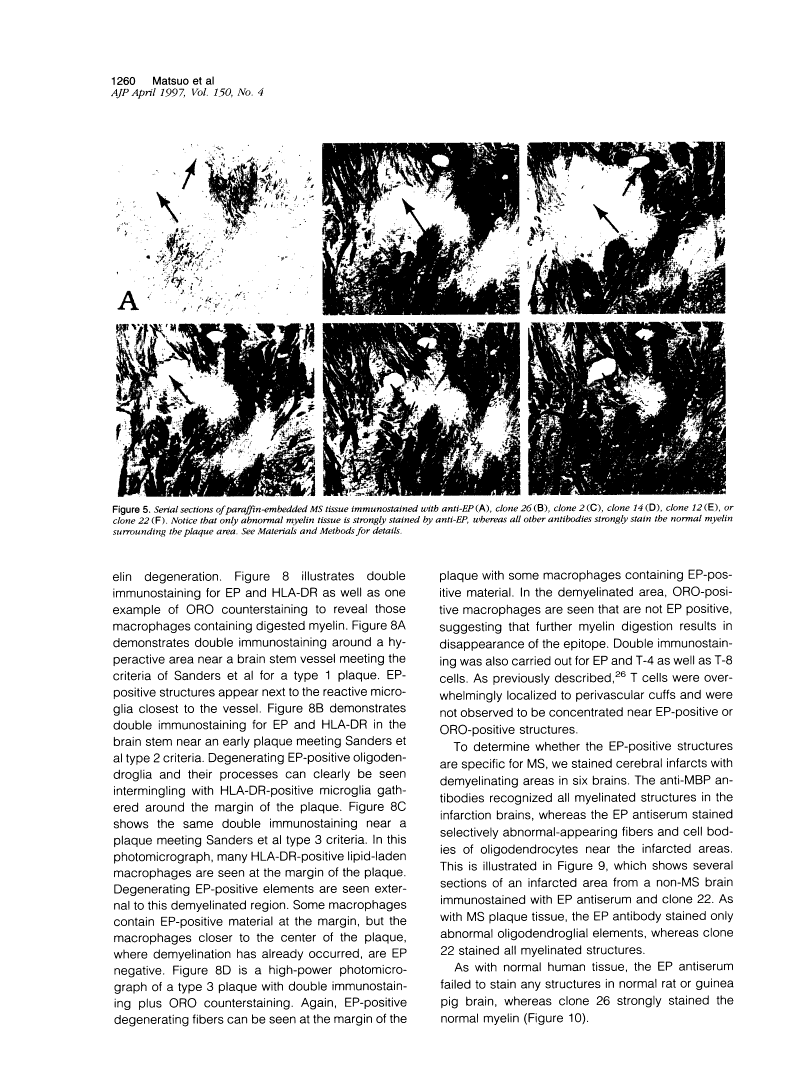
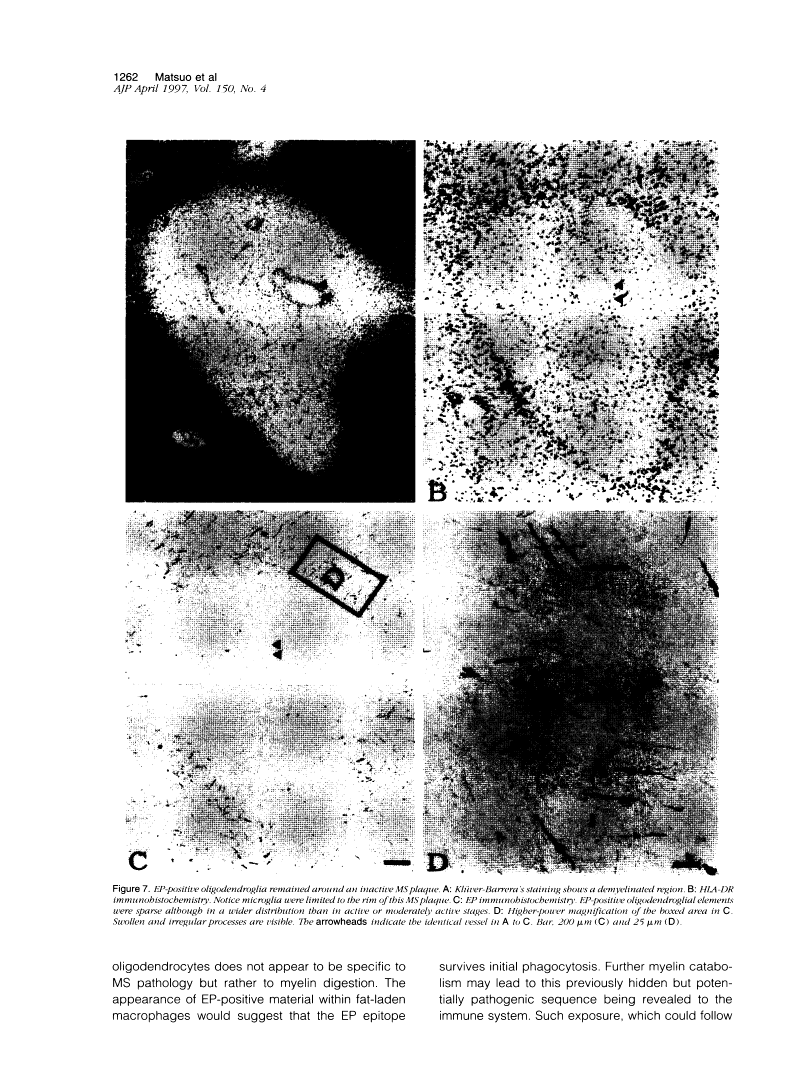
Abstract
Free full text

Unmasking of an unusual myelin basic protein epitope during the process of myelin degeneration in humans: a potential mechanism for the generation of autoantigens.
Abstract
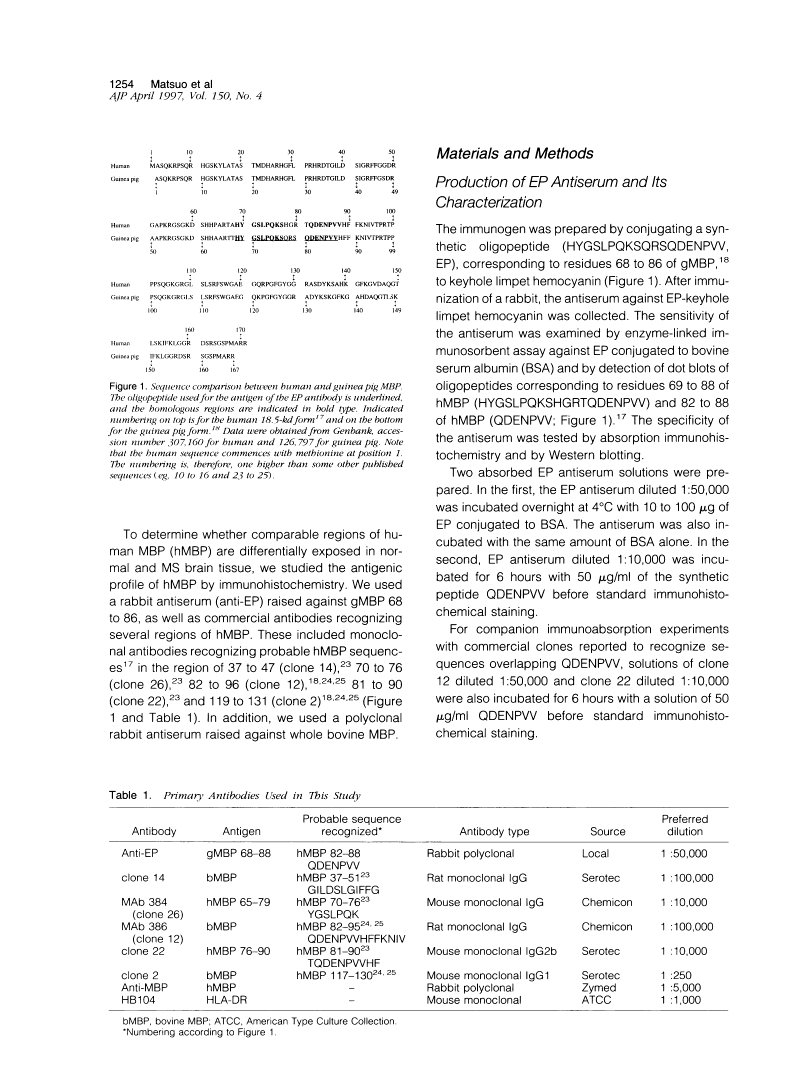
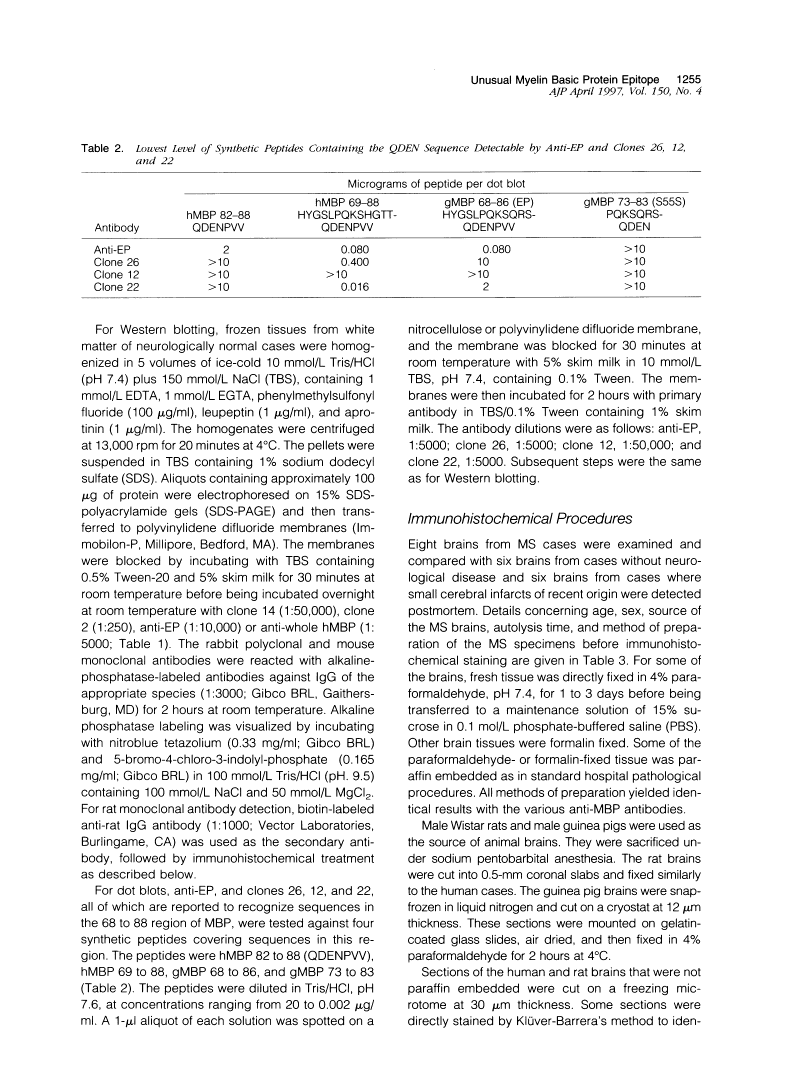
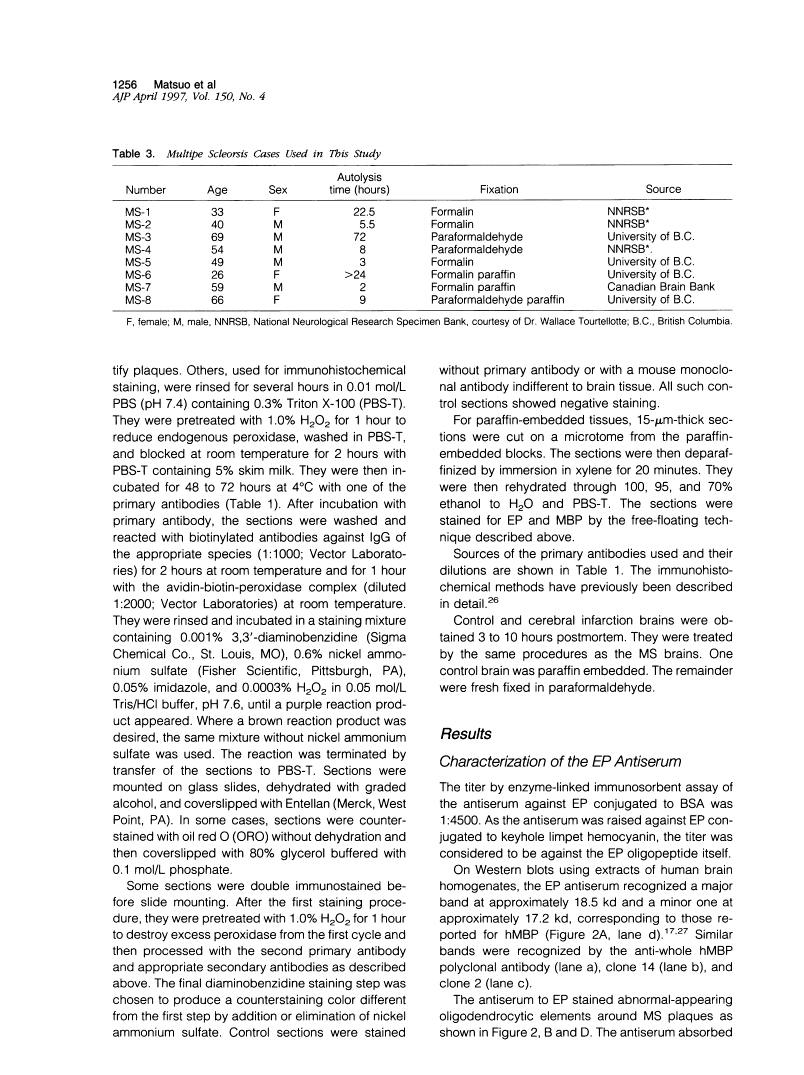
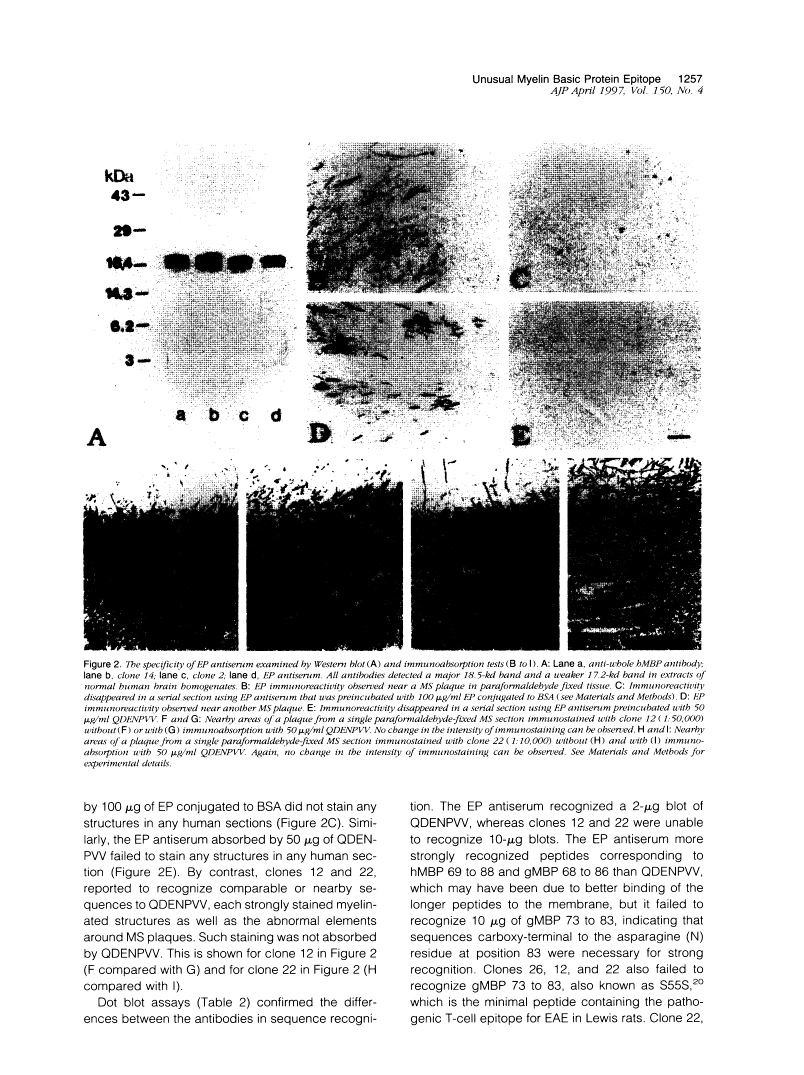
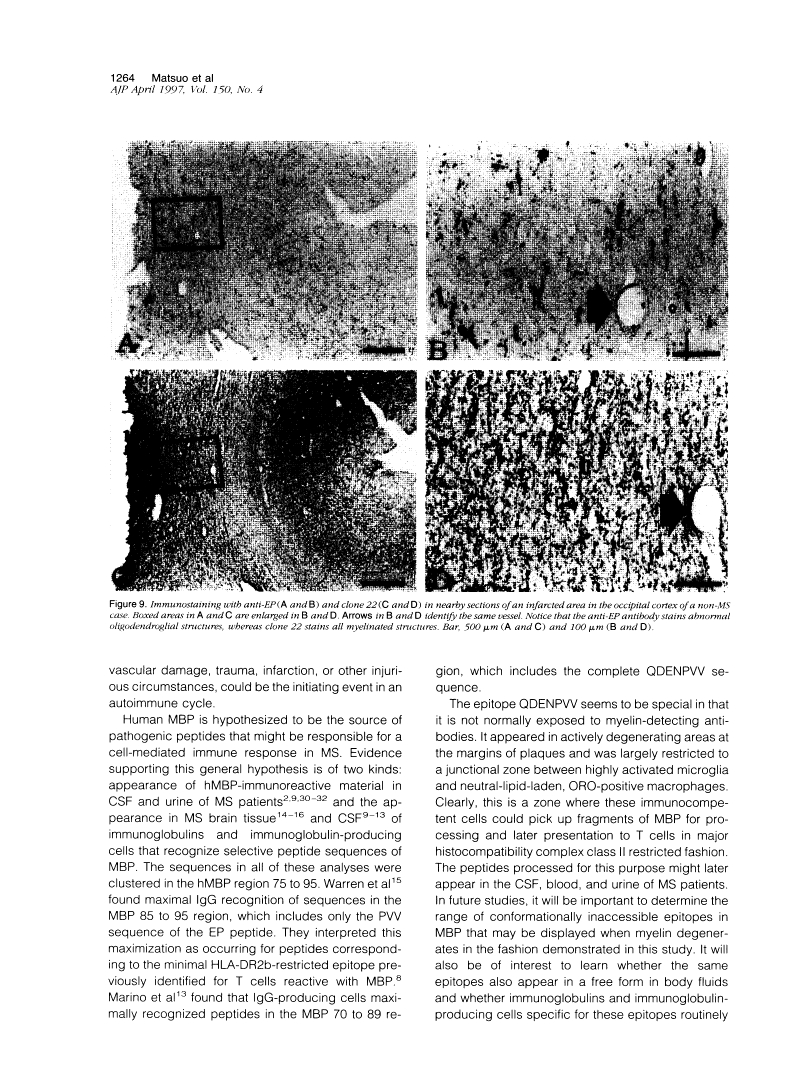
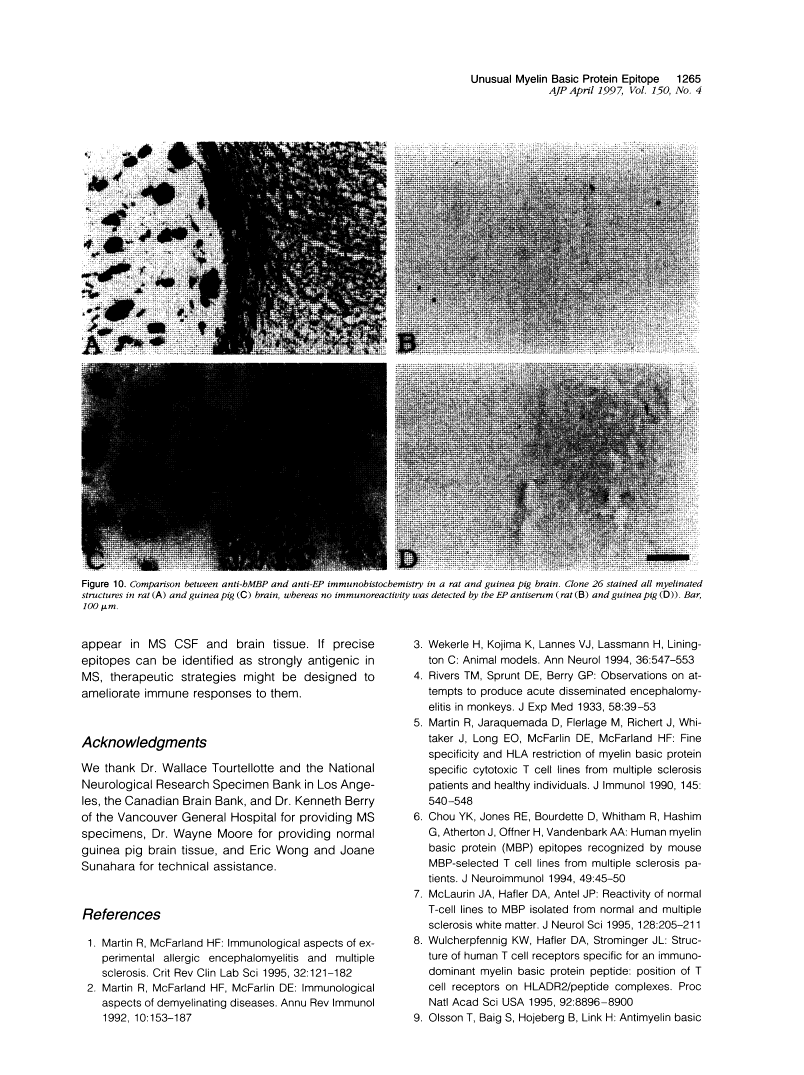
A rabbit antiserum (anti-EP), induced against a synthetic peptide corresponding to residues 68 to 86 of guinea pig myelin basic protein, powerfully immunostained abnormal-appearing oligodendrocytic processes and cell bodies in demyelinating areas associated with multiple sclerosis plaques. However, it failed to recognize any structures in normal human, rat, or guinea pig brain. The antiserum recognized the synthetic peptide QDENPVV, which corresponds to human myelin basic protein residues 82 to 88. Immunoabsorption with this peptide eliminated immunohistochemical staining. By contrast, several commercial antibodies recognizing nearby sequences of human myelin basic protein intensely stained all myelinated structures in both normal and multiple sclerosis tissue. The unusual epitope recognized by anti-EP