Abstract
Free full text

Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications
Abstract
Advancing age is characterized by impairment in the function of the many regulatory processes that provide functional integration between cells and organs. Therefore, there may be a failure to maintain homeostasis under conditions of physiological stress. The reduced homeostatic ability affects different regulatory systems in different subjects, thus explaining at least partly the increased interindividual variability occurring as people get older. Important pharmacokinetic and pharmacodynamic changes occur with advancing age. Pharmacokinetic changes include a reduction in renal and hepatic clearance and an increase in volume of distribution of lipid soluble drugs (hence prolongation of elimination half-life) whereas pharmacodynamic changes involve altered (usually increased) sensitivity to several classes of drugs such as anticoagulants, cardiovascular and psychotropic drugs. This review focuses on the main age-related physiological changes affecting different organ systems and their implications for pharmacokinetics and pharmacodynamics of drugs.
Introduction
Ageing is the progressive accumulation of more or less random changes. This limits the average life expectancy to about 85 years [1], maximum life span to around 122 years [2], and lowers the ability to cope with external stresses. Moreover, the interindividual variability in the physiological responses increases with age. Ageing is not a single entity but a collective term representing the sum of cumulative local effects at the molecular, cellular and tissue level. Ageing is the effect of these underlying changes and not the cause. Although an all-encompassing definition of ageing is not possible, several characteristics are recognized. The most consistent is the time-related loss of functional units. These units are the smallest structures still capable of performing the specific physiological activities characteristic of the organ of which they are a part (e.g. nephrons, alveoli or neurones). A further characteristic is the disruption of some of the regulatory processes that provide functional integration between cells and organs. Consequently, there is a failure to maintain homeostasis under conditions of physiological stress. This loss of functional reserve is associated with a decrease in viability and an increase in vulnerability. Ageing is not solely a progression of functional decline but produces anatomical and physiological changes which might lead to decompensation of the relevant system when they progress beyond a threshold. Some important age-related physiological changes are discussed. This is followed by a detailed description of the age-related changes in pharmacokinetics (drug absorption, distribution, metabolism, and excretion) and pharmacodynamics (the effect of a drug on its target site).
Cardiac structure and function
Ageing produces major cardiovascular changes, including reduced elasticity and compliance of the aorta and great arteries [3]. This results in a higher systolic arterial pressure, increased impedance to left ventricular ejection, and subsequent left ventricular hypertrophy and interstitial fibrosis [4]. A decrease in the rate of myocardial relaxation also occurs. The left ventricle becomes stiffer and takes longer to relax and fill in diastole, thus increasing the importance of a properly timed atrial contraction in contributing to a normal left ventricular end-diastolic volume [5]. The isotonic contraction is prolonged and velocity of shortening reduced.
Ageing is associated with a reduction in the intrinsic heart rate and increased sinoatrial node conduction time [6, 7]. The response to postural changes differs between young and elderly subjects as cardiac output is maintained by increasing heart rate in the young whereas elderly subjects rely on an increase in stroke volume to compensate [8]. During exercise the tachycardic response is reduced. In some subjects, cardiac output is maintained by an increase in stroke volume (relying on the Starling mechanism), in others no compensation occurs and aerobic capacity is reduced [9].
Renal system
Renal mass decreases with age [10]. This reflects the reduction in nephrons [11]. Intra-renal vascular changes also occur, consisting of hyalinization of the vascular tuft leading to reduced blood flow in the afferent arterioles in the cortex [12]. No changes in the medullary vasculature are reported with ageing [13]. Both renal plasma flow and glomerular filtration rate decline with age. The decline is not uniform or consistent, however, [14, 15]. Despite the decline in glomerular filtration rate, there is no concomitant increase in plasma creatinine because of age-related loss of muscle mass. Therefore, creatinine is not a reliable indicator of glomerular filtration rate in the elderly subject. Other markers such as serum cystatin C do not provide significant advantages over creatinine for the measurement of creatinine clearance [16].
