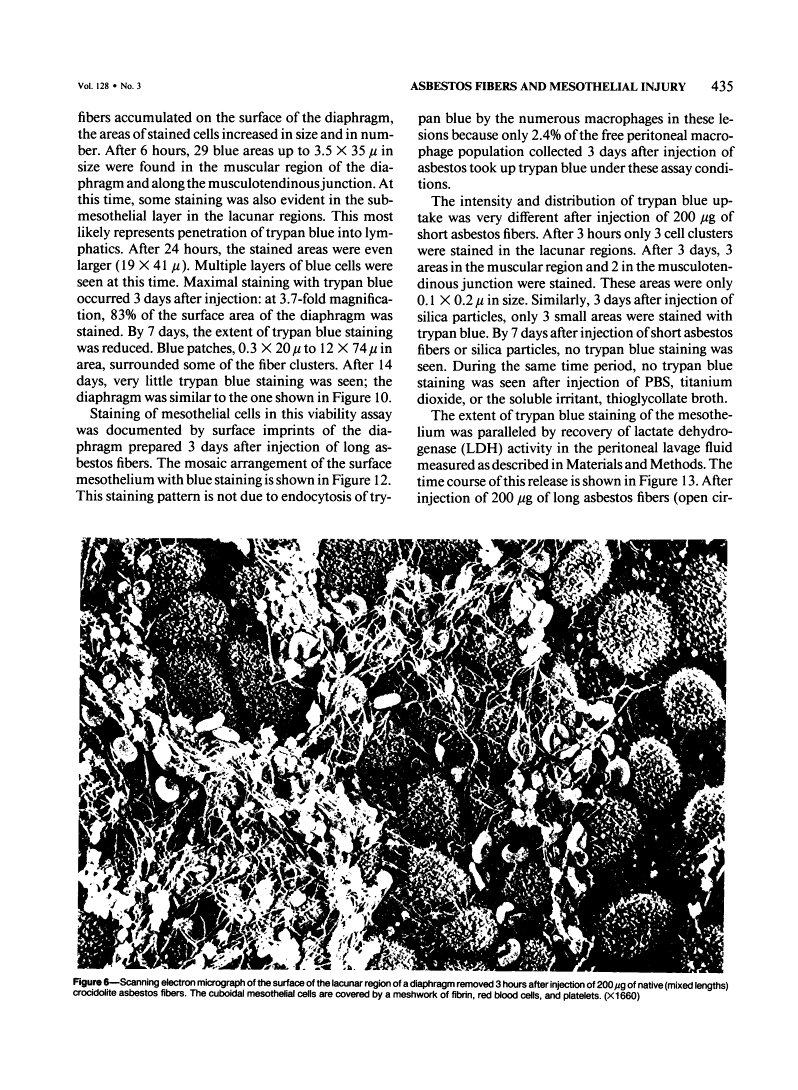
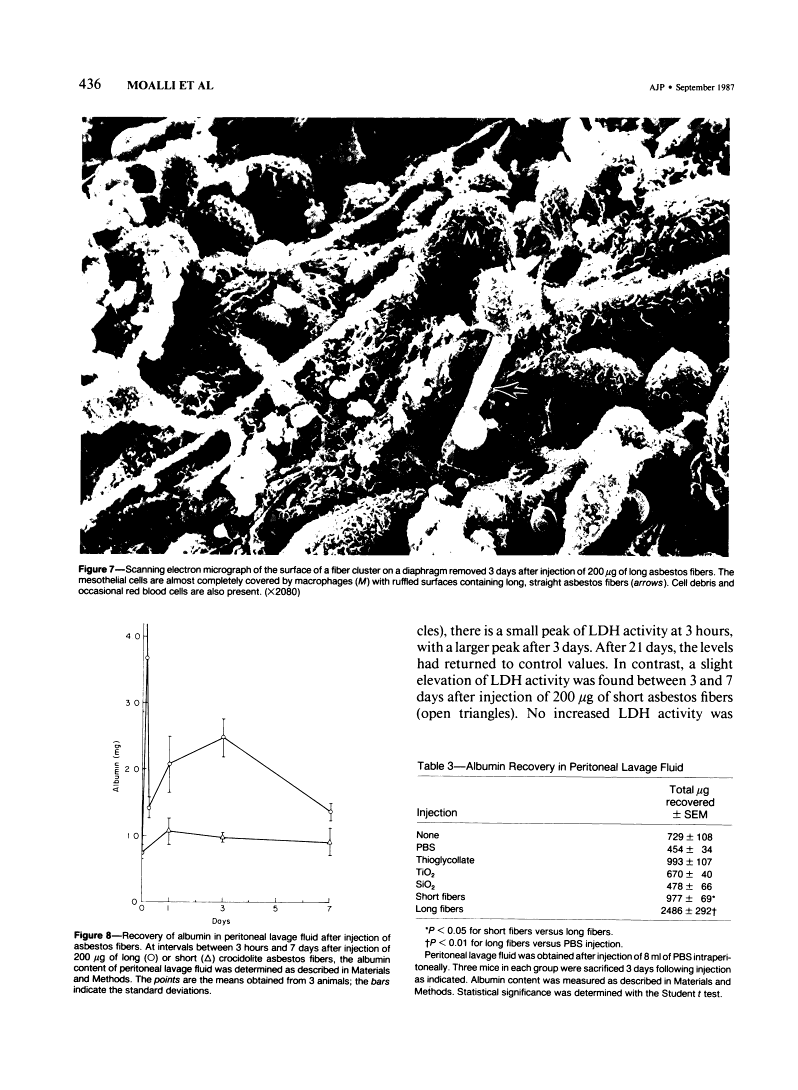