appears to be accessible only in areas of myelin degeneration. If insults occur that repeatedly expose a region of MBP normally sheltered from immunosurveillance, a self-sustaining immune reaction might result.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (7.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32(2):121–182. [Abstract] [Google Scholar]
- Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. [Abstract] [Google Scholar]
- Martin R, Jaraquemada D, Flerlage M, Richert J, Whitaker J, Long EO, McFarlin DE, McFarland HF. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990 Jul 15;145(2):540–548. [Abstract] [Google Scholar]
- Chou YK, Jones RE, Bourdette D, Whitham R, Hashim G, Atherton J, Offner H, Vandenbark AA. Human myelin basic protein (MBP) epitopes recognized by mouse MBP-selected T cell lines from multiple sclerosis patients. J Neuroimmunol. 1994 Jan;49(1-2):45–50. [Abstract] [Google Scholar]
- McLaurin JA, Hafler DA, Antel JP. Reactivity of normal T-cell lines to MBP isolated from normal and multiple sclerosis white matter. J Neurol Sci. 1995 Feb;128(2):205–211. [Abstract] [Google Scholar]
- Wucherpfennig KW, Hafler DA, Strominger JL. Structure of human T-cell receptors specific for an immunodominant myelin basic protein peptide: positioning of T-cell receptors on HLA-DR2/peptide complexes. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8896–8900. [Europe PMC free article] [Abstract] [Google Scholar]
