Abstract
Background
Helicobacter pylori strains possessing the cagA gene are thought to induce interleukin 8 (IL-8) in gastric mucosa. However, it is still unclear whether a relation exists between the cagA gene and the expression patterns of cytokines other than IL-8.Aims
To investigate the relation between the cagA gene and the production of various cytokine proteins using an enzyme linked immunosorbent assay (ELISA).Patients and methods
In 184 patients, the cagA gene was detected by polymerase chain reaction (PCR), and levels of production of IL-1 beta, IL-6, IL-7, IL-8, IL-10, and tumour necrosis factor alpha (TNF-alpha) in antral biopsy specimens were measured by ELISA.Results
Mucosal levels of IL-1 beta, IL-6, IL-8, and TNF-alpha were significantly higher in H pylori positive than in H pylori negative patients. Furthermore, the mucosal levels of IL-1 beta and IL-8 were significantly higher in specimens infected with cagA positive strains than in those infected with cagA negative strains. In H pylori positive patients, the mucosal level of IL-8 was closely correlated with that of IL-1 beta (p < 0.0001), and the mucosal level of IL-6 was closely correlated with that of TNF-alpha (p < 0.0001).Conclusion
These findings suggest that the ability to induce cytokines differs among the strains; cagA+ strains induce various kinds of cytokines and may cause severe inflammation, whereas cagA- strains induce IL-8 and IL-1 beta only weakly and may cause only mild inflammation. However, as most patients infected with the cagA+ strains have gastritis, these strains may not be equivalent to ulcerogenic strains.Free full text

Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains
Abstract
Background—Helicobacter pylori
strains possessing the cagA gene are thought to induce
interleukin 8 (IL-8) in gastric mucosa. However, it is still unclear
whether a relation exists between the cagA gene and the
expression patterns of cytokines other than IL-8.
Aims—To investigate the
relation between the cagA gene and the production
of various cytokine proteins using an enzyme linked immunosorbent
assay (ELISA).
Patients and methods—In 184 patients,
the cagA gene was detected by polymerase chain reaction
(PCR), and levels of production of IL-1β, IL-6, IL-7, IL-8, IL-10,
and tumour necrosis factor α (TNF-α) in antral biopsy specimens
were measured by ELISA.
Results—Mucosal levels of IL-1β, IL-6,
IL-8, and TNF-α were significantly higher in H pylori
positive than in H pylori negative patients.
Furthermore, the mucosal levels of IL-1β and IL-8 were significantly higher in specimens infected with cagA
positive strains than in those infected with cagA
negative strains. In H pylori positive
patients, the mucosal level of IL-8 was closely correlated with
that of IL-1β (p<0.0001), and the mucosal level of IL-6
was closely correlated with that of TNF-α (p<0.0001).
Conclusion—These findings suggest that the
ability to induce cytokines differs among the strains;
cagA+ strains induce various kinds of
cytokines and may cause severe inflammation, whereas
cagA strains induce IL-8 and IL-1β only
weakly and may cause only mild inflammation. However, as most patients
infected with the cagA+ strains have
gastritis, these strains may not be equivalent to ulcerogenic strains.
