Abstract
Free full text

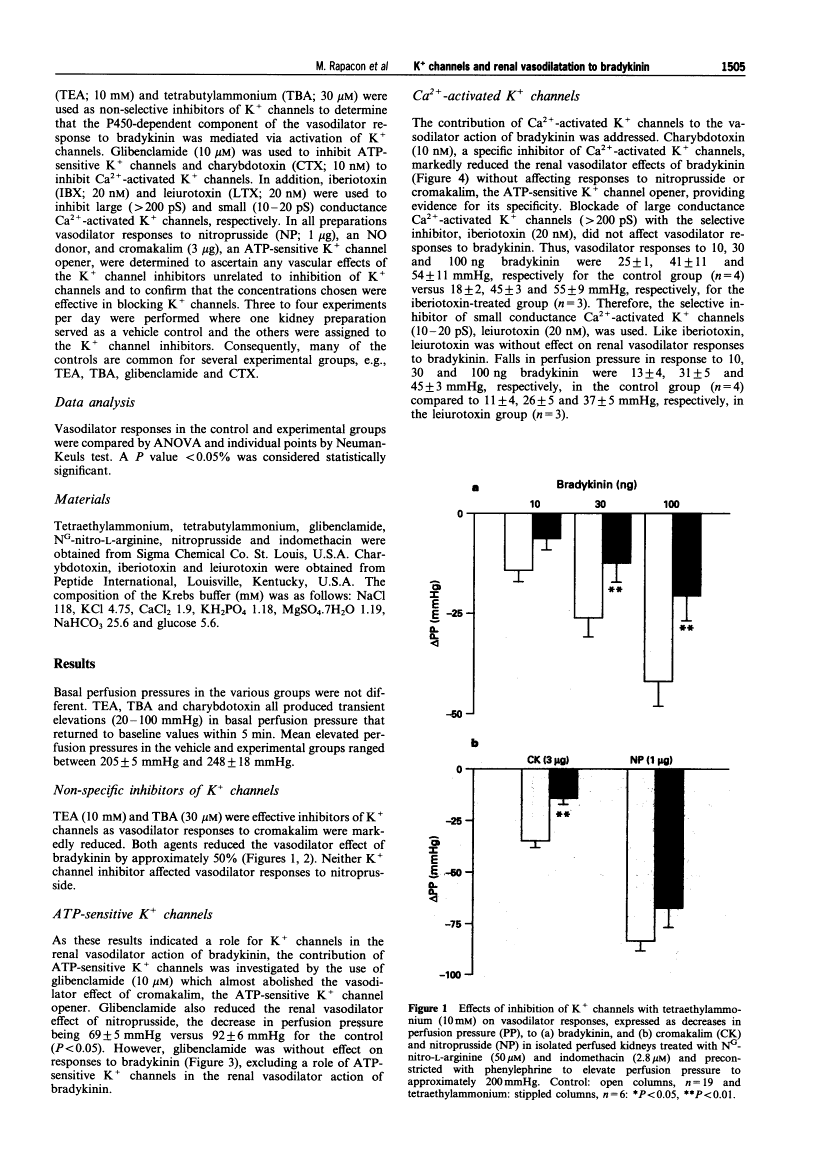
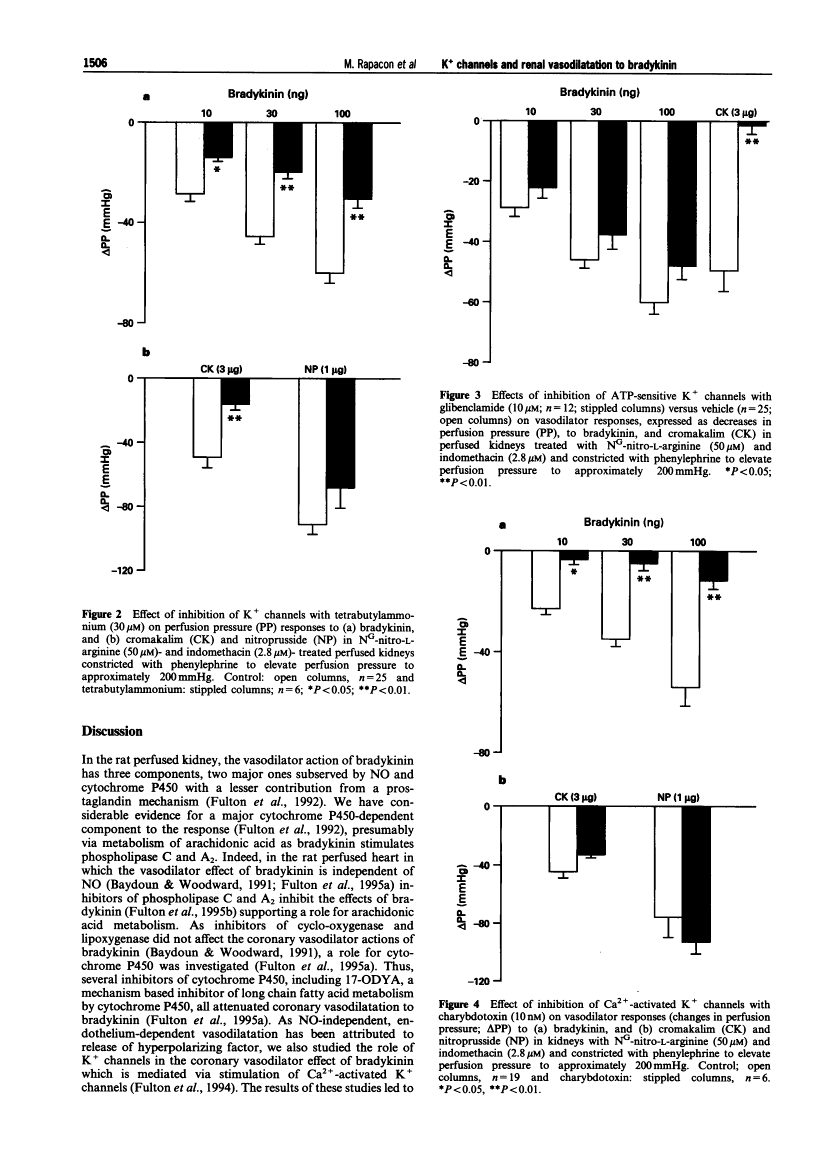
Contribution of calcium-activated potassium channels to the vasodilator effect of bradykinin in the isolated, perfused kidney of the rat.
Abstract
1. NO- and prostaglandin-independent, endothelium-dependent vasodilator responses to bradykinin are attributed to release of a hyperpolarizing factor. Therefore, the contribution of K+ channels to the renal vasodilator effect of bradykinin was examined in rat perfused kidneys that were preconstricted with phenylephrine and treated with NG-nitro-L-arginine (L-NOARG) and indomethacin to inhibit NO and prostaglandin synthesis. 2. The non-specific K+ channel inhibitors, TEA and TBA reduced vasodilator responses to bradykinin and cromakalim but not those to nitroprusside. 3. Glibenclamide, an inhibitor of ATP-sensitive K+ channels, blocked the vasodilator response to cromakalim without affecting responses to bradykinin. 4. Charybdotoxin, a selective inhibitor of Ca(2+)-activated K+ channels, greatly attenuated vasodilator responses to bradykinin without affecting those to cromakalim or nitroprusside. 5. Iberiotoxin and leiurotoxin, inhibitors of large and small conductance Ca(2+)-activated K+ channels, respectively, were without effect on vasodilator responses to bradykinin, cromakalim or nitroprusside. 6. These results implicate K+ channels, specifically Ca(2+)-activated K+ channels of intermediate conductance, in the renal vasodilator effect of bradykinin and, thereby, support a role for a hyperpolarizing factor.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baydoun AR, Woodward B. Effects of bradykinin in the rat isolated perfused heart: role of kinin receptors and endothelium-derived relaxing factor. Br J Pharmacol. 1991 Jul;103(3):1829–1833. [Europe PMC free article] [Abstract] [Google Scholar]
- Bény JL, Brunet PC. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25(6):308–311. [Abstract] [Google Scholar]
