Abstract
Free full text

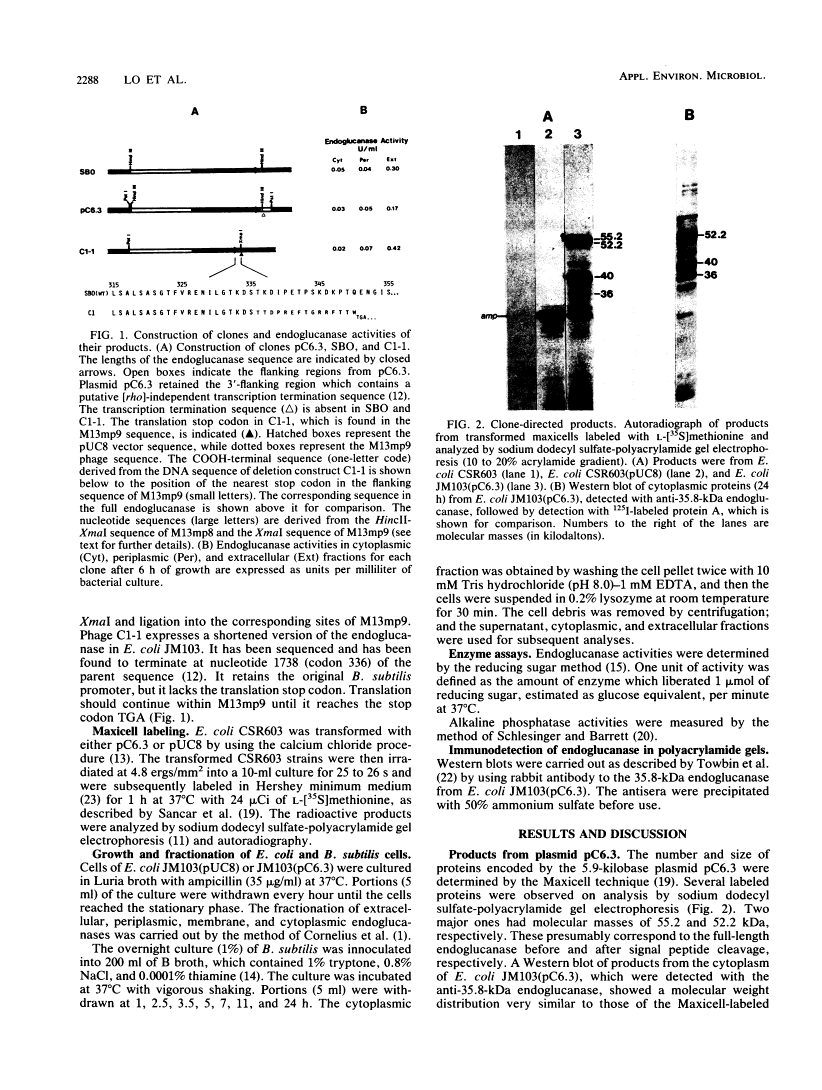
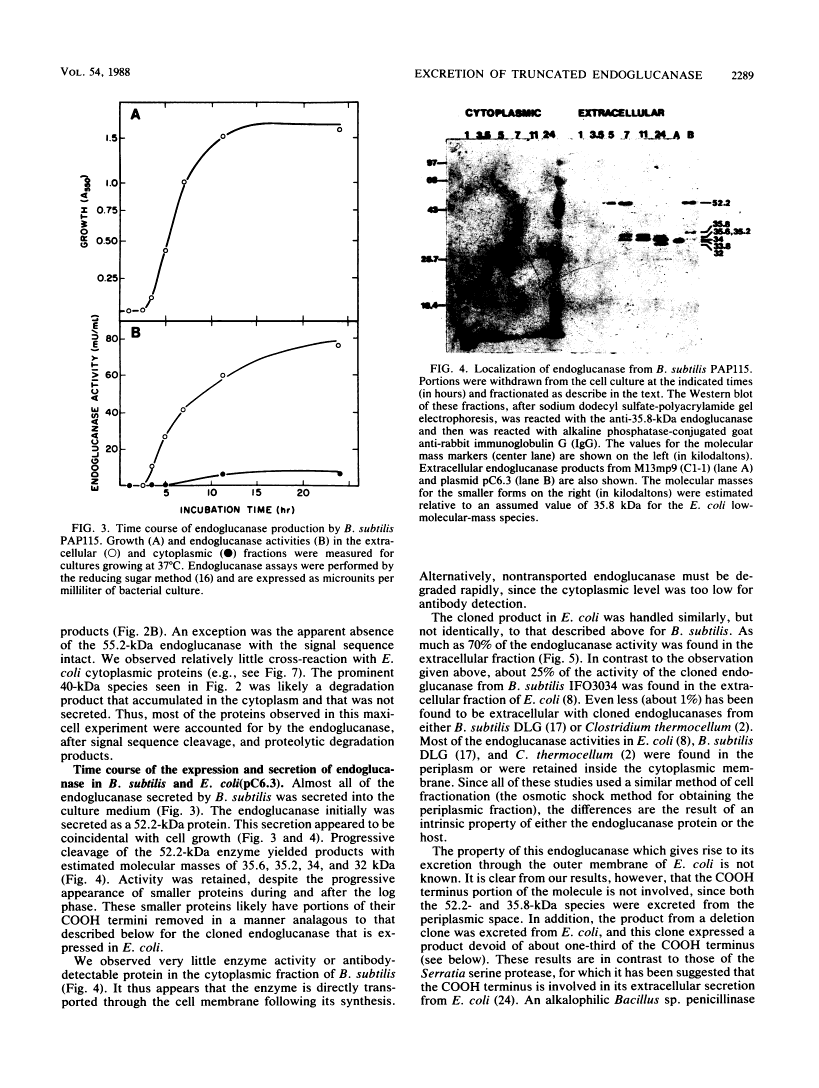
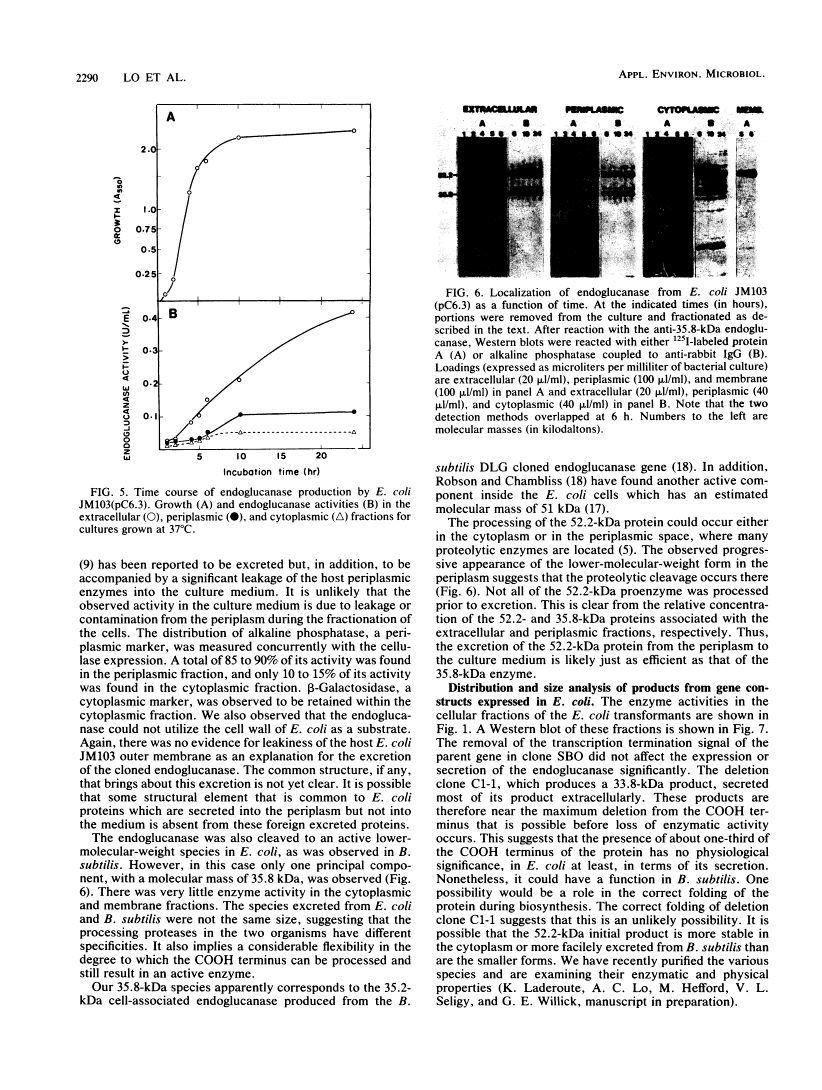
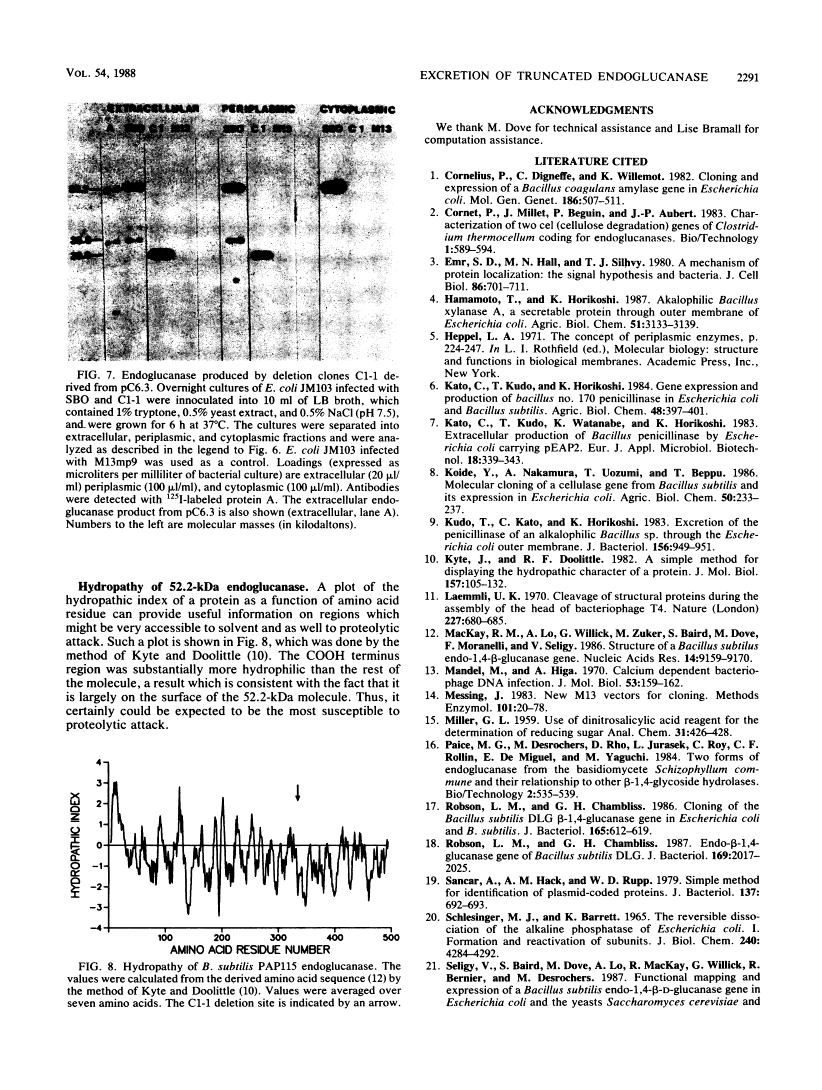
Bacillus subtilis β-1, 4-Endoglucanase Products from Intact and Truncated Genes Are Secreted into the Extracellular Medium by Escherichia coli†
Abstract
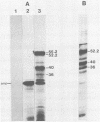
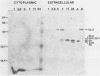
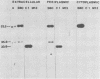
We compared the secretion of a Bacillus subtilis endo-β-1,4-glucanase (EC 3.2.1.4) in B. subtilis and of the product from the cloned gene (pC6.3) expressed in Escherichia coli. The cloned enzyme has been isolated previously as the 52.2-kilodalton (kDa) species predicted from the gene sequence (R. M. MacKay, A. Lo, G. Willick, M. Zuker, S. Baird, M. Dove, F. Moranelli, and V. Seligy, Nucleic Acids Res., 14:9159-9170, 1986); this 52.2-kDa species is then converted to an active 35.8-kDa species. The 35.8-kDa species has a segment removed from