Abstract
Free full text

1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: a novel class of neutrophil stimulants.
Abstract
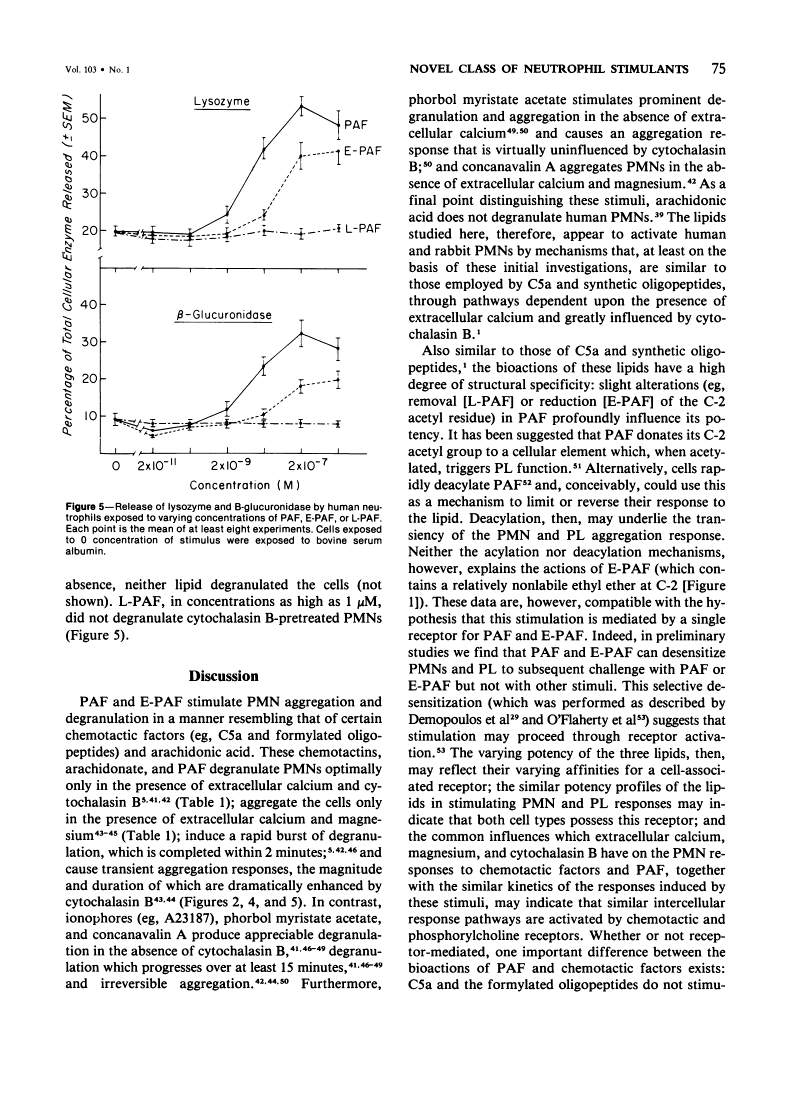
1-O-Alkyl-2-O-acetyl-sn-glyceryl-3-phosphorylcholine aggregates and degranulates platelets and polymorphonuclear neutrophils. Here, the bioactivities of this platelet-activating factor, its 2-O-ethyl, and its 2-lyso derivatives were examined further. Each phospholipid aggregated and degranulated rabbit platelets and neutrophils with relative potencies of about 10,000 1,000, and 1, respectively. For rabbit neutrophils, and 2-O-acetyl compound was active in nanomolar and lower concentrations; required extracellular calcium and magnesium in order to induce aggregation; and required extracellular calcium and cytochlasin B in order to induce optimal degranulation. Furthermore the 2-O-acetyl and 2-O-ethyl compounds, in concentrations about tenfold higher than those required for rabbit neutrophils, aggregated and degranulated human neutrophils. With reference to these human neutrophil responses, degranulation required, and aggregation was dramatically enhanced by, cytochalasin B. The lysoanalog was unable to induce these response in the human cells. Thus, these lipids represent a novel class of neutrophil stimulants that closely resemble certain chemotactic factors (eg, C5a and synthetic oligopeptides) in their ability to aggregate and degranulate neutrophils and in the influences which calcium, magnesium and cytochalasin B have on their bioactions. Because platelet-activating factor circulates in the blood of rabbits and, perhaps, humans during anaphylaxis and is suspected of being involved in other syndromes such as serum sickness, this lipid may have unique biologic significance: it may act to recruit platelets and neutrophils into the lesions of these and similar pathologic syndromes.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- O'Flaherty JT, Ward PA. Chemotactic factors and the neutrophil. Semin Hematol. 1979 Apr;16(2):163–174. [Abstract] [Google Scholar]
- O'Flaherty JT, Cousart S, Lineberger AS, Bond E, Bass DA, DeChatelet LR, Leake ES, McCall CE. Phorbol myristate acetate: in vivo effects upon neutrophils, platelets, and lung. Am J Pathol. 1980 Oct;101(1):79–92. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Flaherty JT, Kreutzer DL, Ward PA. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [Europe PMC free article] [Abstract] [Google Scholar]
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. [Abstract] [Google Scholar]
- Naccache PH, Showell HJ, Becker EL, Sha'afi RI. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. [Abstract] [Google Scholar]
- Stenson WF, Parker CW. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol. 1980 May;124(5):2100–2104. [Abstract] [Google Scholar]
- Henson PM, Cochrane CG. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. [Europe PMC free article] [Abstract] [Google Scholar]
- Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. [Europe PMC free article] [Abstract] [Google Scholar]
- Benveniste J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature. 1974 Jun 7;249(457):581–582. [Abstract] [Google Scholar]
- Benveniste J, Egido J, Gutierrez-Millet V. Evidence for the involvement of IgE-basophil system in acute serum sickness. Clin Exp Immunol. 1976 Dec;26(3):449–456. [Abstract] [Google Scholar]
