Abstract
Free full text

Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors
Abstract
We have developed a useful surrogate assay for monitoring the efficacy of FLT3 inhibition in patients treated with oral FLT3 inhibitors. The plasma inhibitory activity (PIA) for FLT3 correlates with clinical activity in patients treated with CEP-701 and PKC412. Using the PIA assay, along with in vitro phosphorylation and cytotoxicity assays in leukemia cells, we compared PKC412 and its metabolite, CGP52421, with CEP-701. While both drugs could effectively inhibit FLT3 in vitro, CEP-701 was more cytotoxic to primary samples at comparable levels of FLT3 inhibition. PKC412 appears to be more selective than CEP-701 and therefore less effective at inducing cytotoxicity in primary acute myeloid leukemia (AML) samples in vitro. However, the PKC412 metabolite CGP52421 is less selective than its parent compound, PKC412, and is more cytotoxic against primary blast samples at comparable levels of FLT3 inhibition. The plasma inhibitory activity assay represents a useful correlative tool in the development of small-molecule inhibitors. Our application of this assay has revealed that the metabolite CGP52421 may contribute a significant portion of the antileukemia activity observed in patients receiving oral PKC412. Additionally, our results suggest that nonselectivity may constitute an important component of the cytotoxic effect of FLT3 inhibitors in FLT3-mutant AML.
Introduction
FMS-like tyrosine kinase-3 (FLT3) is a receptor tyrosine kinase expressed on the blasts in most cases of acute myeloid leukemia (AML).1 Activating mutations of this receptor, consisting of internal tandem duplications within the juxtamembrane domain (FLT3/ITD) and point mutations within the kinase domain, are found in roughly 30% of de novo AML patients. The FLT3/ITD mutations in particular are associated with an increased relapse rate and a reduced survival and, in light of this, several different small-molecule FLT3 inhibitors are in clinical development.2 Two indolocarbazole derivatives, CEP-701 and PKC412, have shown modest clinical activity as single agents and are currently being tested in combination with chemotherapy in patients with AML who harbor FLT3 mutations.3-6
FLT3 inhibitors are being developed based on the hypothesis that effective, sustained inhibition of FLT3 signaling will be of clinical benefit to a subset of AML patients.7 Kinases in general appear to represent valid therapeutic targets in a wide variety of human malignancies, as demonstrated by the clinical successes of imatinib mesylate and other small-molecule kinase inhibitors.8-11 However, a consistent obstacle encountered in the clinical development of kinase inhibitors, including FLT3 inhibitors, is the absence of a reliable means to confirm that the kinase being targeted has been inhibited in vivo. In the case of solid tumors, the target tissue is often difficult to access, although suitable surrogate tissue can occasionally be used (eg, skin biopsies for epidermal growth factor receptor inhibitors12). In hematologic malignancies such as AML, the tumor tissue is generally more accessible, but the measurement of kinase inhibition in leukemia cells is still problematic in patients with low leukemia cell counts and/or large fractions of normal cells in the peripheral blood. Measurement of plasma drug levels in patients treated with both PKC412 and CEP-701 is often unreliable because the “free” drug level—that which is necessary for biologic activity—is greatly influenced by plasma protein binding, which can vary from patient to patient.13,14
To address this problem in our efforts to incorporate a small-molecule FLT3 inhibitor into leukemia therapy, we have developed a useful surrogate assay for the determination of FLT3 inhibition in patients receiving oral FLT3 inhibitors. By determining the plasma inhibitory activity (PIA) for FLT3 in patients receiving FLT3 inhibitors, we are able to monitor the efficacy of target inhibition for any trial patient at any point during treatment. We show here that the measurement of PIA for FLT3 correlates reliably with clinical response to CEP-701 and PKC412 and provides additional insight into the cytotoxic mechanism of these compounds.
Patients, materials, and methods
Inhibitors
CEP-701 was provided by Cephalon (West Chester, PA). PKC412 and CGP52421 were provided by Novartis (Basel, Switzerland) Compounds were dissolved in DMSO and stored at -80°C as 10 mM stock solutions. Working stocks of 4 to 100 μM were prepared by diluting DMSO stock solutions into RPMI/0.05% BSA. All samples in any given experiment contained identical concentrations of DMSO. Plasma experiments typically contained 0.5%, while all others contained less than 0.01%.
Patient samples
Bone marrow and peripheral blood samples from leukemia patients and healthy donors were obtained through an institutional review board-approved protocol from patients treated on Novartis clinical trial CPKC4122104, a phase 1/2 study of relapsed/refractory FLT3-mutated AML patients treated with PKC412 at a dose of 75 mg 3 times per day continuously, and from Cephalon clinical trial 202, a phase 1/2, open-label trial of single-agent CEP-701 in patients with refractory, relapsed, or poor-risk AML expressing FLT3-activating mutations.3,4 Leukemia cell specimens were provided by the Sidney Kimmel Cancer Center at Johns Hopkins Tumor and Cell Procurement Bank. All patients gave informed consent according to the Declaration of Helsinki. Plasma for the PIA was derived from samples otherwise collected and stored for pharmacokinetic samplings.