Acid-base balance is maintained under physiological conditions but a reduced response to stress is revealed by the inability to deal with acid loads, which may be due to defective renal tubular secretion of ammonium ions [17]. The ability to concentrate the urine during water deprivation is reduced. This is probably due to the inability of the declining number of nephrons to deal with an increased solute load or to the increased perfusion of the juxtamedullary glomeruli producing medullary washout [11, 17]. The response to water loading is also impaired but the mechanisms responsible are unclear. Basal vasopressin secretion is probably normal in elderly subjects. However, both normal and reduced responses to water deprivation have been described [18, 19]. Although the ability to conserve salt is maintained, there is a delay in balancing losses [20]. Changes in salt and water regulation also interact with age-related change in thirst mechanisms. Reduced thirst has been reported in elderly subjects during water deprivation [18], despite considerable rises in plasma osmolality. Possible mechanisms include reduced sensation of mouth dryness and increased activity of the renin-angiotensin-aldosterone axis [18, 21].
Gastrointestinal system
Stomach and duodenum
The main changes involve the secretion of hydrochloric acid and pepsin, which are decreased under basal conditions. This may be the direct consequence of changes in the enzyme secreting cells and organs or hormonal and neural regulatory alterations [22]. By contrast, gastric emptying in elderly subjects is similar to that of young subjects [23].
Small intestine
Advancing age is accompanied by reduced absorption of several substances (e.g. sugar, calcium, iron) while digestion and motility remain relatively unchanged [24, 25].
Colon
The studies investigating the relationship between age and colonic motility have shown conflicting results. In one study, elderly subjects had a slower colonic transit time of radiolabelled plastic particles than young subjects [26]. No significant age-related changes in colonic transit time have been observed in a recent study comparing young and middle-aged subjects [27].
Pancreas
Some uncertainty exists regarding the effects of advancing age upon pancreatic secretion [28]. Of the major enzymes, some (amylase) remain constant whereas others (lipase, trypsin) decrease dramatically [29]. Secretin-stimulated pancreatic juice and bicarbonate concentrations remain unchanged [30].
Liver
Advancing age is associated with a progressive reduction in liver volume and liver blood flow [31]. Alteration of hepatic structure and enzymatic functions with ageing is moderate. In the healthy elderly person, routine tests of liver function involving the metabolism and elimination of specific dyes, radioisotopes, and protein synthesis do not show significant differences between individuals aged 50–69 and 70–89 years [31–33].
Neuroendocrine responses
Ageing is accompanied by changes in neuroendocrine responses to psychosocial or physical stress. In particular, an altered function of the hypothalamic-pituitary-adrenal (HPA) axis has been observed. Excessive HPA activation and hypersecretion of glucocorticoids can lead to dendritic atrophy in neurones in the hippocampus, resulting in learning and memory impairment. Damage or loss of hippocampal neurones results in impaired feedback inhibition of the HPA axis and glucocorticoid secretion, leading to further damage caused by elevated glucocorticoid concentrations. This positive feedback effect on hippocampal neuronal loss is known as the glucocorticoid cascade hypothesis [34]. Thus, glucocorticoids may sensitize hippocampal neurones to cell death and/or functional impairment, indirect effects that are likely to be age-dependent.
Under conditions of chronic stress, there would be insufficient adjustments in HPA axis activity in response to the challenge of sustained glucocorticoid levels. This might be caused by impaired feedback regulation of the HPA axis activity [35].
Body composition
Significant changes in body composition occur with advancing age. There is a progressive reduction in total body water and lean body mass, resulting in a relative increase in body fat [36].
Pharmacokinetic implications
Drug absorption
Although earlier studies reported significant apparently age-related effects including reduced gastric acid secretion [37, 38] and gastric emptying [39], reduced splanchnic blood flow [40], and absorptive capacity of the small intestine [41], probably due to the effects of disease states, more recent reports have not confirmed these findings in healthy subjects [23, 25, 42, 43].
