Abstract
Free full text

First report of donor cell–derived acute leukemia as a complication of umbilical cord blood transplantation
Abstract
Donor cell leukemia is a rare complication after allogeneic hematopoietic stem cell transplantation. A 12-month-old boy underwent unrelated donor umbilical cord blood transplant (UCBT) for refractory Langerhan's cell histiocytosis. Forty months after transplantation, he developed acute myeloid leukemia. Cytogenetic and molecular analysis confirmed donor cell origin. The Cord Blood Bank (CBB) contacted the donor's family and established that the child, now 7 years old, was healthy. This represents the first reported case of donor cell leukemia following UCBT. This case illustrates that donor cell leukemia is a rare but real event after UCBT as with other stem cell sources and highlights the need for CBBs to maintain linkage data between donors and recipients.
Introduction
Donor cell leukemia (DCL) is a rare complication following allogeneic hematopoietic stem cell transplantation, with approximately 25 cases previously reported.1-7 Umbilical cord blood has emerged as an alternative source of hematopoietic stem cells over the past decade, with more than 6000 umbilical cord blood transplants (UCBTs) performed worldwide.8 We report the first case of DCL following UCBT.
The etiology of DCL is unclear, and the reported literature does not suggest a common mechanism. Mechanisms proposed include occult leukemia in the donor, impaired immune surveillance, chemotherapy- or radiation-induced stromal abnormalities, inherent stromal abnormalities, transformation of donor cells by antigenic stimulation through host tissue, and fusion of donor cells with residual leukemic cells, resulting in oncogene transfection.1 The possibility of occult leukemia in the donor raises the question as to whether notification and investigation are warranted.
Patient and methods
This is a single case report with retrospective analysis of clinical and laboratory data. The Institutional Review Board of the University of Minnesota reviewed the protocols and informed consent documents relevant to the individual patient.
Case report
A 12-month-old male presented with a 2-month history of recurrent epistaxes, weight loss, and a 2-week history of fever. Examination demonstrated hepatosplenomegaly, cervical lymphadenopathy, and a petechial rash. Initial investigations revealed anemia, thrombocytopenia, and hypoalbuminemia. A bone marrow biopsy demonstrated histiocytic infiltration with prominent hemophagocytosis. After lymph node biopsy demonstrated histiocytic infiltration and immunohistochemical staining was positive for CD1a, a diagnosis of Langerhans cell histiocytosis was made. Cytogenetic analyses of the bone marrow and lymph node specimens revealed normal 46, XY male karyotypes with no evidence of a clonal chromosomal abnormality. There was no response to therapy with steroids, vinblastine and etoposide, or salvage therapy with 2-chlorodeoxyadenosine. Five months after presentation, a 2-antigen mismatched male donor UCBT was performed with a preparative regimen of busulfan, cyclophosphamide, etoposide, and antithymocyte globulin (ATG). Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine and short-course steroids.9 The total nucleated cell dose was 5.6 × 107/kg. Neutrophil engraftment occurred at day +43 although intermittent granulocyte colony-stimulating factor (G-CSF) therapy was required for 9 months. Infectious complications included multiple episodes of bacteremia, parainfluenza III and adenoviral pneumonia, and cytomegalovirus (CMV) pneumonitis and retinitis, which necessitated 18 months of ganciclovir and foscarnet. Cyclosporine was discontinued 7 months after transplantation with no evidence of GVHD. Molecular studies consistently demonstrated 100% donor engraftment on serial bone marrow examinations from day +21. The histiocytic infiltrate in the bone marrow resolved slowly over the first 12 months. Eighteen months following transplantation, the patient was clinically well and had normal blood counts, a normal bone marrow examination, and normal lymphocyte responses to a panel of mitogens. Cytogenetic analysis at that time revealed a normal 46, XY male karyotype in each of 20 metaphase cells examined. G-band polymorphisms were consistent with these cells being of donor origin. Specifically, in previous studies of the recipient's cells, both 22 chromosomes had short arms that were of similar size with short stalk (band 22p12) regions (Figure 1, study no. 1). In contrast, in donor cells, the stalk region of one chromosome 22 was notably larger than the other and had more prominent terminal satellites (Figure 1, study no. 2). This polymorphic difference could be reliably evaluated in each of the metaphase cells examined.