For both trials, patients were eligible if they (1) had refractory, relapsed, or poor-risk AML; (2) were over the age of 18 years; (3) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; (4) had recovered from the side effects of previous therapies and were at least 4 weeks off previous treatments; (5) had adequate renal function and hepatic parameters; and (6) their leukemia tested positive for an activating mutation of FLT3. For the CEP-701 trial, patients were treated with 40 mg (then subsequently 60 mg) twice daily in 28-day cycles. Patient response assessments were planned based on the completion of 2 28-day treatment cycles. Patients with evidence of clinical activity (complete remission [CR] or hematologic response [HR]) were continued on monthly cycles of CEP-701 until disease progression or evidence of dose-limiting toxicity. For the PKC412 trial, patients were treated with 75 mg 3 times daily until toxicity or progression occurred, with follow-up marrow examinations every 28 days while on therapy. For both trials, responses were determined after the first 28-day treatment period and monthly thereafter. CR was defined as a cellular bone marrow with 5% or fewer leukemic blasts and a normalization of peripheral blood cell counts (more than 109/L granulocytes and more than 100 × 109/L platelets). HR was defined as a more than 50% reduction in the absolute number of peripheral blood blasts (determined by routine peripheral blood differential) or at least a 50% reduction in the percentage of bone marrow blasts.
In the PKC412 trial, whole blood was collected at designated intervals during therapy: before treatment; days 2, 3, 8, and 29; and monthly thereafter, if applicable. In the CEP-701 trial, samples were collected using a similar schedule: before treatment and days 2, 8, and 29. All samples studied here were trough specimens (taken 12 hours after the most recent dose of drug) and were drawn into vacuum tubes containing heparin and delivered to a central location on ice packs (but not frozen). Whole blood samples occasionally were processed as many as 3 days after collection but more commonly within 24 hours. Whole blood samples were centrifuged at 500g in a clinical centrifuge, and the plasma layer was carefully removed, aliquotted, and stored at -80°C. Mononuclear cells were isolated from whole blood or marrow using density gradient centrifugation with Ficoll-Hypaque (Amersham, Piscataway, NJ). When frozen blasts samples were used, the sample was thawed rapidly, incubated in culture medium overnight, and then subjected to another round of density centrifugation (with added DNAse obtained from Amersham) to eliminate cells that had undergone apoptosis from the freeze-thaw cycle. Plasma samples and corresponding drug levels of patients enrolled on the PKC412 trial were supplied by Novartis. Also available were samples and pharmacokinetic (pK) data from Cephalon clinical trial 202.3 Determination of plasma drug levels and FLT3 mutation status was as described.3,4
Cell lines and culture methods
All cell lines and primary blast samples were cultured in RPMI/10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Ba/F3, TF-1, MV4-11, Molm-14, SEMK2, HB119, and 697 cells were obtained from American Type Culture Collection ([ATCC] Manassas, VA) or the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany). BaF3/ITD and TF/ITD cell lines were derived by transfecting Ba/F3 and TF-1 cells (growth factor dependent) with an expression vector containing the FLT3 coding sequence containing an ITD mutation from an AML patient, as described. The resultant BaF3/ITD and TF/ITD lines are growth factor independent and express constitutively phosphorylated FLT3. The IL-3 rescue assay was performed as described.15 IL-3 was from Peprotech (Rocky Hill, NJ).
Cytotoxicity assays
Cytotoxicity was assessed using a dimethyl-thiazol diphenyl tetrazolium bromide (MTT) assay. In selected cases, we also used an annexin V-binding apoptosis assay to confirm that the cytotoxic effect observed (or lack thereof) was in fact accompanied by an equivalent degree of apoptosis. MTT (Roche, Indianapolis, IN) and annexin V (Pharmingen, San Diego, CA) assays were performed as described.15 Cell lines were seeded onto 96-well plates with the indicated reagents at a concentration of 10 000 cells per well. Primary blasts were seeded at 250 000 cells per well.
FLT3 phosphorylation
Leukemia cells were washed in PBS and then lysed by resuspending them in lysis buffer (20 mM Tris [pH 7.4], 100 mM NaCl, 1% Igepal [Sigma, St Louis, MO], 1 mM EDTA, 2 mM NaVO4, plus Complete protease inhibitor [Roche]) for 30 minutes while rocking. The extract was clarified by centrifugation at 14 000 rpm, and the supernatant was assayed for protein (Bio-Rad, Richmond, CA). Anti-FLT3 antibody (S18; Santa Cruz Biotechnology, Santa Cruz, CA) was added to the extract for overnight incubation, and then protein A-Sepharose (Upstate Biotechnology, Lake Placid, NY) was added for 2 additional hours. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transfer to Immobilon membranes (Millipore, Bedford, MA), immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology) to detect phosphorylated FLT3 and then the blots were stripped and reprobed with anti-FLT3 antibody to measure total FLT3. Proteins were visualized using chemiluminescence (ECL; Amersham) and scanned using a Bio-Rad GS800 densitometer.