Keywords: cytokines; Helicobacter pylori; cagA gene; interleukin 8; interleukin 1β
Full Text
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GN. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [Abstract] [Google Scholar]
- Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. [Europe PMC free article] [Abstract] [Google Scholar]
- Crabtree JE, Peichl P, Wyatt JI, Stachl U, Lindley IJ. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993 Jan;37(1):65–70. [Abstract] [Google Scholar]
- Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. [Abstract] [Google Scholar]
- Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995 Dec;30(12):1153–1159. [Abstract] [Google Scholar]
- Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996 Jun;110(6):1744–1752. [Abstract] [Google Scholar]
- Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. [Europe PMC free article] [Abstract] [Google Scholar]
- Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. [Europe PMC free article] [Abstract] [Google Scholar]
- Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. [Abstract] [Google Scholar]
- Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. [Europe PMC free article] [Abstract] [Google Scholar]
- Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJ, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995 Jan;48(1):41–45. [Europe PMC free article] [Abstract] [Google Scholar]
- Sharma SA, Tummuru MK, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995 May;63(5):1681–1687. [Europe PMC free article] [Abstract] [Google Scholar]
- Tummuru MK, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. [Abstract] [Google Scholar]
- Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991 May-Jun;6(3):209–222. [Abstract] [Google Scholar]
- Hoshina S, Kahn SM, Jiang W, Green PH, Neu HC, Chin N, Morotomi M, LoGerfo P, Weinstein IB. Direct detection and amplification of Helicobacter pylori ribosomal 16S gene segments from gastric endoscopic biopsies. Diagn Microbiol Infect Dis. 1990 Nov-Dec;13(6):473–479. [Abstract] [Google Scholar]
- Moss SF, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994 Nov;35(11):1567–1570. [Europe PMC free article] [Abstract] [Google Scholar]
- Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995 Feb;48(2):133–136. [Europe PMC free article] [Abstract] [Google Scholar]
- Peek RM, Jr, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995 Dec;73(6):760–770. [Abstract] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. [Abstract] [Google Scholar]
- Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. [Abstract] [Google Scholar]
- Nakamura H, Yoshimura K, McElvaney NG, Crystal RG. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992 May;89(5):1478–1484. [Europe PMC free article] [Abstract] [Google Scholar]
- Suzuki M, Miura S, Suematsu M, Fukumura D, Kurose I, Suzuki H, Kai A, Kudoh Y, Ohashi M, Tsuchiya M. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol. 1992 Nov;263(5 Pt 1):G719–G725. [Abstract] [Google Scholar]
- Weel JF, van der Hulst RW, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat GN, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. [Abstract] [Google Scholar]
- Huang J, O'Toole PW, Doig P, Trust TJ. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995 May;63(5):1732–1738. [Europe PMC free article] [Abstract] [Google Scholar]
- Gionchetti P, Vaira D, Campieri M, Holton J, Menegatti M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Miglioli M, et al. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol. 1994 Jun;89(6):883–887. [Abstract] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. [Europe PMC free article] [Abstract] [Google Scholar]
- Alderson MR, Tough TW, Ziegler SF, Grabstein KH. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991 Apr 1;173(4):923–930. [Europe PMC free article] [Abstract] [Google Scholar]
Figures and Tables
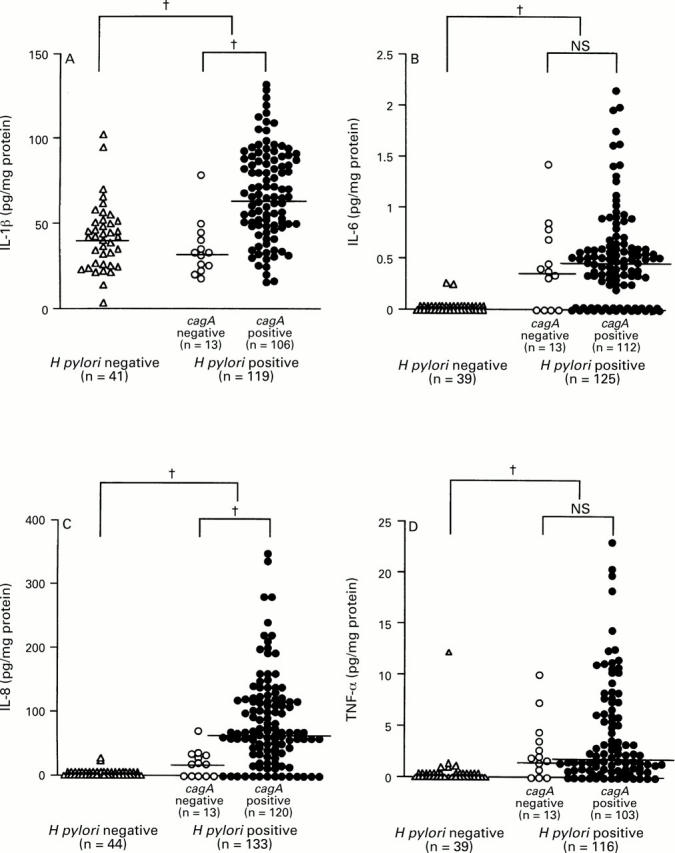
: Production of (A) IL-1β, (B) IL-6, (C)
IL-8, and (D) TNF-α and H pylori infection. Large brackets indicate
the comparison between H pylori positive and negative specimens and
small brackets the comparison between cagA+ and
cagA- specimens. Bars indicate median values for each
group. †p<0.0001 by Mann-Whitney U test.