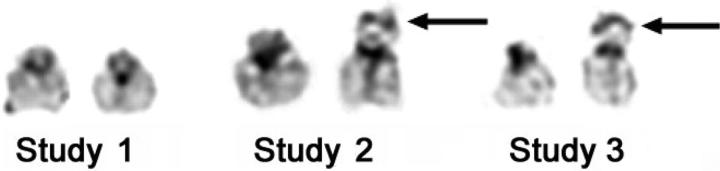
Cytogenetic studies demonstrating donor origin of leukemic cells. Composites of the 22 chromosomes from pretransplantation (study no. 1), 6 months after transplantation, when molecular studies demonstrated 100% donor chimerism (study no. 2), and following diagnosis of AML (study no. 3), illustrating informative G-band polymorphism. One donor chromosome 22 has an enlarged short arm and satellite region, designated by the arrow. This polymorphism is seen in studies 2 and 3, suggesting donor origin of the leukemic cells. G-band polymorphisms represent regions that differ between individuals but have no clinical significance. They are most commonly seen on acrocentric chromosomes such as chromosome 22.
Forty months after UCBT, the patient became thrombocytopenic (38 × 109/L), and bone marrow examination revealed 25% blasts with morphologic features of acute myeloid leukemia (AML) with multilineage dysplasia. Immunophenotyping revealed a distinct population within the blast gate (weak CD45 expression) comprising 20% of nucleated cells. This gated population showed moderate to strong expression of CD34, HLA-DR, CD117, CD38, CD13, and CD33, consistent with neoplastic myeloblasts. Cytogenetic and molecular studies were undertaken to determine the cell of origin and for evaluation of a clonal chromosomal abnormality (Figures (Figures1,1, ,2,2, ,3).3). The patient failed to achieve a remission following attempted treatment with chemotherapy and a phase I agent and died from infectious complications 10 months after the diagnosis of leukemia.
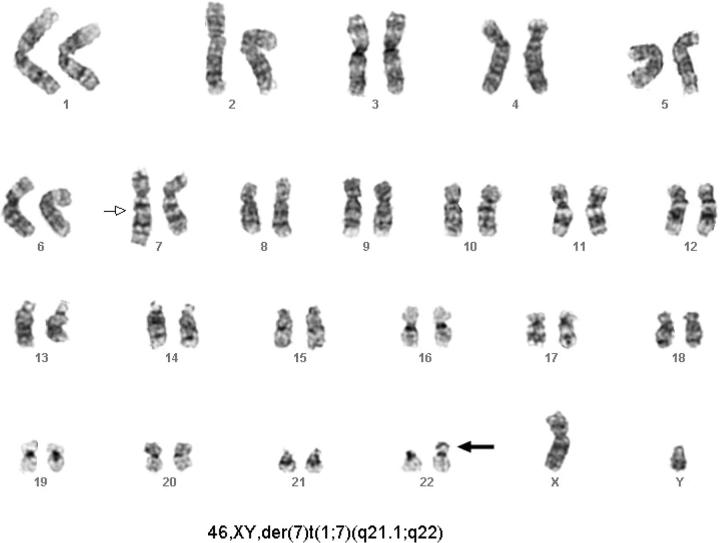
Cytogenetic studies demonstrating clonal abnormality. Studies performed after transplantation at diagnosis of AML revealed a clone characterized by a derivative chromosome 7 (marked by arrow). The chromosome 22 polymorphisms are consistent with donor origin of these cells (marked by bold arrow).