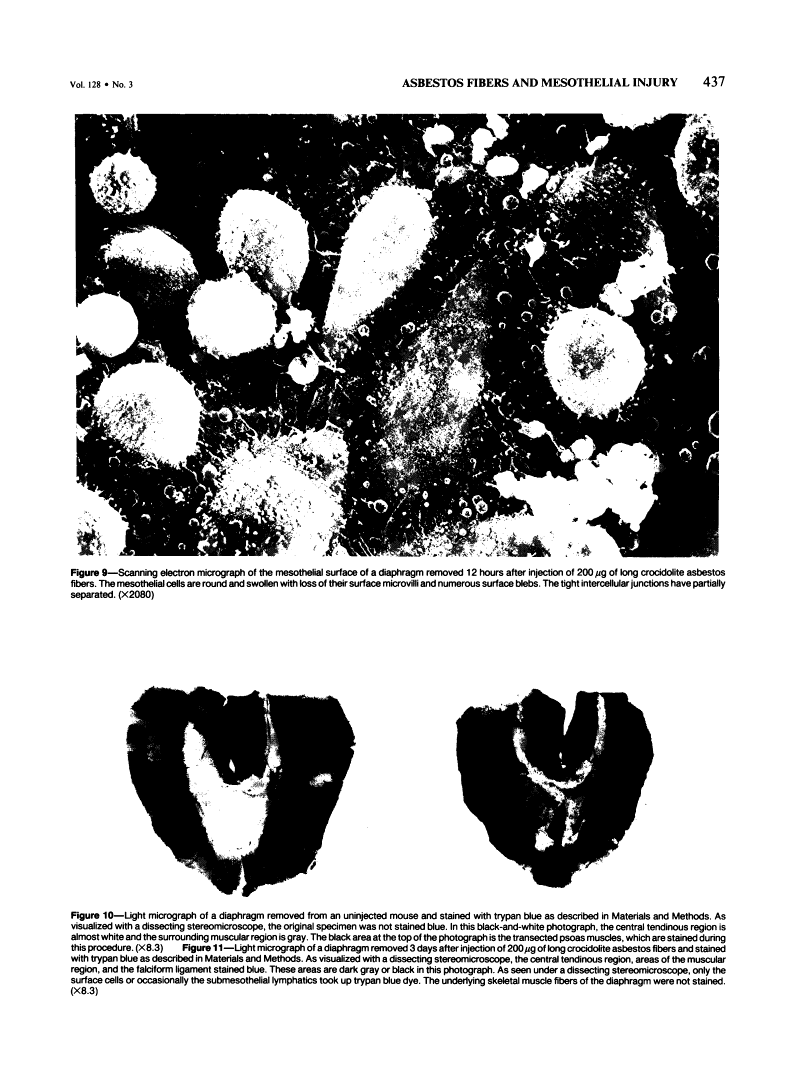
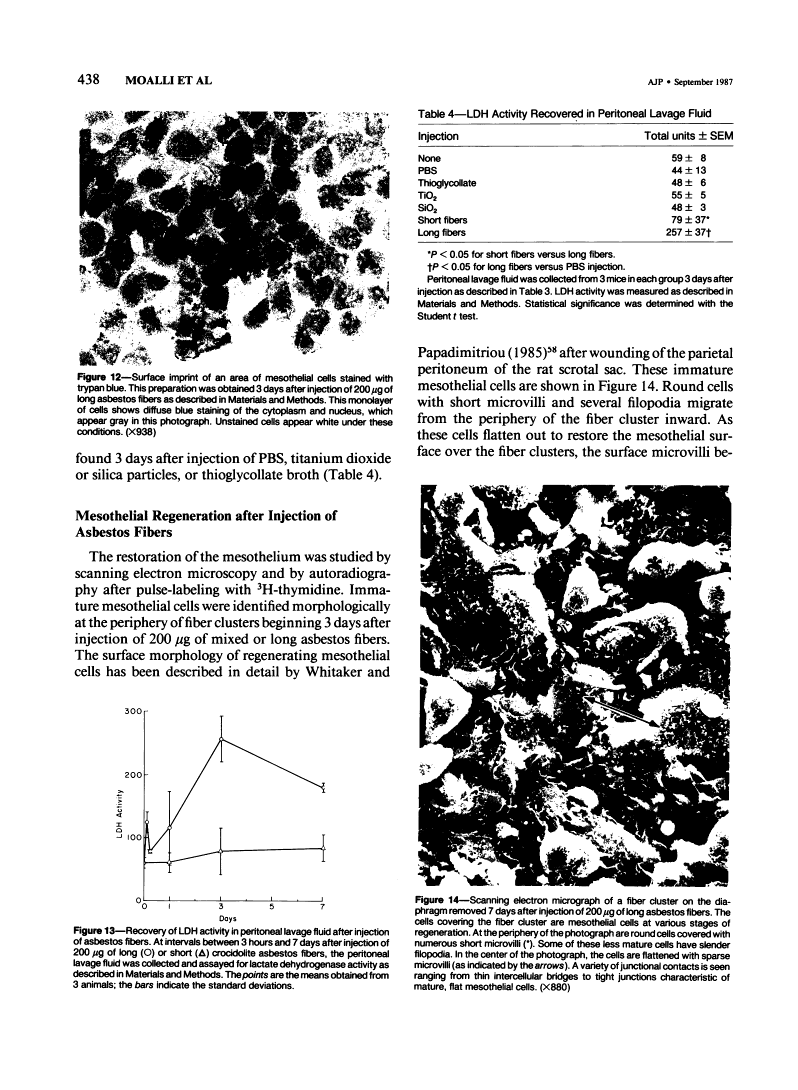
Abstract
Free full text

Acute injury and regeneration of the mesothelium in response to asbestos fibers.