Plasma inhibitory activity
Frozen plasma samples were thawed and clarified by centrifugation at 16 000g for 2 minutes. There was no difference in results when fresh plasma was used in comparison with frozen/thawed, nor was there any difference in results if samples were allowed to incubate at room temperature (protected from light) for as much as 1 week (data not shown). We have, however, found that there is some loss of plasma inhibitory activity in CEP-701/plasma samples stored at -80°C for more than 12 months (data not shown). All assays described herein were performed within 12 months of collection. For each time point, 4 × 106 TF/ITD cells were incubated with 1 mL plasma at 37°C for 2 hours. We have found that a 2-hour incubation period yields the most consistent results with the indolocarbazole FLT3 inhibitors. For other, more soluble, inhibitors, a 1-hour incubation period is sufficient. Pretreatment plasma is used as a baseline. The TF/ITD cells were washed twice with ice-cold PBS and then lysed and analyzed by immunoprecipitation for FLT3 and antiphosphotyrosine immunoblotting as described above, under “FLT3 phosphorylation.” After immunoblotting, densitometric analysis was performed on the bands, and the PIA for a given plasma sample was calculated by expressing the density of its corresponding band as a percent decrease from the density of the baseline band, which was arbitrarily set at 100%. For example, if the densitometric analysis of a sample band reveals it to be 10% of the pretreatment/baseline sample, the FLT3 PIA for that sample is 90%. While we have performed this assay successfully and with equivalent results using other human cell lines (Molm-14, EOL-1, SEMK2), the results shown here are for TF/ITD cells only.
Statistical analysis
Statistical analysis was performed using a 2-tailed t test.
Results
CEP-701 and PKC412 are highly protein bound in plasma compared with medium
CEP-701 potently inhibits FLT3/ITD autophosphorylation with a half maximal inhibitory concentration (IC50) of approximately 2 nM. This value was estimated using antiphosphotyrosine immunoblotting of FLT3 immunoprecipitated from extracts of cells exposed to the drug in culture medium containing 10% fetal bovine serum (FBS).16 This inhibition reflects an equilibrium of the drug between serum proteins and cells suspended in the medium. When the same experiment is performed with cells incubated in 100% FBS, the FLT3 inhibition curve shifts to the right (Figure 1A), reflecting the higher levels of protein present in serum compared with culture medium. When the cells are exposed to CEP-701 in 100% human plasma, the curve shifts far to the right and the IC50 for inhibition of FLT3 is approximately 700 nM (Figure 1B). This is most likely due to the high degree of protein binding (primarily to alpha-1-acid glycoprotein) of CEP-701 in plasma (Cephalon, unpublished data). Inhibition of FLT3 to 15% of its baseline level of autophosphorylation (the level of inhibition required to induce a significant cytotoxic effect on FLT3-dependent cell lines) occurs at a concentration of roughly 5 μM. Likewise, PKC412 potently inhibits FLT3 in culture medium (Figure 1C) but requires micromolar concentrations to achieve the same effect in plasma (Figure 1D). This is thought to be due in part to approximately 98% protein binding of PKC412 in human plasma (primarily to alpha-1-acid glycoprotein17). The calculated IC50 of 1.8 μM from this curve is slightly higher than the value estimated previously (1 μM).4 Thus, there is an approximately 300-fold difference in potency for both of these FLT3 inhibitors in medium compared with plasma when analyzed with this method.
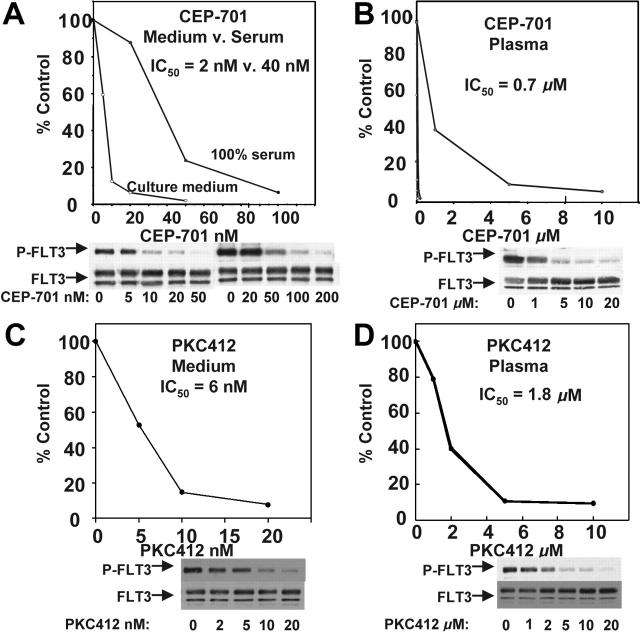
FLT3 inhibition by CEP-701 and PKC412 in culture medium compared with plasma. TF/ITD cells were incubated with the indicated concentrations of drug for 2 hours, and then FLT3 was immunoprecipitated from cell lysates and resolved by electrophoresis as described in “Patients, materials, and methods,” under “FLT3 phosphorylation.” Immunoblots were probed with antiphosphotyrosine and then stripped and reprobed with anti-FLT3. Densitometry results from the immunoblots were plotted as percent untreated. The calculated IC50 values shown were derived using linear regression analysis. (A) CEP-701, medium versus serum. (B) CEP-701, plasma. (C) PKC412, medium versus serum. (D) PKC412, plasma.