- Warren KG, Catz I. Synthetic peptide specificity of anti-myelin basic protein from multiple sclerosis cerebrospinal fluid. J Neuroimmunol. 1992 Jul;39(1-2):81–89. [Abstract] [Google Scholar]
- Warren KG, Catz I. Increased synthetic peptide specificity of tissue-CSF bound anti-MBP in multiple sclerosis. J Neuroimmunol. 1993 Mar;43(1-2):87–96. [Abstract] [Google Scholar]
- Warren KG, Catz I. Relative frequency of autoantibodies to myelin basic protein and proteolipid protein in optic neuritis and multiple sclerosis cerebrospinal fluid. J Neurol Sci. 1994 Jan;121(1):66–73. [Abstract] [Google Scholar]
- Martino G, Olsson T, Fredrikson S, Hojeberg B, Kostulas V, Grimaldi LM, Link H. Cells producing antibodies specific for myelin basic protein region 70-89 are predominant in cerebrospinal fluid from patients with multiple sclerosis. Eur J Immunol. 1991 Dec;21(12):2971–2976. [Abstract] [Google Scholar]
- Warren KG, Catz I. Autoantibodies to myelin basic protein within multiple sclerosis central nervous system tissue. J Neurol Sci. 1993 Apr;115(2):169–176. [Abstract] [Google Scholar]