Abstract
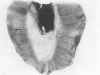
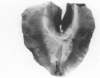
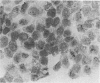
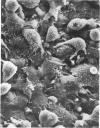
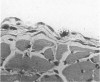
The mesothelium is a target of the toxic and carcinogenic effects of asbestos fibers. Fibers greater than 8 mu in length and less than 0.25 mu in diameter have been found to be highly tumorigenic in rodents, while shorter asbestos fibers or spherical mineral particles have not been shown to produce mesotheliomas. For investigation of early mesothelial reactions associated with the development of mesotheliomas, C57BL/6 mice were given intraperitoneal injections of 200 micrograms of short or long crocidolite asbestos fibers, toxic silica particles, or nontoxic titanium dioxide particles. At intervals between 3 hours and 21 days after a single injection, the mesothelial surface of the diaphragm was examined by stereomicroscopy, scanning electron microscopy, and autoradiography. Within 6 hours after injection of asbestos fibers, mesothelial cells in the lacunar regions of the diaphragm retracted opening stomata 10.7 +/- 2.3 mu in diameter leading to the submesothelial lymphatic plexus. Short asbestos fibers (90.6% less than or equal to 2 mu in length), silica, or titanium dioxide particles (less than or equal to 5 mu in diameter) were cleared through these stomata without provoking an inflammatory reaction or mesothelial injury. In contrast, long asbestos fibers (60.3% greater than or equal to 2 mu in length) were trapped at the lymphatic stomata in the lacunar regions on the peritoneal surface of the diaphragm. At these sites, an intense inflammatory reaction developed with accumulation of activated macrophages and a 5.5-fold increase in albumin recovered in the peritoneal lavage fluid after 3 days. As early as 12 hours after injection of long asbestos fibers, the adjacent mesothelial cells were unable to exclude trypan blue and lost their surface microvilli, developed blebs, and detached. Recovery of lactate dehydrogenase activity in the peritoneal lavage fluid was increased 5.8-fold after 3 days and returned to normal levels after 14 days. Regenerating mesothelial cells appeared at the periphery of asbestos fiber clusters 3 days after injection. Maximal incorporation of 3H-thymidine by mesothelial cells occurred after 7 days, followed by partial restoration of the mesothelial lining after 14-21 days. As late as 6 months after a single injection of crocidolite asbestos fibers, clusters of fibers remained in the lacunar regions, partially covered by mesothelium but surrounded by macrophages and regenerating mesothelial cells. The anatomic distribution and size of lymphatic stomata on the peritoneal surface of the diaphragm account for the selective accumulation of long asbestos fibers in these regions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (6.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craighead JE, Mossman BT. The pathogenesis of asbestos-associated diseases. N Engl J Med. 1982 Jun 17;306(24):1446–1455. [Abstract] [Google Scholar]
- Davis JM. The pathology of asbestos related disease. Thorax. 1984 Nov;39(11):801–808. [Europe PMC free article] [Abstract] [Google Scholar]
- Stanton MF, Laynard M, Tegeris A, Miller E, May M, Kent E. Carcinogenicity of fibrous glass: pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst. 1977 Mar;58(3):587–603. [Abstract] [Google Scholar]
- Lee KP, Trochimowicz HJ, Reinhardt CF. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol. 1985 Jun 30;79(2):179–192. [Abstract] [Google Scholar]