Pharmacokinetic studies on the effect of ageing on drug absorption have provided conflicting results. While some studies have not shown significant age-related differences in absorption rates for different drugs [23, 44–49], the absorption of vitamin B12, iron and calcium through active transport mechanisms is reduced [22, 50] whereas the absorption of levodopa is increased. The latter is probably secondary to a reduced amount of dopadecarboxylase in the gastric mucosa [51]. Some of the discrepancy in the results obtained from these studies might be due to different methods of assessing drug absorption.
First-pass metabolism and bioavailability
Ageing is associated with a reduction in first-pass metabolism. This is probably due to a reduction in liver mass and blood flow [52]. As a result, the bioavailability of drugs undergoing extensive first-pass metabolism such as propranolol and labetalol can be significantly increased [53–55]. On the other hand, several ACE inhibitors such as enalapril and perindopril are pro-drugs and need to be activated in the liver. Therefore, their first-pass activation might be slowed or reduced with advancing age [56, 57].
Drug distribution
As a consequence of the age-related changes in body composition [36], polar drugs that are mainly water-soluble tend to have smaller volumes of distribution (V) resulting in higher serum levels in older people. Gentamicin, digoxin, ethanol, theophylline, and cimetidine fall into this category [58–60]. Loading doses of digoxin need to be reduced to accommodate these changes [60]. On the other hand, nonpolar compounds tend to be lipid-soluble and so their V increases with age. The main effect of the increased V is a prolongation of half-life. Increased V and t1/2 have been observed for drugs such as diazepam, thiopentone, lignocaine, and chlormethiazole [61–66]. The reduction in V for water-soluble drugs tends to be balanced by a reduction in renal clearance (CL) with little net effect on t1/2,z, as shown in the following equation:
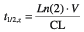
where t1/2,z= elimination half-life, Ln(2) = natural log of 2 (0.693), V = apparent volume of distribution, and CL = clearance.
Protein binding
Acidic compounds (diazepam, phenytoin, warfarin, salicylic acid) bind principally to albumin whereas basic drugs (lignocaine, propranolol) bind to α1-acid glycoprotein. Although no substantial age-related changes in the concentrations of both these proteins have been observed [33, 67], albumin is commonly reduced in malnutrition or acute illness whereas α1-acid glycoprotein is increased during acute illness. However, the importance of such changes remains to be elucidated as the main factor determining drug effect is the free concentration of the drug. Although plasma protein binding might theoretically contribute to drug interactions or physiological effects for drugs that are highly protein-bound, its clinical relevance is probably limited. The reason for this is related to the fact that the initial and transient effect of protein binding on free plasma concentration is rapidly counterbalanced by its effects on clearance [68].
Drug clearance
Kidney
Reduction in renal function in elderly subjects, particularly glomerular filtration rate, affects the clearance of many drugs such as water-soluble antibiotics [69, 70], diuretics [71], digoxin [72], water-soluble β-adrenoceptor blockers [73], lithium [74], and nonsteroidal anti-inflammatory drugs [75, 76]. The clinical importance of such reductions of renal excretion is dependent on the likely toxicity of the drug. Drugs with a narrow therapeutic index like aminoglycoside antibiotics, digoxin, and lithium are likely to have serious adverse effects if they accumulate only marginally more than intended. However, a recent study has questioned the importance of age-related reduction in renal function in affecting pharmacokinetics. Although creatinine clearance was slightly reduced in healthy elderly subjects, excretion of atenolol, hydrochlorothiazide and triamterene was similar to young subjects [77].
Liver
The drug clearance by the liver depends on the capacity of the liver to extract the drug from the blood passing through the organ and the amount of hepatic blood flow, as illustrated by the following formula:
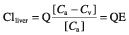
where E = steady-state extraction ratio, Q = liver blood flow (sum of hepatic portal and hepatic arterial blod flow), [Ca] = concentration of drug in portal vein and hepatic artery; [Cv] = concentration of drug leaving the liver in the hepatic vein, and CLliver = clearance by the liver. Therefore, the clearance by the liver depends on both the blood flow and the extraction ratio. The latter is dependent on the metabolizing capacity of the liver.