- Cachofeiro V, Nasjletti A. Increased vascular responsiveness to bradykinin in kidneys of spontaneously hypertensive rats. Effect of N omega-nitro-L-arginine. Hypertension. 1991 Nov;18(5):683–688. [Abstract] [Google Scholar]
- Chicchi GG, Gimenez-Gallego G, Ber E, Garcia ML, Winquist R, Cascieri MA. Purification and characterization of a unique, potent inhibitor of apamin binding from Leiurus quinquestriatus hebraeus venom. J Biol Chem. 1988 Jul 25;263(21):10192–10197. [Abstract] [Google Scholar]
- Cowan CL, Cohen RA. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and -independent responses. Am J Physiol. 1991 Sep;261(3 Pt 2):H830–H835. [Abstract] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. [Europe PMC free article] [Abstract] [Google Scholar]
- Fulton D, Mahboubi K, McGiff JC, Quilley J. Cytochrome P450-dependent effects of bradykinin in the rat heart. Br J Pharmacol. 1995 Jan;114(1):99–102. [Europe PMC free article] [Abstract] [Google Scholar]
- Fulton D, McGiff JC, Quilley J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br J Pharmacol. 1992 Nov;107(3):722–725. [Europe PMC free article] [Abstract] [Google Scholar]
- Fulton D, McGiff JC, Quilley J. Role of K+ channels in the vasodilator response to bradykinin in the rat heart. Br J Pharmacol. 1994 Nov;113(3):954–958. [Europe PMC free article] [Abstract] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [Abstract] [Google Scholar]
- Johns A, Lategan TW, Lodge NJ, Ryan US, Van Breemen C, Adams DJ. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. [Abstract] [Google Scholar]
- Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995 Sep;47(3):387–573. [Abstract] [Google Scholar]
- Oyekan AO, McGiff JC, Rosencrantz-Weiss P, Quilley J. Relaxant responses of rabbit aorta: influence of cytochrome P450 inhibitors. J Pharmacol Exp Ther. 1994 Jan;268(1):262–269. [Abstract] [Google Scholar]
- Rusko J, Tanzi F, van Breemen C, Adams DJ. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. [Abstract] [Google Scholar]
- Vane JR, Anggård EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. [Abstract] [Google Scholar]
Associated Data
Articles from British Journal of Pharmacology are provided here courtesy of The British Pharmacological Society