- Adamson IY, Bowden DH. Crocidolite-induced pulmonary fibrosis in mice. Cytokinetic and biochemical studies. Am J Pathol. 1986 Feb;122(2):261–267. [Europe PMC free article] [Abstract] [Google Scholar]
- Bowden DH, Adamson IY. The role of cell injury and the continuing inflammatory response in the generation of silicotic pulmonary fibrosis. J Pathol. 1984 Nov;144(3):149–161. [Abstract] [Google Scholar]
- Wagner JC, Berry G, Skidmore JW, Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. [Europe PMC free article] [Abstract] [Google Scholar]
- Wagner JC, Berry G. Mesotheliomas in rats following inoculation with asbestos. Br J Cancer. 1969 Sep;23(3):567–581. [Europe PMC free article] [Abstract] [Google Scholar]
- Wagner JC, Berry G, Timbrell V. Mesotheliomata in rats after inoculation with asbestos and other materials. Br J Cancer. 1973 Aug;28(2):173–185. [Europe PMC free article] [Abstract] [Google Scholar]
- Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981 Nov;67(5):965–975. [Abstract] [Google Scholar]
- Monchaux G, Bignon J, Jaurand MC, Lafuma J, Sebastien P, Masse R, Hirsch A, Goni J. Mesotheliomas in rats following inoculation with acid-leached chrysotile asbestos and other mineral fibres. Carcinogenesis. 1981;2(3):229–236. [Abstract] [Google Scholar]
- Chamberlain M, Tarmy EM. Asbestos and glass fibres in bacterial mutation tests. Mutat Res. 1977 May;43(2):159–164. [Abstract] [Google Scholar]
- Huang SL, Saggioro D, Michelmann H, Malling HV. Genetic effects of crocidolite asbestos in Chinese hamster lung cells. Mutat Res. 1978 May;57(2):225–232. [Abstract] [Google Scholar]
- Reiss B, Solomon S, Tong C, Levenstein M, Rosenberg SH, Williams GM. Absence of mutagenic activity of three forms of asbestos in liver epithelial cells. Environ Res. 1982 Apr;27(2):389–397. [Abstract] [Google Scholar]
- Fornace AJ., Jr Detection of DNA single-strand breaks produced during the repair of damage by DNA-protein cross-linking agents. Cancer Res. 1982 Jan;42(1):145–149. [Abstract] [Google Scholar]
- Denizeau F, Marion M, Chevalier G, Côté MG. Inability of chrysotile asbestos fibers to modulate the 2-acetylaminofluorene-induced UDS in primary cultures of rat hepatocytes. Mutat Res. 1985 Jan-Feb;155(1-2):83–90. [Abstract] [Google Scholar]
- Sincock A, Seabright M. Induction of chromosome changes in Chinese hamster cells by exposure to asbestos fibres. Nature. 1975 Sep 4;257(5521):56–58. [Abstract] [Google Scholar]
- Barrett JC, Hesterberg TW, Oshimura M, Tsutsui T. Role of chemically induced mutagenic events in neoplastic transformation of Syrian hamster embryo cells. Carcinog Compr Surv. 1985;9:123–137. [Abstract] [Google Scholar]
- Jaurand MC, Kaplan H, Thiollet J, Pinchon MC, Bernaudin JF, Bignon J. Phagocytosis of chrysotile fibers by pleural mesothelial cells in culture. Am J Pathol. 1979 Mar;94(3):529–538. [Europe PMC free article] [Abstract] [Google Scholar]
- Rajan KT, Wagner JC, Evans PH. The response of human pleura in organ culture to asbestos. Nature. 1972 Aug 11;238(5363):346–347. [Abstract] [Google Scholar]
- Lechner JF, Tokiwa T, LaVeck M, Benedict WF, Banks-Schlegel S, Yeager H, Jr, Banerjee A, Harris CC. Asbestos-associated chromosomal changes in human mesothelial cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3884–3888. [Europe PMC free article] [Abstract] [Google Scholar]
- Jaurand MC, Kheuang L, Magne L, Bignon J. Chromosomal changes induced by chrysotile fibres or benzo-3,4-pyrene in rat pleural mesothelial cells. Mutat Res. 1986 Mar;169(3):141–148. [Abstract] [Google Scholar]
- Mossman BT, Kessler JB, Ley BW, Craighead JE. Interaction of crocidolite asbestos with hamster respiratory mucosa in organ culture. Lab Invest. 1977 Feb;36(2):131–139. [Abstract] [Google Scholar]