- Warren KG, Catz I, Steinman L. Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: the minimal B-cell epitope and a model of its features. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11061–11065. [Europe PMC free article] [Abstract] [Google Scholar]
- Gerritse K, Deen C, Fasbender M, Ravid R, Boersma W, Claassen E. The involvement of specific anti myelin basic protein antibody-forming cells in multiple sclerosis immunopathology. J Neuroimmunol. 1994 Jan;49(1-2):153–159. [Abstract] [Google Scholar]
- Kamholz J, de Ferra F, Puckett C, Lazzarini R. Identification of three forms of human myelin basic protein by cDNA cloning. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4962–4966. [Europe PMC free article] [Abstract] [Google Scholar]
- Hashim GA. Experimental allergic encephalomyelitis in Lewis rats: chemical synthesis of disease-inducing determinant. Science. 1977 Jun 10;196(4295):1219–1221. [Abstract] [Google Scholar]
- Hashim GA, Day ED, Carvalho E, Abdelaal A. Experimental allergic encephalomyelitis (EAE): role of B cell and T cell epitopes in the development of EAE in Lewis rats. J Neurosci Res. 1987;17(4):375–383. [Abstract] [Google Scholar]
- Chou CH, Fritz RB, Chou FC, Kibler RF. The immune response of Lewis rats to peptide 68-88 of guinea pig myelin basic protein. I. T cell determinants. J Immunol. 1979 Oct;123(4):1540–1543. [Abstract] [Google Scholar]