- Camussi G, Mencia-Huerta JM, Benveniste J. Release of platelet-activating factor and histamine. I. Effect of immune complexes, complement and neutrophils on human and rabbit mastocytes and basophils. Immunology. 1977 Oct;33(4):523–534. [Abstract] [Google Scholar]
- Benveniste J, Camussi J, Polonsky J. Platelet-activating factor. Monogr Allergy. 1977;12:138–142. [Abstract] [Google Scholar]
- Barbaro JF, Zvaifler NJ. Antigen induced histamine release from platelets of rabbits producing homologous PCA antibody. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1245–1247. [Abstract] [Google Scholar]
- Henson PM. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. [Europe PMC free article] [Abstract] [Google Scholar]
- Halonen M, Fisher HK, Blair C, Butler C, 2nd, Pinckard RN. IgE-induced respiratory and circulatory changes during systemic anaphylaxis in the rabbit. Am Rev Respir Dis. 1976 Nov;114(5):961–970. [Abstract] [Google Scholar]
- Halonen M, Pinckard RN. Intravascular effects of IgE antibody upon basophils,, neutrophils, platelets and blood coagulation in the rabbit. J Immunol. 1975 Aug;115(2):519–524. [Abstract] [Google Scholar]
- Henson PM, Pinckard RN. Basophil-derived platelet-activating factor (PAF) as an in vivo mediator of acute allergic reactions: demonstration of specific desensitization of platelets to PAF during IgE-induced anaphylaxis in the rabbit. J Immunol. 1977 Dec;119(6):2179–2184. [Abstract] [Google Scholar]
- Pinckard RN, Halonen M, Palmer JD, Butler C, Shaw JO, Henson PM. Intravascular aggregation and pulmonary sequestration of platelets during IgE-induced systemic anaphylaxis in the rabbit: abrogation of lethal anaphylactic shock by platelet depletion. J Immunol. 1977 Dec;119(6):2185–2193. [Abstract] [Google Scholar]
- Henson PM, Pinckard RN. Platelet activating factor (PAF). A possible direct mediator of anaphylaxis in the rabbit and a trigger for the vascular deposition of circulating immune complexes. Monogr Allergy. 1977;12:13–26. [Abstract] [Google Scholar]
- Pinckard RN, Farr RS, Hanahan DJ. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol. 1979 Oct;123(4):1847–1857. [Abstract] [Google Scholar]
- Siraganian RP, Osler AG. Destruction of rabbit platelets in the allergic response of sensitized leukocytes. I. Demonstration of a fluid phase intermediate. J Immunol. 1971 May;106(5):1244–1251. [Abstract] [Google Scholar]
- Lewis RA, Goetzl EJ, Wasserman SI, Valone FH, Rubin RH, Austen KF. The release of four mediators of immediate hypersensitivity from human leukemic basophils. J Immunol. 1975 Jan;114(1 Pt 1):87–92. [Abstract] [Google Scholar]
- Henson PM. Activation and desensitization of platelets by platelet-activating factor (PAF) derived from IgE-sensitized basophils. I. Characteristics of the secretory response. J Exp Med. 1976 Apr 1;143(4):937–952. [Europe PMC free article] [Abstract] [Google Scholar]
- Clark PO, Hanahan DJ, Pinckard RN. Physical and chemical properties of platelet-activating factor obtained from human neutrophils and monocytes and rabbit neutrophils and basophils. Biochim Biophys Acta. 1980 Feb 21;628(1):69–75. [Abstract] [Google Scholar]
- Kravis TC, Henson PM. IgE-induced release of a platelet-activating factor from rabbit lung. J Immunol. 1975 Dec;115(6):1677–1681. [Abstract] [Google Scholar]