- Landesman JM, Mossman BT. Induction of ornithine decarboxylase in hamster tracheal epithelial cells exposed to asbestos and 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982 Sep;42(9):3669–3675. [Abstract] [Google Scholar]
- Woodworth CD, Mossman BT, Craighead JE. Induction of squamous metaplasia in organ cultures of hamster trachea by naturally occurring and synthetic fibers. Cancer Res. 1983 Oct;43(10):4906–4912. [Abstract] [Google Scholar]
- Paterour MJ, Bignon J, Jaurand MC. In vitro transformation of rat pleural mesothelial cells by chrysotile fibres and/or benzo[a]pyrene. Carcinogenesis. 1985 Apr;6(4):523–529. [Abstract] [Google Scholar]
- Chamberlain M. The influence of mineral dusts on metabolic co-operation between mammalian cells in tissue culture. Carcinogenesis. 1982;3(3):337–339. [Abstract] [Google Scholar]
- Donaldson K, Cullen RT. Chemiluminescence of asbestos-activated macrophages. Br J Exp Pathol. 1984 Feb;65(1):81–90. [Europe PMC free article] [Abstract] [Google Scholar]
- Goodglick LA, Kane AB. Role of reactive oxygen metabolites in crocidolite asbestos toxicity to mouse macrophages. Cancer Res. 1986 Nov;46(11):5558–5566. [Abstract] [Google Scholar]
- Mossman BT, Marsh JP, Shatos MA. Alteration of superoxide dismutase activity in tracheal epithelial cells by asbestos and inhibition of cytotoxicity by antioxidants. Lab Invest. 1986 Feb;54(2):204–212. [Abstract] [Google Scholar]
- Weitzman SA, Stossel TP. Mutation caused by human phagocytes. Science. 1981 May 1;212(4494):546–547. [Abstract] [Google Scholar]
- Weitberg AB, Weitzman SA, Destrempes M, Latt SA, Stossel TP. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983 Jan 6;308(1):26–30. [Abstract] [Google Scholar]
- Weitzman SA, Weitberg AB, Clark EP, Stossel TP. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. [Abstract] [Google Scholar]
- Cerutti PA. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. [Abstract] [Google Scholar]
- Davis JM. An electron microscope study of the response of mesothelial cells to the intrapleural injection of asbestos dust. Br J Exp Pathol. 1974 Feb;55(1):64–70. [Europe PMC free article] [Abstract] [Google Scholar]
- Davis JM. Histogenesis and fine structure of peritoneal tumors produced in animals by injections of asbestos. J Natl Cancer Inst. 1974 Jun;52(6):1823–1837. [Abstract] [Google Scholar]
- Brody AR, George G, Hill LH. Interactions of chrysotile and crocidolite asbestos with red blood cell membranes. Chrysotile binds to sialic acid. Lab Invest. 1983 Oct;49(4):468–475. [Abstract] [Google Scholar]
- Macdonald JL, Kane AB. Identification of asbestos fibers within single cells. Lab Invest. 1986 Aug;55(2):177–185. [Abstract] [Google Scholar]
- Leak LV. Interaction of mesothelium to intraperitoneal stimulation. I. Aggregation of peritoneal cells. Lab Invest. 1983 Apr;48(4):479–491. [Abstract] [Google Scholar]
- Churg A, Warnock ML. Asbestos fibers in the general population. Am Rev Respir Dis. 1980 Nov;122(5):669–678. [Abstract] [Google Scholar]
- Harrison CJ, Allen TD, Britch M, Harris R. High-resolution scanning electron microscopy of human metaphase chromosomes. J Cell Sci. 1982 Aug;56:409–422. [Abstract] [Google Scholar]
- Björkerud S, Bondjers G. Endothelial integrity and viability in the aorta of the normal rabbit and rat as evaluated with dye exclusion tests and interference contrast microscopy. Atherosclerosis. 1972 May-Jun;15(3):285–300. [Abstract] [Google Scholar]