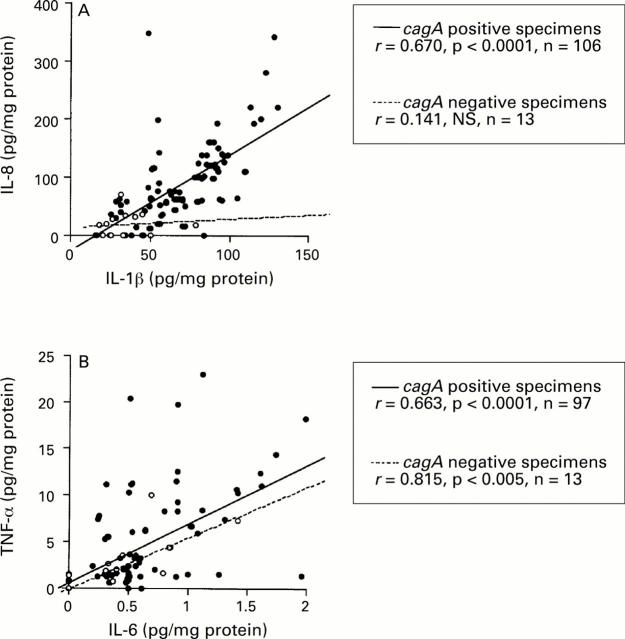
: Correlation between (A) IL-1β and IL-8, and (B)
IL-6, and TNF-α production. Filled circles, cagA+
specimens; open circles, cagA specimens. Correlation
coefficients were calculated with the Spearman rank
test.
: Relation between the production of (A)
IL-1β, (B) IL-6, (C) IL-8, and (D) TNF-α and MNC infiltration in
patients with H pylori infection. Filled circles, cagA+
specimens; open circles, cagA specimens. Correlation
coefficients were calculated with the Spearman rank test.
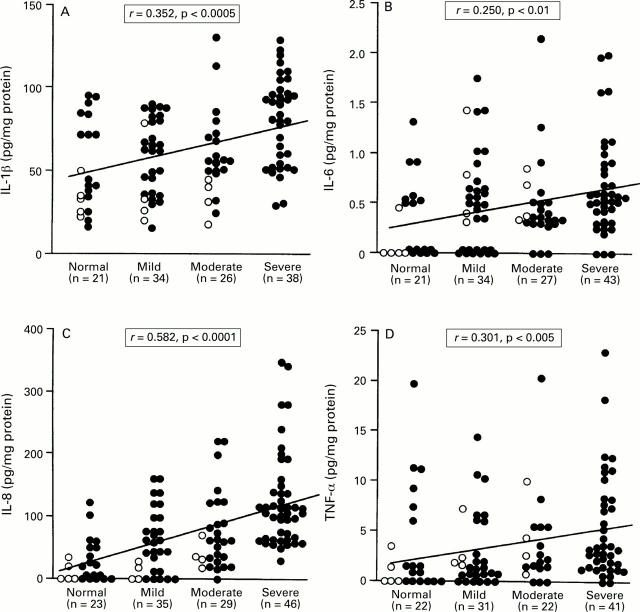
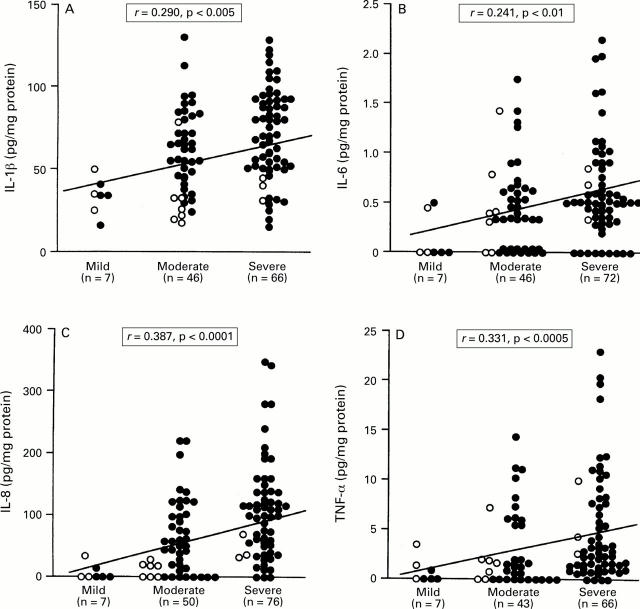
: Relation between the production of (A)
IL-1β, (B) IL-6, (C) IL-8, and (D) TNF-α and PMN infiltration in
patients with H pylori infection. Filled circles, cagA+
specimens; open circles, cagA specimens. Correlation
coefficients were calculated with the Spearman rank test.
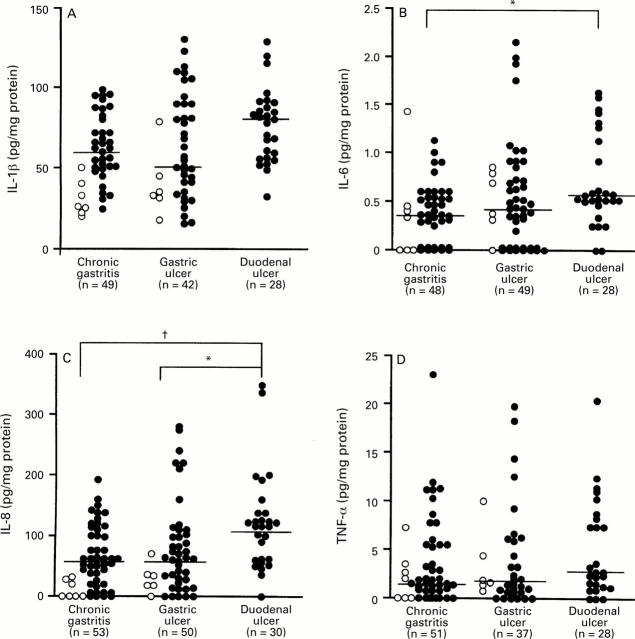
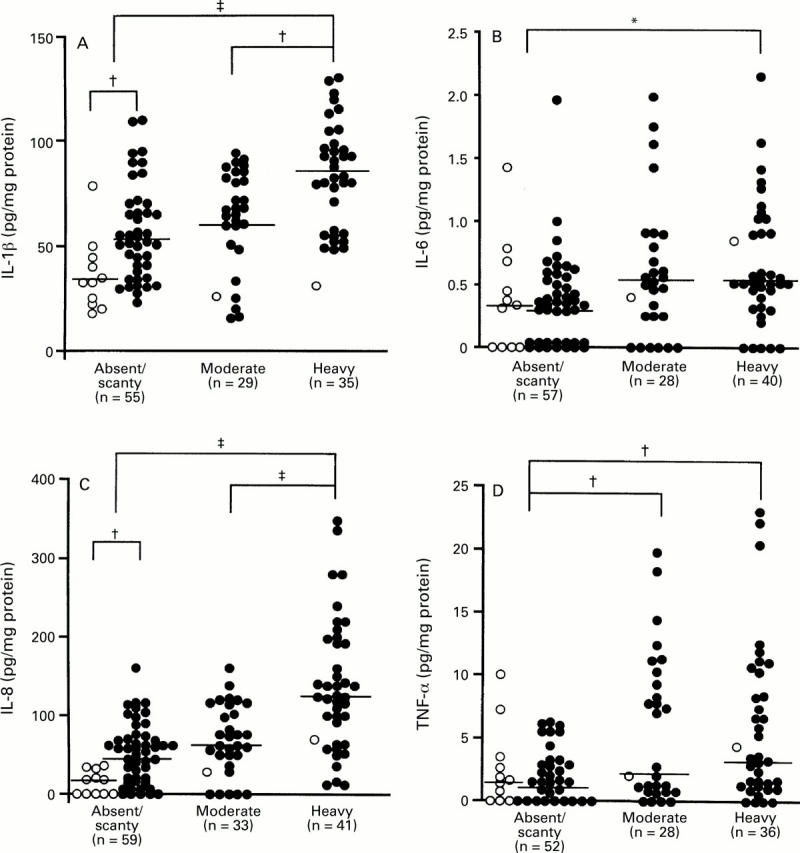
: Production of (A) IL-1β, (B) IL-6, (C)
IL-8, and (D) TNF-α and endoscopic findings in patients with H pylori
infection. Filled circles, cagA+ specimens; open circles,