Plasma inhibitory activity for FLT3 correlates with clinical activity
Given that CEP-701 is highly protein bound, we used the FLT3 PIA as a surrogate measure of FLT3 inhibition in patients treated with CEP-701. In a clinical trial of CEP-701 in relapsed/refractory AML patients who harbored FLT3 mutations, a PIA of 85% or more correlated with clinical activity.3 To confirm that the PIA was associated with clinical response for PKC412, we determined the PIA of plasma samples from 10 of the patients treated with oral PKC412 in the phase 2 trial (Figure 2).4 In 8 of the 10 patients, the PIA for all samples tested was 85% or greater, suggesting adequate FLT3 inhibition. Six of these 8 patients were noted to have a clinical response to the drug, as defined by either a more than 2-log reduction in absolute blast count for more than 4 weeks or a more than 50% reduction in bone marrow blast percentage.4
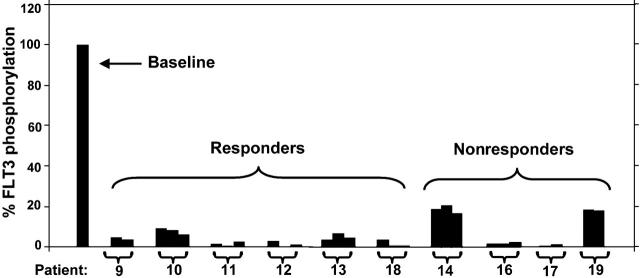
PIA assay for FLT3 using plasma samples from patients treated with PKC412. Trough plasma samples from 10 patients receiving 75 mg PKC412 orally every 8 hours were used in a PIA assay for FLT3. The results are expressed as percent baseline. Each bracket marks a set of samples from the same patient, and the patient numbers correspond to the numbering used by Stone et al.4 For each patient, PIA results from day 3, day 8, and day 28 of treatment with PKC412 are shown. A day-8 sample was not available for patient 9, and a day-28 sample was not available for patient 19. For some samples,11,12 some of the columns are not visible because FLT3 phosphorylation was completely suppressed.
The PIA for 2 of the 4 nonresponders in this sample group was less than 85% (ie, FLT3 phosphorylation still more than 15% of baseline), indicating inadequate FLT3 inhibition (Figure 2, patients 14 and 19). The other 2 nonresponding patients appeared to achieve adequate FLT3 inhibition by this assay. This lack of response despite adequate PIA levels may be explained by the results of the CEP-701 trial. In that trial, 25% of FLT3-mutant AML cases were intrinsically resistant to CEP-701, as determined by in vitro cytotoxicity assays.3 This same approximate rate of resistance appears to hold true in the PKC412 trial as well and may well be due to the presence of other mutations downstream rendering cells resistant to the cytotoxic effects of the kinase inhibitor.
Because of the small sample size, the data shown in Figure 2 are statistically inadequate to suggest a correlation between the PIA assay results and clinical activity. This was likewise the case with the CEP-701 trial, in which 14 samples were analyzed in identical fashion. Because both trials used essentially identical response criteria, and the PIA assay was performed on plasma samples from both trials in identical fashion, we felt it was valid to combine the results and ask if there was a correlation between the PIA assay results and the clinical responses of patients treated with oral indolocarbazole FLT3 inhibitors. When this is done, the sample size is 24 patients (14 from the CEP-701 trial, 10 from the PKC412 trial). Of these 24 samples, 16 had PIA results of 85% or greater. Eleven of the 16 patients achieving a PIA result of 85% or more showed a clinical response (69%), while none of the 8 patients failing to achieve this level of FLT3 inhibition in the PIA assay responded. Thus, the comparison is 11 of 16 versus 0 of 8, for a P value of .002. The PIA assay for FLT3 therefore correlates with clinical activity for both CEP-701 and PKC412 in that clinical responses are observed only when the PIA is at least 85%.