- Mannie MD, Paterson PY, U'Prichard DC, Flouret G. Induction of experimental allergic encephalomyelitis in Lewis rats with purified synthetic peptides: delineation of antigenic determinants for encephalitogenicity, in vitro activation of cellular transfer, and proliferation of lymphocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5515–5519. [Europe PMC free article] [Abstract] [Google Scholar]
- Groome N, Dawkes A, Barry R, Hruby S, Alvord E., Jr New monoclonal antibodies reactive with defined sequential epitopes in human myelin basic protein. J Neuroimmunol. 1988 Oct;19(4):305–315. [Abstract] [Google Scholar]
- Hruby S, Alvord EC, Jr, Groome NP, Dawkes A, Martenson RE. Monoclonal antibodies reactive with myelin basic protein. Mol Immunol. 1987 Dec;24(12):1359–1364. [Abstract] [Google Scholar]
- Groome NP, Dawkes A, Gales M, Hruby S, Alvord EC., Jr Region-specific immunoassays for human myelin basic protein. J Neuroimmunol. 1986 Oct;12(4):253–264. [Abstract] [Google Scholar]
- Boyle EA, McGeer PL. Cellular immune response in multiple sclerosis plaques. Am J Pathol. 1990 Sep;137(3):575–584. [Europe PMC free article] [Abstract] [Google Scholar]
- Kerlero De Rosbo N, Carnegie PR, Bernard CC, Linthicum DS. Detection of various forms of brain myelin basic protein in vertebrates by electroimmunoblotting. Neurochem Res. 1984 Oct;9(10):1359–1369. [Abstract] [Google Scholar]