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1476-5381.1996.tb15566.x
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/j.1476-5381.1996.tb15566.x
Citations & impact
Impact metrics
Citations of article over time
Article citations
Role of renal vascular potassium channels in physiology and pathophysiology.
Acta Physiol (Oxf), 221(1):14-31, 25 Apr 2017
Cited by: 13 articles | PMID: 28371470
Review
KCa 3.1 channels maintain endothelium-dependent vasodilatation in isolated perfused kidneys of spontaneously hypertensive rats after chronic inhibition of NOS.
Br J Pharmacol, 167(4):854-867, 01 Oct 2012
Cited by: 16 articles | PMID: 22646737 | PMCID: PMC3575784
Role of vascular potassium channels in the regulation of renal hemodynamics.
Am J Physiol Renal Physiol, 302(5):F505-18, 14 Dec 2011
Cited by: 24 articles | PMID: 22169005
Review
Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses.
Pflugers Arch, 459(6):897-914, 09 Apr 2010
Cited by: 96 articles | PMID: 20379740
Review
K(+)-induced vasodilation in the rat kidney is dependent on the endothelium and activation of K+ channels.
Eur J Pharmacol, 508(1-3):193-199, 07 Jan 2005
Cited by: 5 articles | PMID: 15680271
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
NO-independent vasodilation to acetylcholine in the rat isolated kidney utilizes a charybdotoxin-sensitive, intermediate-conductance Ca(++)-activated K+ channel.
J Pharmacol Exp Ther, 285(2):659-664, 01 May 1998
Cited by: 14 articles | PMID: 9580610
Role of K+ channels in the vasodilator response to bradykinin in the rat heart.
Br J Pharmacol, 113(3):954-958, 01 Nov 1994
Cited by: 37 articles | PMID: 7858891 | PMCID: PMC1510451
Involvement of nitric oxide and potassium channels in the bradykinin-induced vasodilatation in the rat kidney perfused ex situ.
Regul Pept, 105(3):155-162, 01 May 2002
Cited by: 3 articles | PMID: 11959369
Role of non-nitric oxide non-prostaglandin endothelium-derived relaxing factor(s) in bradykinin vasodilation.
Braz J Med Biol Res, 31(9):1229-1235, 01 Sep 1998
Cited by: 10 articles | PMID: 9876291
Review