The PIA correlates with plasma drug levels for CEP-701 but not for PKC412
While patients receiving either CEP-701 or PKC412 appear to achieve adequate PIA for FLT3, more of those taking PKC412 in particular showed a high level of sustained FLT3 inhibition (8 of 10 with PKC412 versus 8 of 14 with CEP-7013,4), possibly accounting for the greater rate of clinical activity observed in the PKC412 trial. We next compared the results of the PIA assays with the conventional pharmacokinetic data from both trials. For both the CEP-701 and PKC412 trials, high-performance liquid chromatography (HPLC) was used to determine plasma drug levels. For each plasma sample used to assay for PIA, HPLC was used to determine the actual concentration of drug present. When the PIA results are plotted against the measured concentrations of CEP-701, the points fall closely along the standard curve for FLT3 inhibition by CEP-701 in plasma (Figure 3A). This suggests that all of the PIA for FLT3 measured in the assay is accounted for by CEP-701 alone, as expected. However, we did not find this to hold true for PKC412. When the PIA results are plotted against PKC412 plasma concentrations, the points fall well to the left of the standard curve (Figure 3B).
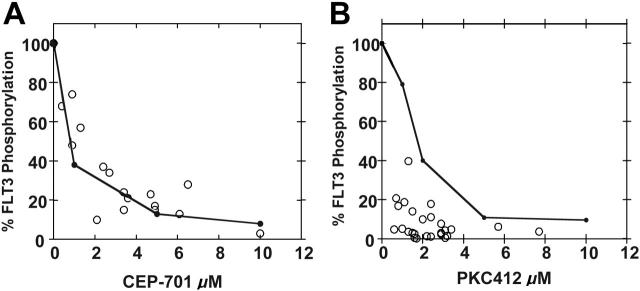
PIA for FLT3 plotted against plasma drug levels. Shown are the standard curves (solid black lines) for inhibition of FLT3 autophosphorylation in plasma by CEP-701 (A) and PKC412 (B). These are the same curves from Figure 1B,D. Superimposed over the curves are PIA assay results (○) for individual samples from patients treated with CEP-701 (A) and PKC412 (B). Each circle plots the level of FLT3 phosphorylation in the PIA assay against the measurement of the actual drug level from that plasma sample.
PKC412 undergoes cytochrome P450-mediated metabolism and is present in plasma along with 2 major metabolites, CGP62221 and CGP52421 (Figure 4A). The pK analysis from the PKC412 trial suggested that, in most patients, PKC412 (and its active metabolite, CGP62221) reached micromolar concentrations during the first week of treatment, and then the levels of both parent drug and metabolite fell rapidly (Figure 4B). The concentration of the other major metabolite, CGP52421, rose continuously through day 28 and remained relatively stable thereafter.4 Notably, the plasma concentration of CGP52421 at steady state (20 to 25 μM) is roughly 7-fold more than that of either the parent drug or CGP62221.
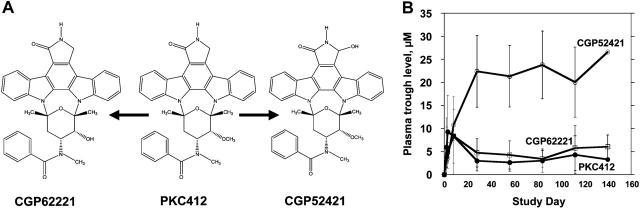
PKC412 and metabolites. (A) CGP6221 and CGP52421 are generated from PKC412 via P450 liver enzyme metabolism.17 (B) Trough plasma concentrations of PKC412 (•), CGP62221 (□), and CGP52421 (○) averaged for patients treated with 75 mg PKC412 orally every 8 hours. Error bars represent standard deviations.
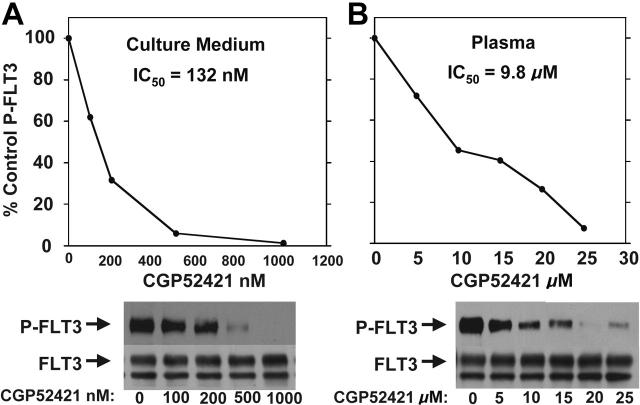
CGP52421 may be less protein bound in plasma compared with PKC412
We first adjusted the curve shown in Figure 3B to account for the presence of the active metabolite CGP62221 (by simply adding its concentration to that of PKC412), but the PIA data points still fell to the left of this adjusted curve (not shown). Because of this continuing discrepancy between the PIA assay results and the pharmacokinetic data, and because of the remarkably high levels of CGP52421 in the plasma of trial patients, we further investigated the FLT3-inhibitory properties of CGP52421. In culture medium, CGP52421 inhibits FLT3 autophosphorylation 22-fold less potently than the parent compound, with an IC50 of approximately 132 nM (Figure 5A). In plasma, the IC50 of CGP52421 is 9.8 μM, compared with 1.8 μM for PKC412 (compare Figure 5B with Figure 1D). Therefore, while CGP52421 is 22-fold less potent than PKC412 in culture medium containing 10% serum, it is roughly 5-fold less potent than the parent compound in plasma, possibly because it is less protein bound. CGP52421 levels at steady state ranged from 20 to 30 μM, 2- to 3-fold over the IC50 and likely sufficient to contribute significantly to FLT3 inhibition. Using a formula of the concentration of PKC412 plus the concentration of CGP6221 plus the concentration of CGP52421/5.4, the levels of PKC412 and its metabolites can be expressed as an adjusted value in terms of FLT3 inhibition. When the PIA results are plotted against the adjusted values, the points now fall along the standard curve for PKC412-mediated FLT3 inhibition in plasma (Figure 6A). When the results of all of the PIA assays are averaged and plotted along with the adjusted plasma concentrations, the remarkably effective, sustained FLT3 inhibition by PKC412 and its metabolites is illustrated (Figure 6B-C).