- Lynch JM, Lotner GZ, Betz SJ, Henson PM. The release of a platelet-activating factor by stimulated rabbit neutrophils. J Immunol. 1979 Sep;123(3):1219–1226. [Abstract] [Google Scholar]
- Lotner GZ, Lynch JM, Betz SJ, Henson PM. Human neutrophil-derived platelet activating factor. J Immunol. 1980 Feb;124(2):676–684. [Abstract] [Google Scholar]
- Demopoulos CA, Pinckard RN, Hanahan DJ. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [Abstract] [Google Scholar]
- Benveniste J, Tencé M, Varenne P, Bidault J, Boullet C, Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [Abstract] [Google Scholar]
- Blank ML, Snyder F, Byers LW, Brooks B, Muirhead EE. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. [Abstract] [Google Scholar]
- Cochrane CG, Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. [Abstract] [Google Scholar]
- McManus LM, Hanahan DJ, Demopoulos CA, Pinckard RN. Pathobiology of the intravenous infusion of acetyl glyceryl ether phosphorylcholine (AGEPC), a synthetic platelet-activating factor (PAF), in the rabbit. J Immunol. 1980 Jun;124(6):2919–2924. [Abstract] [Google Scholar]
- Gupta CM, Radhakrishnan R, Khorana HG. Glycerophospholipid synthesis: improved general method and new analogs containing photoactivable groups. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4315–4319. [Europe PMC free article] [Abstract] [Google Scholar]
- Rouser G, Siakotos AN, Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. [Abstract] [Google Scholar]
- O'Flaherty JT, Showell HJ, Ward PA, Becker EL. A possible role of arachidonic acid in human neutrophil aggregation and degranulation. Am J Pathol. 1979 Sep;96(3):799–810. [Europe PMC free article] [Abstract] [Google Scholar]
- Wykle RL, Kraemer WF, Schremmer JM. Specificity of lysophospholipase D. Biochim Biophys Acta. 1980 Jul 14;619(1):58–67. [Abstract] [Google Scholar]
- O'Flaherty JT. Involvement of bivalent cations and arachidonic acid in neutrophil aggregation. Inflammation. 1980 Jun;4(2):181–194. [Abstract] [Google Scholar]
- Bentwood BJ, Henson PM. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [Abstract] [Google Scholar]
- Hoffstein S, Soberman R, Goldstein I, Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. [Europe PMC free article] [Abstract] [Google Scholar]
- White JG, Estensen RD. Cytochemical electron microscopic studies of the action of phorbol myristate acetate on platelets. Am J Pathol. 1974 Mar;74(3):453–466. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldstein IM, Horn JK, Kaplan HB, Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. [Abstract] [Google Scholar]
- O'Flaherty JT, Dechatelet LR, McCall CE, Bass DA. Neutrophil aggregation: evidence for a different mechanism of action by phorbol myristate acetate. Proc Soc Exp Biol Med. 1980 Nov;165(2):225–232. [Abstract] [Google Scholar]
- Hanahan DJ, Demopoulos CA, Liehr J, Pinckard RN. Identification of platelet activating factor isolated from rabbit basophils as acetyl glyceryl ether phosphorylcholine. J Biol Chem. 1980 Jun 25;255(12):5514–5516. [Abstract] [Google Scholar]
- O'Flaherty JT, Kreutzer DL, Showell HJ, Vitkauskas G, Becker EL, Ward PA. Selective neutrophil desensitization to chemotactic factors. J Cell Biol. 1979 Mar;80(3):564–572. [Europe PMC free article] [Abstract] [Google Scholar]