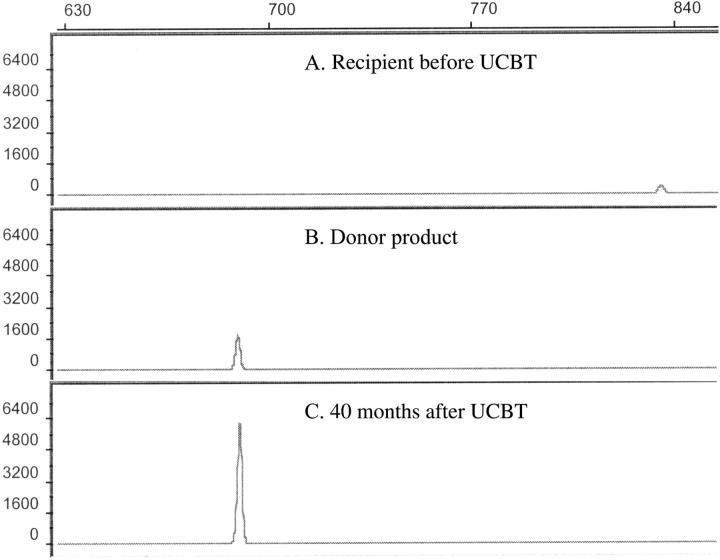
Assessment of donor chimerism. Electrophoretic profiles of informative VNTR PCR products of the recipient before transplantation (allele size 836 bp) (A), the donor product before transplantation (allele size 690 bp) (B), and the recipient following the diagnosis of leukemia demonstrating 100% donor cells (C). The donor and recipient were both homozygous for the respective alleles.
Cytogenetics
Cytogenetic evaluation involved G-banded analysis of 20 metaphase cells from unstimulated, cultured (24 hours) bone marrow aspirates. All numerical and structural chromosomal abnormalities were characterized according to the International System for Human Cytogenetic Nomenclature, 1995.10
Molecular diagnostics
Genomic DNA was extracted from the bone marrow sample and amplified by polymerase chain reaction (PCR) using a series of fluorescently labeled oligonucleotide primers specific for highly polymorphic genetic markers (VNTR) according to standard methods.11 Pretransplantation samples from the donor and recipient had been analyzed previously for informative markers. The resulting products were analyzed on a Model 3100/Genescan system (Applied Biosystems, Weiterstadt, Germany) from which the pretransplantation and posttransplantion specimens were compared.
Results
Of 20 metaphase cells examined, 19 comprised a clone characterized by a derivative chromosome 7, with its distal long arm at band 7q21.1 replaced by an extra copy of most of the long arm of a chromosome 1. Thus, this abnormality, designated as 46, XY, der(7)t(1;7)(q21.1;q22), results in monosomy for the region extending from 7q21.1 to the 7q telomere, and trisomy for the 1q region extending from 1q22 to the 1q telomere (Figure 2). Cytogenetic analysis of the patient's karyotype before and after UCBT demonstrated an informative G-band polymorphism on the short arm of chromosome 22. The polymorphism of chromosome 22 showed the posttransplantation cells to be of donor origin, including the leukemic cells that harbored the derivative chromosome 7 (Figures (Figures1,1, ,2).2). Molecular analysis demonstrated 100% donor cells (Figure 3).