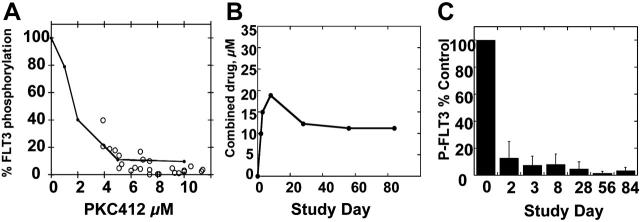
PIA assay results compared with combined PKC412, CGP6221, and CGP52421 levels. (A) Standard curve for inhibition of FLT3 autophosphorylation by PKC412 in plasma (Figures (Figures1D1D and and3B).3B). Superimposed over this graph are PIA assay results (○) plotted against the “adjusted” PKC412 level, calculated by adding the concentration of PKC412 and CGP6221 and value of the CGP52421 level divided by 5.4. (B) The average “adjusted” concentrations of PKC412 for patients on the PKC412 trial. (C) PIA results, averaged for each time point, for patients on the PKC412 trial. Error bars represent standard deviations.
CGP52421 is less selective but more cytotoxic than PKC412
Because CGP52421 contributes significantly to FLT3 inhibition in patients treated with PKC412, we wished to better characterize its cytotoxic properties against leukemia cells. We first tested it against BaF3-ITD cells, a model cell line derived by transfecting murine Ba/F3 cells, which are IL-3 dependent, with a FLT3/ITD construct.16 While the resultant BaF3-ITD cells are IL-3 independent, IL-3 can be used to “rescue” the cells from the cytotoxic effects of FLT3 inhibitors. Using an MTT assay, we determined that PKC412 induces a cytotoxic effect in BaF3-ITD cells with an IC50 of 29 nM in the absence of IL-3 and 155 nM in the presence of IL-3. The cytotoxic effects seen in the presence of IL-3 are presumably due to inhibition of other, unidentified targets of PKC412. Using the same assay, CGP52421 induces cytotoxicity in BaF3-ITD cells with an IC50 of 613 nM in the absence of IL-3 and 1053 nM in the presence of IL-3. The difference between the IC50 values in the absence and presence of IL-3—4.3-fold versus 1.7-fold higher for PKC412 and CGP52421, respectively—suggests that CGP52421 is less selective than PKC412 at concentrations that are inhibitory for FLT3. For CEP-701 the IC50 is 8 nM in the absence of IL-3 and 24 nM in the presence of IL-3, which places it between PKC412 and CGP52421 in this assessment of selectivity. The results of the IL-3 rescue assays are summarized in Table 1.
Table 1.
IL-3 rescue assay
| IC50, nM
| |||
|---|---|---|---|
| Drug | Without IL-3 | With IL-3 | +IL-3/-IL-3 |
| CEP-701 | 8 | 24 | 3 |
| PKC412 | 29 | 155 | 5.3 |
| CGP52421 | 613 | 1053 | 1.7 |
BaF3/ITD cells were seeded onto 96-well plates containing increasing concentrations of CEP-701, PKC412, and CGP52421, both in the presence and absence of 1 ng/mL IL-3. MTT assay was performed at 48 hours, and the results were plotted as percent untreated control. Regression analysis of the resulting curves yielded the displayed IC50 values.
+IL3/-IL3 refers to the ratio of the IC50 values obtained in the presence and absence of IL-3
We next wished to compare the cytotoxic effects of these inhibitors on blasts isolated from AML patients. MTT assays of CEP-701 and PKC412 were performed on 2 groups of primary AML samples, one group harboring wild-type FLT3 (25 samples) and the other group harboring FLT3/ITD mutations (21 samples). As summarized in Table 2, CEP-701 induced a cytotoxic response in both wild-type and FLT3/ITD samples, with a greater effect seen in the mutant samples, as described previously.16,18 However, PKC412 induced virtually no cytotoxicity in the wild-type AML samples and induced a much more modest effect in the mutant samples (Table 2). While this difference in cytotoxicity in vitro between CEP-701 and PKC412 has been observed by others,19 this is nonetheless an unexpected result given that PKC412 was actually more effective than CEP-701 in clearing peripheral blasts from patients.3,4 We next selected 5 samples from each group that differed in cytotoxic response to CEP-701 compared with PKC412 (Figure 7A,E versus Figure 7B,F) and examined the response of those samples to CGP52421 (Figure 7C,G). Over the dose range 100 to 500 nM (corresponding to the range over which FLT3 inhibition occurs; Figure 5A) CGP52421 is more cytotoxic than PKC412. When PKC412 and CGP52421 are combined (at levels that approximate what might be present in a patient) in the cytotoxic assay, there is no difference in effect with CGP52421 alone as compared with the combination (Figure 7D,H). Thus, at levels that correspond to achievable levels in plasma, CGP52421 is more cytotoxic to AML blasts than its parent compound, PKC412. This greater cytotoxic effect may be the result of its lesser selectivity, a property that it appears to share with CEP-701.