- Lee RM, Chambers C, O'Brodovich H, Forrest JB. Trypan blue method for the identification of areas of damage to airway epithelium due to mechanical trauma. Scan Electron Microsc. 1984;(Pt 3):1267–1271. [Abstract] [Google Scholar]
- Whitaker D, Papadimitriou JM, Walters MN. The mesothelium; techniques for investigating the origin, nature and behaviour of mesothelial cells. J Pathol. 1980 Nov;132(3):263–271. [Abstract] [Google Scholar]
- Williams MA. Autoradiography: its methodology at the present time. J Microsc. 1982 Oct;128(Pt 1):79–94. [Abstract] [Google Scholar]
- Beck BD, Brain JD, Bohannon DE. An in vivo hamster bioassay to assess the toxicity of particulates for the lungs. Toxicol Appl Pharmacol. 1982 Oct;66(1):9–29. [Abstract] [Google Scholar]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971 Jan;31(1):87–96. [Abstract] [Google Scholar]
- CABAUD PG, WROBLEWSKI F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol. 1958 Sep;30(3):234–236. [Abstract] [Google Scholar]
- Leak LV, Rahil K. Permeability of the diaphragmatic mesothelium: the ultrastructural basis for "stomata". Am J Anat. 1978 Apr;151(4):557–593. [Abstract] [Google Scholar]
- Tsilibary EC, Wissig SL. Absorption from the peritoneal cavity: SEM study of the mesothelium covering the peritoneal surface of the muscular portion of the diaphragm. Am J Anat. 1977 May;149(1):127–133. [Abstract] [Google Scholar]
- Roser BJ. The origin and significance of macrophages in thoracic duct lymph. Aust J Exp Biol Med Sci. 1976 Dec;54(6):541–550. [Abstract] [Google Scholar]
- COURTICE FC, HARDING J, STEINBECK AW. The removal of free red blood cells from the peritoneal cavity of animals. Aust J Exp Biol Med Sci. 1953 Jun;31(3):215–225. [Abstract] [Google Scholar]
- ALLEN L, WEATHERFORD T. Role of fenestrated basement membrane in lymphatic absorption from peritoneal cavity. Am J Physiol. 1959 Sep;197:551–554. [Abstract] [Google Scholar]
- Miller K, Kagan E. The in vivo effects of asbestos on macrophage membrane structure and population characteristics of macrophages: a scanning electron microscope study. J Reticuloendothel Soc. 1976 Aug;20(2):159–170. [Abstract] [Google Scholar]
- Whitaker D, Papadimitriou J. Mesothelial healing: morphological and kinetic investigations. J Pathol. 1985 Feb;145(2):159–175. [Abstract] [Google Scholar]
- Corry D, Kulkarni P, Lipscomb MF. The migration of bronchoalveolar macrophages into hilar lymph nodes. Am J Pathol. 1984 Jun;115(3):321–328. [Europe PMC free article] [Abstract] [Google Scholar]
- Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. [Abstract] [Google Scholar]
- Kagan E, Oghiso Y, Hartmann DP. Enhanced release of a chemoattractant for alveolar macrophages after asbestos inhalation. Am Rev Respir Dis. 1983 Oct;128(4):680–687. [Abstract] [Google Scholar]
- Warheit DB, George G, Hill LH, Snyderman R, Brody AR. Inhaled asbestos activates a complement-dependent chemoattractant for macrophages. Lab Invest. 1985 May;52(5):505–514. [Abstract] [Google Scholar]
- Brody AR, Hill LH. Interstitial accumulation of inhaled chrysotile asbestos fibers and consequent formation of microcalcifications. Am J Pathol. 1982 Oct;109(1):107–114. [Europe PMC free article] [Abstract] [Google Scholar]
- Bowden DH, Adamson IY. Bronchiolar and alveolar lesions in the pathogenesis of crocidolite-induced pulmonary fibrosis in mice. J Pathol. 1985 Dec;147(4):257–267. [Abstract] [Google Scholar]
- Hamilton JA. Macrophage stimulation and the inflammatory response to asbestos. Environ Health Perspect. 1980 Feb;34:69–74. [Europe PMC free article] [Abstract] [Google Scholar]
- Werb Z. How the macrophage regulates its extracellular environment. Am J Anat. 1983 Mar;166(3):237–256. [Abstract] [Google Scholar]