Discussion
Cooley et al1 reviewed 18 published cases of DCL. Subsequent to that report, we identified 6 further reported cases.2-7 Analysis of these cases does not suggest a common mechanism, and we can only speculate as to the etiology of this rare complication. Of 24 cases, 21 occurred following transplantation for leukemia, but there have been cases reported after transplantation for nonmalignant conditions.1,6 Deletion of 7q and gain of 1q, as seen in this case, are well-documented recurring abnormalities in both de novo and therapy-associated myelodysplastic syndrome (MDS) and AML (t-MDS/AML).12 Of the 24 cases of DCL previously described, 3 developed AML after antecedent MDS.1,4,7 Our patient had received only 600 mg/m2 of etoposide and 200 mg/kg of cyclophosphamide, much less than doses usually associated with t-MDS/AML. The fact that the risk of t-MDS/AML is higher after autologous transplantation than after conventional chemotherapy and radiation therapy13 suggests that the transplantation process itself may potentiate t-MDS/AML, although the mechanism remains unclear. Furthermore, in contrast to the autologous setting, t-MDS/AML is very rare following allogeneic transplantation.14,15 Indeed, if chemotherapy- or radiation-induced stromal changes were important in the development of DCL, one would expect a higher frequency than that observed following allogeneic transplantation. It is of interest that patients with Langerhans cell histiocytosis (LCH) have a higher risk of malignancy beyond that expected because of treatment with epipodophyllotoxins and that LCH has been associated with myelodysplasia.16,17 This raises the possibility that particular hosts may have an intrinsic predisposition to the development of leukemia mediated by factors such as the marrow microenvironment or cytokine profiles that are not ameliorated by hematopoietic stem cell transplantation.
We were unable to identify any reported cases of a donor developing leukemia following the development of DCL. However, studies identifying clonotypic gene fusion sequences in the neonatal blood spots of children who subsequently developed leukemias characterized by MLL-AF4, TEL-AML1, and AML1-ETO translocations highlight the fact that transmission of leukemic or preleukemic cells from a neonatal donor could occur.18-20 Several of these children were older than 10 years at the time of leukemia diagnosis. This protracted postnatal latency would suggest that additional events or exposures are required for the development of leukemia. A follow-up study that demonstrated that the TEL-AML1 translocation was detectable at frequencies consistent with clonal expansion in 6 of 567 cord blood samples screened is consistent with this hypothesis21 but also suggests that preleukemic cells may be infused more commonly than expected during UCBT. It has already been suggested that these data argue against the use of stored cord blood in an autologous setting for leukemia patients.21
The possibility of an occult leukemia in the donor raises questions regarding the ethical responsibilities of the CBB to the donor. In the case of adult donors, the National Marrow Donor Program inquires as to their health if they are notified of a case of DCL. No specific policy exists in the case of UCBT. In this case, the parents of the donor, who is now 7 years old, were contacted and asked whether their child had any health concerns and specifically whether the child had been diagnosed with any blood disorders. The child remained well. However, such inquiries could create anxiety for the donor family and may not always be possible, given the difficulties in maintaining current contact information. No stored sample of the cord blood unit was available to test for the presence of the cytogenetic clone. A decision was made not to request a blood sample from the child, because of the lack of evidence of occult leukemia as a cause of this rare complication, the absence of any prophylactic treatment or validated screening strategy, and a desire to not create undue anxiety.
This case serves as a reminder that there are a number of ethical issues that are unique to UCBT. The recently released Institute of Medicine report on establishing a National Cord Blood Stem Cell Bank Program dedicates a chapter to these issues and highlights the fact that there is no uniformity in practices across different CBBs and transplant centers.22 The informed consent process is central to ensuring that donors understand issues regarding potential outcomes of their donation, including the possibility that screening or additional testing could reveal unanticipated information about the mother's or her child's health that may otherwise have gone unnoticed. The most commonly cited examples include infectious or metabolic diseases, but as this case illustrates, there are other possibilities, including malignancy. While in some situations this information may be beneficial, in others it has the potential to result in emotional, social, and financial hardship. A recent study demonstrated that many donors do not fully comprehend the medical, legal, and ethical issues despite having granted “informed consent.”23 These ethical considerations are further complicated by the fact that, by necessity, consent to voluntary donation is granted by the parents on behalf of one of the major stakeholders, the neonatal donor. In the United States and Europe, cord blood banks are required to maintain linkage data between each unit and the demographic details of the donors. This practice has been debated in terms of potential benefit to the recipient, as it could facilitate removal of unsuitable units prior to UCBT but may cause potential harm to the donor, as it may compromise donor privacy and raises the possibility of requests for second donations.22,24 This case illustrates that scenarios may arise where maintenance of linkage could potentially benefit the donor.