Drugs can be classified into three groups according to their extraction ratio: high (E > 0.7, such as chlormethiazole, dextropropoxyphene, glyceryl nitrate, lignocaine, pethidine, and propranolol), intermediate (E 0.3–0.7, such as aspirin, codeine, morphine, and triazolam), and low extraction ratio (E < 0.3, such as carbamazepine, diazepam, phenytoin, theophylline, and warfarin). When E is high, CL is rate-limited by perfusion. When E is low, Cv is similar to Ca and changes in blood flow produce little changes in CL. Therefore, the reduction in liver blood flow with ageing will mainly affect the clearance of drugs with a high extraction ratio.
Several studies have shown significant reductions in the clearance of many drugs metabolized by phase-1 pathways in the liver [78, 79]. The main factor is probably represented by the age-related changes in liver size and hepatic blood flow as the activity of drug metabolizing enzymes is preserved [80]. Studies on human liver tissue showed that mono-oxygenase activities are maintained even in advanced old age [81]. These results have been confirmed by in vivo studies using radiolabelled erythromycin breath tests as probes for CYP3A activity [82]. It is unclear whether enzyme responsiveness changes with ageing in man. Some pharmacokinetic studies have reported that factors such as cigarette smoking do not induce drug metabolism in older people to the same extent as in younger people [83]. Other authors reported similar theophylline clearances in old and young smokers [84]. Conflicting reports also exist regarding the inducing effects of various drugs [85, 86]. The evidence regarding enzyme inhibition in ageing is more consistent, most of the human studies showing enzyme inhibition similar to young subjects [87, 88]. Much less effort has been directed into investigating the effects of ageing on conjugative metabolism. In general, studies reported no major effects of ageing in the pathways of conjugation [89–93].
Recently, it has been observed that a reduction in renal function may significantly affect not only renally excreted drugs but also drugs undergoing extensive metabolism in the liver [94–96]. A decrease in liver cytochrome P450 activity, secondary to reduced gene expression, has been observed in renal failure [96]. Therefore, the age-associated reduction in renal function might potentially affect drug metabolism in the liver. Further research is needed to clarify this issue.
Age-related pharmacokinetic changes in specific clinical situations
Congestive heart failure
Studies investigating possible age-related differences in cardiovascular function in patients with congestive heart failure show a progressive decrease in heart rate and an increase in systemic vascular resistance in older patients [97]. This is associated with increased plasma noradrenaline and serum creatinine concentrations [97]. The therapeutic implications of some pharmacokinetic changes involving the main agents used for the treatment of this condition are discussed.
Digoxin
Digoxin is well absorbed in the gastrointestinal tract. However, the time to peak plasma concentrations is prolonged with advancing age from a mean of 38 h in younger subjects to 69 h in elderly subjects [60]. Therefore, the time to reach steady-state plasma concentrations increases from 7 to 12 days in elderly subjects. The volume of distribution is decreased in elderly patients. As a result, loading doses should be reduced by approximately 20%[60].
Because digoxin is cleared mainly through the kidneys and digoxin clearance is proportional to creatinine clearance [98], the systemic clearance of digoxin is reduced with age [60]. As clearance is the main determinant of the maintenance dose, the daily dose of digoxin should be reduced. This should be guided by renal function and body weight.
Diuretics
Several studies investigated the effect of ageing on the pharmacokinetics of frusemide administered intravenously [99, 100]. The volume of distribution was similar in older subjects as compared with younger individuals. This was associated with a reduced renal clearance and a prolonged half-life in elderly subjects [99, 100]. The reduced effects of frusemide with ageing seem to be due mainly to a decrease in tubular secretion. The latter may be caused by a reduction in renal plasma flow.