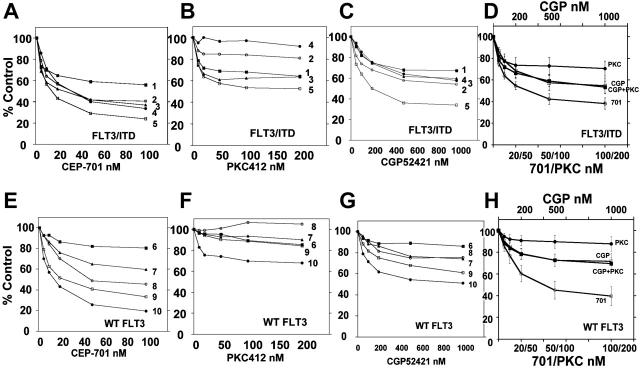
Cytotoxicity assays for CEP-701, PKC412, and CGP52421. MTT assays were performed on 10 primary AML samples, 5 with FLT3/ITD mutations and 5 with wild-type FLT3. (A-C) FLT3/ITD samples tested against increasing concentrations of CEP-701, PKC412, and CGP52421. (E-G) Samples with wild-type FLT3 tested against increasing concentrations of CEP-701, PKC412, and CGP52421. (D) Four dose-response curves are shown. Three represent the combined results of the values obtained in the experiments shown in panels A-C for the 3 drugs CEP-701, PKC412, and CGP52421 as single agents (error bars depict SEM). The fourth curve, which virtually overlaps the CGP52421 curve, represents the results when these same samples were exposed to an increasing concentration of CGP52421 in the presence of a fixed concentration of PKC412 (10 nM) so as to mimic the presence of both compounds found in plasma simultaneously. (H) As in panel D, using wild-type FLT3 samples.
Table 2.
Summary of MTT assay results for primary AML samples treated with CEP-701 versus PKC412
| Drug, dose | WT FLT3 | FLT3/ITD |
|---|---|---|
| CEP-701 | ||
   20 nM 20 nM | 76.1 ± 2.0 | 66.4 ± 4.8 |
   50 nM 50 nM | 60.8 ± 2.6 | 56.6 ± 5.1 |
| PKC412 | ||
   50 nM 50 nM | 96.4 ± 2.2 | 82.2 ± 4.8 |
   100 nM 100 nM | 96.0 ± 2.7 | 79.2 ± 5.3 |
Primary AML samples harboring wild-type FLT3 (25 samples) and FLT3/ITD mutations (21 samples) were incubated with increasing concentrations of CEP-701 or PKC412, and the MTT assay was performed after 72 hours. The results are expressed as percent untreated control, and the values for all results from a given concentration point are expressed as a mean ± SEM.
WT indicates wild-type
Discussion
More than half a century ago, investigators developing dosing regimens for penicillin cultured bacteria directly in the plasma from patients treated with penicillin as an assay for plasma bacteriocidal effect.20 The PIA assay described here, which mimics this approach, offers a unique advantage over conventional pharmacokinetic analysis because it focuses on the target of the drug rather than the drug itself or its metabolites. While it is not a substitute for the measurement of drug levels, it complements the pharmacokinetic data and in this case has revealed an important aspect of the mechanism of action of PKC412. With the PIA assay, accessibility of the tumor tissue is no longer a limiting factor in determining if a target is being affected by a small-molecule inhibitor (as long as a human cell line expressing the target is available). This assay may therefore be generally useful in the development of inhibitors of targets in solid tumors. We are currently using it in a multicenter trial of CEP-701 administered in sequence with chemotherapy for AML patients with FLT3 mutations.5
PKC412 and CEP-701 are structurally related, orally available compounds that are in similar stages of clinical development as FLT3 inhibitors. Most published data on PKC412 constitute experiments using cell lines often engineered to be dependent on FLT3 signaling (eg, the Ba/F3 transfectants).21-24 In such experiments, PKC412 appears to behave similarly to CEP-701 in terms of inducing cytotoxicity. However, the PIA assay results combined with the available pharmacokinetic data suggest that the parent compounds are quite different in their effects on leukemia cells. This highlights the importance of testing these agents against primary leukemia cells. Others have previously examined the effects of PKC412 on primary blasts, including one group that compared the effects of PKC412 with CEP-701 and also noted CEP-701 to be more cytotoxic at equivalent levels of FLT3 inhibition.19,24,25 The best explanation for these findings is that selective inhibition of FLT3 is in most cases insufficient to kill AML cells. The inhibition of other cellular targets by CGP52421 and CEP-701 in combination with FLT3 inhibition is the likely means of inducing cell death by FLT3 inhibitors. In other words, lack of selectivity (or “multitargeting”) may be important in FLT3 inhibitor-mediated leukemia cell killing.