- Sanders V, Conrad AJ, Tourtellotte WW. On classification of post-mortem multiple sclerosis plaques for neuroscientists. J Neuroimmunol. 1993 Jul;46(1-2):207–216. [Abstract] [Google Scholar]
- Whitaker JN, Moscarello MA, Herman PK, Epand RM, Surewicz WK. Conformational correlates of the epitopes of human myelin basic protein peptide 80-89. J Neurochem. 1990 Aug;55(2):568–576. [Abstract] [Google Scholar]
- Whitaker JN. The presence of immunoreactive myelin basic protein peptide in urine of persons with multiple sclerosis. Ann Neurol. 1987 Nov;22(5):648–655. [Abstract] [Google Scholar]
- Whitaker JN, Layton BA, Herman PK, Kachelhofer RD, Burgard S, Bartolucci AA. Correlation of myelin basic protein-like material in cerebrospinal fluid of multiple sclerosis patients with their response to glucocorticoid treatment. Ann Neurol. 1993 Jan;33(1):10–17. [Abstract] [Google Scholar]
- Whitaker JN, Williams PH, Layton BA, McFarland HF, Stone LA, Smith ME, Kachelhofer RD, Bradley EL, Burgard S, Zhao G, et al. Correlation of clinical features and findings on cranial magnetic resonance imaging with urinary myelin basic protein-like material in patients with multiple sclerosis. Ann Neurol. 1994 May;35(5):577–585. [Abstract] [Google Scholar]
Associated Data
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/150/4/1253
Citations & impact