A slight reduction in the renal clearance of thiazide diuretics and triamterene, alone or in combination, has also been observed with advancing age [101, 102]. The latter findings have been disproved by a recent study [77].
ACE inhibitors
Some of the drugs of this class are active compounds (i.e. lisinopril) but most are pro-drugs undergoing activation in the liver (i.e. enalapril, perindopril). This biotransformation might be impaired in patients with severe heart failure and hepatic congestion. Most of the ACE inhibitors are excreted through the kidney by glomerular filtration and tubular secretion. In the presence of renal impairment their plasma concentration increases [103, 104]. Therefore, the dose needs to be adjusted accordingly, especially when the creatinine clearance is below 30 ml min−1[103, 104]. Some of the newer ACE inhibitors, such as benazepril, fosinopril, spirapril, and zofenopril, are also eliminated by the biliary route thus potentially compensating for the reduced renal clearance in elderly subjects [104].
Pharmacodynamic implications
Some important pharmacodynamic age-related changes are illustrated in Table 1. Since the effect of age on drug sensitivities varies with the drug studied and the response measured, generalizations are often difficult. Studies of drug sensitivity require measurement of concentrations of drug in plasma as differences in pharmacokinetics with increasing age may increase or decrease differences in response to the drug.
Table 1
Selected pharmacodynamic changes with ageing.
| Drug | Pharmacodynamic effect | Age-related change |
|---|---|---|
| Adenosine | Heart-rate response | ↔ |
| Diazepam | Sedation, postural sway | ↑ |
| Diltiazem | Acute and chronic antihypertensive effect | ↑ |
| Acute PR interval prolongation | ↓ | |
| Diphenhydramine | Postural sway | ↔ |
| Enalapril | ACE inhibition | ↔ |
| Furosemide | Peak diuretic response | ↓ |
| Heparin | Anticoagulant effect | ↔ |
| Isoproterenol | Chronotropic effect | ↓ |
| Morphine | Analgesic effect | ↑ |
| Respiratory depression | ↔ | |
| Phenylephrine | α1-adrenergic responsiveness | ↔ |
| Propranolol | Antagonism of chronotropic effects of isoproterenol | ↓ |
| Scopolamine | Cognitive function | ↓ |
| Temazepam | Postural sway | ↑ |
| Verapamil | Acute antihypertensive effect | ↑ |
| Warfarin | Anticoagulant effect | ↑ |
↑ = increase; ↓ = decrease; ↔ = no significant change; ACE = angiotensin-converting enzyme.
Anticoagulants
There is evidence of a greater inhibition of synthesis of vitamin K-dependent clotting factors at similar plasma concentrations of warfarin in elderly compared with young patients. However, the exact mechanisms responsible for the increased sensitivity are unknown [105]. By contrast, the relationship between plasma heparin concentration and anticoagulant effect does not change with increasing age [106].
Cardiovascular and respiratory drugs
Although elderly subjects are less sensitive to the effects of verapamil on cardiac conduction, the effect on blood pressure and heart rate tends to be greater in older than in younger patients [107]. This might be explained by an increased sensitivity to the negative inotropic and vasodilator effect of verapamil as well as diminished baroreceptor sensitivity. The acute intravenous administration of diltiazem causes greater prolongation of the PR interval (dromotropic effect) in young than in elderly subjects [108].
Reduced β-adrenoceptor function is observed with advancing age. Elderly patients are less sensitive to the chronotropic effect of isoprenaline [109]. The impaired response, however, is due primarily to an age-related reduction in the influence of reflex cardiovascular effects on heart rate rather than reduced β-adrenoceptor sensitivity [110]. On the other hand, both salbutamol (β2-adrenoceptor agonist) and propranolol (β-adrenoceptor antagonist) show reduced responses with age. This is secondary to impaired β-receptor function due to reduced synthesis of cyclic AMP following receptor stimulation. The total number of receptors seems to be maintained but the postreceptor events are changed because of alterations of the intracellular environment [109]. The responsiveness of α-adrenoceptors, on the other hand, is preserved with advancing age [111, 112].