- Craddock PR, Fehr J, Dalmasso AP, Brighan KL, Jacob HS. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Flaherty JT, Craddock PR, Jacob HS. Effect of intravascular complement activation on granulocyte adhesiveness and distribution. Blood. 1978 Apr;51(4):731–739. [Abstract] [Google Scholar]
- Craddock PR, Hammerschmidt D, White JG, Dalmosso AP, Jacob HS. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Flaherty JT, Kreutzer DL, Ward PA. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [Abstract] [Google Scholar]
- Silver MJ, Hoch W, Kocsis JJ, Ingerman CM, Smith JB. Arachidonic acid causes sudden death in rabbits. Science. 1974 Mar 15;183(4129):1085–1087. [Abstract] [Google Scholar]
- Willis AL. Isolation of a chemical trigger for thrombosis. Prostaglandins. 1974 Jan 10;5(1):1–25. [Abstract] [Google Scholar]
- Willis AL, Vane FM, Kuhn DC, Scott CG, Petrin M. An endoperoxide aggregator (Lass), formed in platelets in response to thrombotic stimuli: purification, identification and unique biological significance. Prostaglandins. 1974 Dec 25;8(6):453–507. [Abstract] [Google Scholar]
- Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. [Europe PMC free article] [Abstract] [Google Scholar]
- Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980 May 3;1(8175):947–949. [Abstract] [Google Scholar]
- Hammerschmidt DE, Bowers TK, Lammi-Keefe CJ, Jacob HS, Craddock PR. Granulocyte aggregometry: a sensitive technique for the detection of C5a and complement activation. Blood. 1980 Jun;55(6):898–902. [Abstract] [Google Scholar]
Associated Data
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/103/1/70
Citations & impact
Impact metrics
Citations of article over time
Article citations
Human neutrophil formyl peptide receptor phosphorylation and the mucosal inflammatory response.
J Leukoc Biol, 97(1):87-101, 13 Nov 2014
Cited by: 15 articles | PMID: 25395303 | PMCID: PMC4377828
Immunomodulatory effects of the ether phospholipid edelfosine in experimental autoimmune encephalomyelitis.
J Neuroimmunol, 274(1-2):111-124, 21 Jul 2014
Cited by: 5 articles | PMID: 25086877
Platelet-activating factor (PAF) receptor binding activity of the roots of Enicosanthellum pulchrum.
Pharm Biol, 50(3):284-290, 21 Nov 2011
Cited by: 5 articles | PMID: 22103812
Role of platelets in neuroinflammation: a wide-angle perspective.
J Neuroinflammation, 7:10, 03 Feb 2010
Cited by: 56 articles | PMID: 20128908 | PMCID: PMC2829540
Review Free full text in Europe PMC
Platelet-activating factor interaction with the human erythrocyte membrane.
J Biochem Mol Toxicol, 23(5):345-348, 01 Sep 2009
Cited by: 10 articles | PMID: 19827129
Go to all (131) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Role of extracellular calcium and neutrophil degranulation responses to 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine.
Am J Pathol, 105(2):107-113, 01 Nov 1981
Cited by: 14 articles | PMID: 6271016 | PMCID: PMC1903872
Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor).
J Immunol, 127(3):1250-1255, 01 Sep 1981
Cited by: 211 articles | PMID: 6267133
Selective desensitization of neutrophils: further studies with 1-O-alkyl-sn-glycero-3-phosphocholine analogues.
J Immunol, 127(2):731-737, 01 Aug 1981
Cited by: 38 articles | PMID: 7252157
Platelet activating factor (1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine). Activity of analogs lacking oxygen at the 2-position.
FEBS Lett, 141(1):29-32, 01 May 1982
Cited by: 12 articles | PMID: 7084476