A further related question is whether there is a need for active prospective follow-up of donors. Currently, policies regarding this differ between CBBs, and such programs, which require significant resources, are not mandated by regulatory authorities. However, unrelated UCBT does not allow clinical or laboratory assessment of the donor immediately prior to transplantation. A program of active prospective follow-up at 6 months instituted by the Milan CBB identified 5 of 2315 (0.2%) cords that needed to be discarded on the basis of postnatal history, although there were no cases of malignancy.25 CBBs rely upon the donor's parents to contact the bank should their child develop a medical condition that would make the unit unsuitable for transplantation. However, in one study, almost 25% of women who had provided informed consent for cord blood donation did not know how to contact the CBB.23
This first report of DCL after UCBT demonstrates that DCL is a risk following allogeneic transplantation irrespective of the graft source. Given the lack of evidence of occult leukemia in the donor as a cause of this rare complication and the absence of prophylactic treatment, we would argue that donor notification and investigation is not warranted. However, this case serves as a reminder of the ethical dilemmas that will undoubtedly be faced as the number of UCBTs performed increases and of the need for standardization of regulations governing CBBs.
Notes
Supported in part by grants from the National Cancer Institute (PO1-CA65493) (J.E.W.) and the Children's Cancer Research Fund (C.J.F., J.P.N., K.S.B., J.E.W.).
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-06-2551.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2005-06-2551
Read article for free, from open access legal sources, via Unpaywall:
https://ashpublications.org/blood/article-pdf/106/13/4377/465118/zh802405004377.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Outcomes of subsequent neoplasms after umbilical cord blood transplantation in Europe.
Blood Adv, 7(10):1976-1986, 01 May 2023
Cited by: 0 articles | PMID: 36350759 | PMCID: PMC10189403
Donor-Derived Leukemia in a Recipient of Double-Unit Cord Blood Transplantation for Acute Myeloid Leukemia: A Case Study and Literature Review.
Oncol Ther, 10(1):75-84, 07 Feb 2022
Cited by: 0 articles | PMID: 35129793 | PMCID: PMC9098757
Review Free full text in Europe PMC
Genetics of donor cell leukemia in acute myelogenous leukemia and myelodysplastic syndrome.
Bone Marrow Transplant, 56(7):1535-1549, 08 Mar 2021
Cited by: 14 articles | PMID: 33686252
Review
Multiple donor-derived leukemias in a recipient of allogeneic hematopoietic cell transplantation for myeloid malignancy.
Blood Adv, 4(19):4798-4801, 01 Oct 2020
Cited by: 7 articles | PMID: 33022063 | PMCID: PMC7556138
Jumping translocations of 1q in donor cell-derived myelodysplastic syndrome after cord blood transplantation: Case report and review of the literature.
Mol Clin Oncol, 12(4):365-373, 06 Feb 2020
Cited by: 0 articles | PMID: 32190321 | PMCID: PMC7058023
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mutation of the NPM1 gene contributes to the development of donor cell-derived acute myeloid leukemia after unrelated cord blood transplantation for acute lymphoblastic leukemia.
Hum Pathol, 44(8):1696-1699, 01 Mar 2013
Cited by: 10 articles | PMID: 23465275
Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high-risk acute lymphoblastic leukemia.
Int J Cancer, 139(9):2106-2115, 15 Jul 2016
Cited by: 26 articles | PMID: 27356906
Unrelated donor umbilical cord blood transplant versus unrelated hematopoietic stem cell transplant in patients with acute leukemia: A meta-analysis and systematic review.
Blood Rev, 32(3):192-202, 15 Nov 2017
Cited by: 1 article | PMID: 29174416
Review
Cord blood transplantation for acute leukemia.
Expert Opin Biol Ther, 20(10):1223-1236, 22 Jun 2020
Cited by: 8 articles | PMID: 32529854
Review