Impact metrics
Citations of article over time
Article citations
Hypoxic oligodendrocyte precursor cell-derived VEGFA is associated with blood-brain barrier impairment.
Acta Neuropathol Commun, 11(1):128, 07 Aug 2023
Cited by: 5 articles | PMID: 37550790 | PMCID: PMC10405482
IFN-Induced Protein with Tetratricopeptide Repeats 2 Limits Autoimmune Inflammation by Regulating Myeloid Cell Activation and Metabolic Activity.
J Immunol, 210(6):721-731, 01 Mar 2023
Cited by: 3 articles | PMID: 36695771
Early microglial response, myelin deterioration and lethality in mice deficient for very long chain ceramide synthesis in oligodendrocytes.
Glia, 71(4):1120-1141, 30 Dec 2022
Cited by: 8 articles | PMID: 36583573 | PMCID: PMC10952316
Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination.
Mol Neurodegener, 17(1):75, 23 Nov 2022
Cited by: 24 articles | PMID: 36419137 | PMCID: PMC9682675
The gut microbiome molecular mimicry piece in the multiple sclerosis puzzle.
Front Immunol, 13:972160, 15 Aug 2022
Cited by: 8 articles | PMID: 36045671 | PMCID: PMC9420973
Review Free full text in Europe PMC
Go to all (53) article citations
Protocols & materials
Related Immune Epitope Information - Immune Epitope Database and Analysis Resource
Ximbio (4)
 https://ximbio.com/reagent/153641/anti-myelin-basic-protein-region-82-87-mbp12