cagA specimens. Bars indicate median values for each
group. *p<0.05; †p<0.005 by Mann-Whitney U test.
Articles from Gut are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/gut.41.4.442
Read article for free, from open access legal sources, via Unpaywall:
https://gut.bmj.com/content/gutjnl/41/4/442.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/126009592
Article citations
Triggers for the onset and recurrence of psoriasis: a review and update.
Cell Commun Signal, 22(1):108, 12 Feb 2024
Cited by: 7 articles | PMID: 38347543 | PMCID: PMC10860266
Review Free full text in Europe PMC
Inflammatory microenvironment in gastric premalignant lesions: implication and application.
Front Immunol, 14:1297101, 15 Nov 2023
Cited by: 5 articles | PMID: 38035066 | PMCID: PMC10684945
Review Free full text in Europe PMC
Inflammation and Digestive Cancer.
Int J Mol Sci, 24(17):13503, 31 Aug 2023
Cited by: 6 articles | PMID: 37686307 | PMCID: PMC10487643
Review Free full text in Europe PMC
The role of gastric microecological dysbiosis in gastric carcinogenesis.
Front Microbiol, 14:1218395, 31 Jul 2023
Cited by: 4 articles | PMID: 37583514 | PMCID: PMC10423824
Review Free full text in Europe PMC
Current Worldwide Trends in Pediatric Helicobacter pylori Antimicrobial Resistance.
Children (Basel), 10(2):403, 18 Feb 2023
Cited by: 5 articles | PMID: 36832532 | PMCID: PMC9954810
Review Free full text in Europe PMC
Go to all (244) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of the CagA gene positive Helicobacter pylori and tissue levels of interleukin-17 and interleukin-8 in gastric ulcer patients.
Egypt J Immunol, 19(1):51-62, 01 Jan 2012
Cited by: 4 articles | PMID: 23888551
Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2.
Int J Clin Lab Res, 26(3):207-210, 01 Jan 1996
Cited by: 64 articles | PMID: 8905454
Relationship between mucosal levels of Helicobacter pylori-specific IgA, interleukin-8 and gastric inflammation.
Clin Sci (Lond), 96(4):409-414, 01 Apr 1999
Cited by: 8 articles | PMID: 10087249
Helicobacter pylori infection and gastric carcinoma: Not all the strains and patients are alike.
World J Gastrointest Oncol, 8(1):40-54, 01 Jan 2016
Cited by: 20 articles | PMID: 26798436 | PMCID: PMC4714145
Review Free full text in Europe PMC