In summary, the plasma inhibitory activity (PIA) assay is a useful surrogate assay for the determination of FLT3 inhibition in vivo and could likely be used for other inhibitors targeted at a wide variety of malignancies. Our findings using this assay have shown the importance of nonselectivity of FLT3 inhibitors in inducing leukemia cell death and have revealed that the metabolite CGP52421 contributes a significant proportion of FLT3 inhibition and clinical activity in patients treated with PKC412.
Notes
Prepublished online as Blood First Edition Paper, July 20, 2006; DOI 10.1182/blood-2006-04-015743.
Supported by grants from the National Cancer Institute (NCI Leukemia Special Program of Research Excellence [SPORE] grants CA100632, CA095600-02, CA90668, CA70970, and P30 CA 006973-44), the Sidney Kimmel Research Foundation, the Leukemia and Lymphoma Society, the Burroughs Wellcome Fund, and the Regional Oncology Research Center (grant 2 P30 CA 006973-44).
Several of the authors (P.C., Y.W., J.R.) are employed by Novartis Oncology, whose potential product (PKC412) was studied in the present work.
M.L. conceived, designed, and performed the FLT3 plasma inhibitory activity assay, performed cytotoxicity assays and immunoblots, and wrote the manuscript; M.L. and D.S. designed the study; D.S. edited the manuscript; A.S. and R.P. performed cytotoxic assays and IL-3 rescue assays; P.B., B.D.S., and J.E.K. provided primary AML samples; E.E. assisted with the statistical analysis; R.S., D.D., I.G., F.G., E.E., and H.K. accrued patients to the PKC412 trial and helped edit the manuscript; and P.C., Y.W., and J.R. provided the pharmacokinetic data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
References
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2006-04-015743
Read article for free, from open access legal sources, via Unpaywall:
http://www.bloodjournal.org/content/108/10/3477.full.pdf
Citations & impact
Impact metrics
Article citations
Ningetinib, a novel FLT3 inhibitor, overcomes secondary drug resistance in acute myeloid leukemia.
Cell Commun Signal, 22(1):355, 08 Jul 2024
Cited by: 0 articles | PMID: 38978049
Functional and Structural Characterization of Clinical-Stage Janus Kinase 2 Inhibitors Identifies Determinants for Drug Selectivity.
J Med Chem, 67(12):10012-10024, 06 Jun 2024
Cited by: 1 article | PMID: 38843875 | PMCID: PMC11215726
A phase 1 study of the irreversible FLT3 inhibitor FF-10101 in relapsed or refractory acute myeloid leukemia.
Blood Adv, 8(10):2527-2535, 01 May 2024
Cited by: 2 articles | PMID: 38502195 | PMCID: PMC11131057
Foretinib Is Effective in Acute Myeloid Leukemia by Inhibiting FLT3 and Overcoming Secondary Mutations That Drive Resistance to Quizartinib and Gilteritinib.
Cancer Res, 84(6):905-918, 01 Mar 2024
Cited by: 1 article | PMID: 38231480 | PMCID: PMC10940854
BRCC36 associates with FLT3-ITD to regulate its protein stability and intracellular signaling in acute myeloid leukemia.
Cancer Sci, 115(4):1196-1208, 30 Jan 2024
Cited by: 3 articles | PMID: 38288901 | PMCID: PMC11007003
Go to all (146) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases.
Blood, 108(10):3494-3503, 25 Jul 2006
Cited by: 85 articles | PMID: 16868253
Gö6976, a FLT3 kinase inhibitor, exerts potent cytotoxic activity against acute leukemia via inhibition of survivin and MCL-1.
Biochem Pharmacol, 90(1):16-24, 13 Apr 2014
Cited by: 10 articles | PMID: 24735609
Divergent cytotoxic effects of PKC412 in combination with conventional antileukemic agents in FLT3 mutation-positive versus -negative leukemia cell lines.
Leukemia, 21(5):1005-1014, 01 Mar 2007
Cited by: 37 articles | PMID: 17330105
Midostaurin/PKC412 for the treatment of newly diagnosed FLT3 mutation-positive acute myeloid leukemia.
Expert Rev Hematol, 10(12):1033-1045, 30 Oct 2017
Cited by: 10 articles | PMID: 29069942
Review
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: CA095600-02
Grant ID: CA90668
Grant ID: CA70970
Grant ID: P30 CA 006973-44
Grant ID: CA100632
Grant ID: 2P30 CA 006973-44