Psychotropic drugs
Elderly patients are particularly vulnerable to adverse effects from neuroleptics, including delirium, extrapyramidal symptoms, arrhythmias, and postural hypotension [113, 114]. Advancing age is also associated with increased sensitivity to the central nervous system effects of benzodiazepines [115]. Sedation is induced by diazepam at lower doses and lower plasma concentrations in elderly subjects [116, 117]. Advancing age is also associated with increased sensitivity to the effects of nitrazepam, flurazepam, and loprazolam [118–120]. The exact mechanisms responsible for the increased sensitivity to benzodiazepines with ageing are unknown, however.
Conclusions
The ageing process is characterized by structural and functional changes affecting all organ systems and results in reduced homeostatic capacity. Although the function of a particular system may be maintained during resting conditions, the reduction of functional reserve is responsible for an increased vulnerability to stress. Changes in body composition, hepatic and renal function are responsible for an increase in the volume of distribution of lipid soluble drugs, reduced clearance of lipid soluble and water soluble drugs, respectively. All these changes lead to a prolongation of plasma elimination half-life. Significant pharmacodynamic changes also occur which, in general, tend to increase sensitivity to drugs. The reduced functional reserve itself also leads to an increase in sensitivity by impairing homeostatic compensatory mechanisms. A better understanding of the effects of ageing on the clinical pharmacology of therapeutic agents would enhance the quality of prescribing.
References
Articles from British Journal of Clinical Pharmacology are provided here courtesy of British Pharmacological Society
Full text links
Read article at publisher's site: https://doi.org/10.1046/j.1365-2125.2003.02007.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1884408?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1046/j.1365-2125.2003.02007.x
Article citations
Darolutamide in Japanese patients with metastatic hormone-sensitive prostate cancer: Phase 3 ARASENS subgroup analysis.
Cancer Med, 13(21):e70029, 01 Nov 2024
Cited by: 0 articles | PMID: 39527466 | PMCID: PMC11552649
Patients with dementia: prevalence and type of drug-drug interactions.
Front Pharmacol, 15:1472932, 28 Oct 2024
Cited by: 0 articles | PMID: 39529888 | PMCID: PMC11550964
Randomized controlled trial parallel-group on optimizing community pharmacist's care for the elderly: The influence of WhatsApp-Email delivered clinical case scenarios.
PLoS One, 19(10):e0308448, 22 Oct 2024
Cited by: 0 articles | PMID: 39436887 | PMCID: PMC11495638
Patients' knowledge of the indications for their medications - a scoping review.
BMC Health Serv Res, 24(1):1195, 08 Oct 2024
Cited by: 0 articles | PMID: 39375664 | PMCID: PMC11460199
Review Free full text in Europe PMC
Tacrolimus Dose Requirement in <i>De Novo</i> Adult Kidney Transplant Patients Treated With Adoport<sup>®</sup> Can Be Anticipated.
Transpl Int, 37:13495, 14 Oct 2024
Cited by: 0 articles | PMID: 39469664 | PMCID: PMC11513580
Go to all (747) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Age-related changes in pharmacokinetics.
Curr Drug Metab, 12(7):601-610, 01 Sep 2011
Cited by: 128 articles | PMID: 21495970
Review
Pharmacokinetic-pharmacodynamic crisis in the elderly.
Am J Ther, 14(5):488-498, 01 Sep 2007
Cited by: 59 articles | PMID: 17890940
Review
Pharmacokinetics in older persons.
Am J Geriatr Pharmacother, 2(4):274-302, 01 Dec 2004
Cited by: 119 articles | PMID: 15903286
Review
When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly.
Exp Gerontol, 38(8):843-853, 01 Aug 2003
Cited by: 212 articles | PMID: 12915206
Review