- Whitaker D, Papadimitriou JM, Walters MN. The mesothelium and its reactions: a review. Crit Rev Toxicol. 1982 Apr;10(2):81–144. [Abstract] [Google Scholar]
- Raftery AT. Regeneration of parietal and visceral peritoneum in the immature animal: a light and electron microscopical study. Br J Surg. 1973 Dec;60(12):969–975. [Abstract] [Google Scholar]
- Bolen JW, Hammar SP, McNutt MA. Reactive and neoplastic serosal tissue. A light-microscopic, ultrastructural, and immunocytochemical study. Am J Surg Pathol. 1986 Jan;10(1):34–47. [Abstract] [Google Scholar]
- Winkler GC, Rüttner JR. Early fibrogenicity of asbestos fibers in visceral peritoneum. Exp Cell Biol. 1983;51(1):1–8. [Abstract] [Google Scholar]
- Hillerdal G. The pathogenesis of pleural plaques and pulmonary asbestosis: possibilities and impossibilities. Eur J Respir Dis. 1980 Jun;61(3):129–138. [Abstract] [Google Scholar]
- Herbert A. Pathogenesis of pleurisy, pleural fibrosis, and mesothelial proliferation. Thorax. 1986 Mar;41(3):176–189. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamilton J, Vassalli JD, Reich E. Macrophage plasminogen activator: induction by asbestos is blocked by anti-inflammatory steroids. J Exp Med. 1976 Dec 1;144(6):1689–1694. [Europe PMC free article] [Abstract] [Google Scholar]
- Whitaker D, Papadimitriou JM, Walters M. The mesothelium: its fibrinolytic properties. J Pathol. 1982 Apr;136(4):291–299. [Abstract] [Google Scholar]
Associated Data
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/128/3/426
Citations & impact
Impact metrics
Citations of article over time
Article citations
Quantitative Assessment of Asbestos Fibers in Abdominal Organs: A Scoping Review.
Med Lav, 114(6):e2023048, 07 Dec 2023
Cited by: 0 articles | PMID: 38060208 | PMCID: PMC10731569
Review Free full text in Europe PMC
Late Inflammation Induced by Asbestiform Fibers in Mice Is Ameliorated by a Small Molecule Synthetic Lignan.
Int J Mol Sci, 22(20):10982, 12 Oct 2021
Cited by: 1 article | PMID: 34681644 | PMCID: PMC8537122
A liquid biopsy for detecting circulating mesothelial precursor cells: A new biomarker for diagnosis and prognosis in mesothelioma.
EBioMedicine, 61:103031, 09 Oct 2020
Cited by: 6 articles | PMID: 33045471 | PMCID: PMC7553233
3D-Printed Lab-on-a-Chip Diagnostic Systems-Developing a Safe-by-Design Manufacturing Approach.
Micromachines (Basel), 10(12):E825, 28 Nov 2019
Cited by: 3 articles | PMID: 31795128 | PMCID: PMC6969929
Review Free full text in Europe PMC
The Biology of Malignant Mesothelioma and the Relevance of Preclinical Models.
Front Oncol, 10:388, 25 Mar 2020
Cited by: 19 articles | PMID: 32269966 | PMCID: PMC7109283
Review Free full text in Europe PMC
Go to all (72) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pathogenesis of mesothelial reactions to asbestos fibers. Monocyte recruitment and macrophage activation.
Pathobiology, 61(3-4):154-163, 01 Jan 1993
Cited by: 9 articles | PMID: 8216837
Induction of angiogenesis by intraperitoneal injection of asbestos fibers.
FASEB J, 3(6):1747-1752, 01 Apr 1989
Cited by: 14 articles | PMID: 2467835
A study of surface property changes in rat mesothelial cells induced by asbestos using aqueous two-phase polymer solutions.
Biochim Biophys Acta, 1181(3):223-232, 01 Jun 1993
Cited by: 5 articles | PMID: 7686399
Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: pathological evidence.
Int J Hyg Environ Health, 208(3):201-210, 01 Jan 2005
Cited by: 58 articles | PMID: 15971859
Review
Funding
Funders who supported this work.
NIEHS NIH HHS (3)
Grant ID: R01 ES 03189
Grant ID: R01 ES 03721
Grant ID: KO4 ES 00127