https://ximbio.com/reagent/153641/anti-myelin-basic-protein-region-82-87-mbp12 https://ximbio.com/reagent/153642/anti-myelin-basic-protein-region-36-50-mbp14
https://ximbio.com/reagent/153642/anti-myelin-basic-protein-region-36-50-mbp14 https://ximbio.com/reagent/153639/anti-myelin-basic-protein-region-119-131-mbp2
https://ximbio.com/reagent/153639/anti-myelin-basic-protein-region-119-131-mbp2 https://ximbio.com/reagent/153643/anti-myelin-basic-protein-mbp40
https://ximbio.com/reagent/153643/anti-myelin-basic-protein-mbp40
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Myelin degeneration in multiple system atrophy detected by unique antibodies.
Am J Pathol, 153(3):735-744, 01 Sep 1998
Cited by: 75 articles | PMID: 9736024 | PMCID: PMC1853025
Identification of antibodies in anti-CNS and anti-PNS myelin sera by immunoblot, characterization by immunohistochemistry, and their effect in tissue culture.
Brain Res, 307(1-2):191-200, 01 Jul 1984
Cited by: 10 articles | PMID: 6205724
Sites in myelin basic protein that react with monoclonal antibodies.
J Neurochem, 44(2):637-650, 01 Feb 1985
Cited by: 20 articles | PMID: 2578184
Molecular "negativity" may underlie multiple sclerosis: role of the myelin basic protein family in the pathogenesis of MS.
Int Rev Neurobiol, 79:149-172, 01 Jan 2007
Cited by: 25 articles | PMID: 17531841
Review