the COOH terminus. Endoglucanase products were identified by use of an antibody directed to the 35.8-kDa enzyme. Time course studies of the secretion in B. subtilis showed that the enzyme was first secreted as a 52.2-kDa proenzyme. This was cleaved progressively to a product of about 32 kDa. Time course analysis of the expression of the cloned product from pC6.3 in E. coli showed that about 70% of the endoglucanase activity was found extracellularly. Analysis of active products from three deletion clones showed that the expression pattern of the endoglucanase was not affected by removal of the transcription termination signal and that neither expression nor secretion was substantially altered by removal of a region coding for up to 163 residues of the carboxyl terminus.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornelis P, Digneffe C, Willemot K. Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet. 1982;186(4):507–511. [Abstract] [Google Scholar]
- Emr SD, Hall MN, Silhavy TJ. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. [Europe PMC free article] [Abstract] [Google Scholar]
- Kudo T, Kato C, Horikoshi K. Excretion of the penicillinase of an alkalophilic Bacillus sp. through the Escherichia coli outer membrane. J Bacteriol. 1983 Nov;156(2):949–951. [Europe PMC free article] [Abstract] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- MacKay RM, Lo A, Willick G, Zuker M, Baird S, Dove M, Moranelli F, Seligy V. Structure of a Bacillus subtilis endo-beta-1,4-glucanase gene. Nucleic Acids Res. 1986 Nov 25;14(22):9159–9170. [Europe PMC free article] [Abstract] [Google Scholar]
- Mandel M, Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. [Abstract] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. [Abstract] [Google Scholar]
- Robson LM, Chambliss GH. Cloning of the Bacillus subtilis DLG beta-1,4-glucanase gene and its expression in Escherichia coli and B. subtilis. J Bacteriol. 1986 Feb;165(2):612–619. [Europe PMC free article] [Abstract] [Google Scholar]
- Robson LM, Chambliss GH. Endo-beta-1,4-glucanase gene of Bacillus subtilis DLG. J Bacteriol. 1987 May;169(5):2017–2025. [Europe PMC free article] [Abstract] [Google Scholar]
- Sancar A, Hack AM, Rupp WD. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. [Europe PMC free article] [Abstract] [Google Scholar]
- Schlesinger MJ, Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [Abstract] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. [Europe PMC free article] [Abstract] [Google Scholar]
- Worcel A, Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. [Abstract] [Google Scholar]
- Yanagida N, Uozumi T, Beppu T. Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli. J Bacteriol. 1986 Jun;166(3):937–944. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Applied and Environmental Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aem.54.9.2287-2292.1988
Read article for free, from open access legal sources, via Unpaywall:
https://aem.asm.org/content/aem/54/9/2287.full.pdf
Free to read at aem.asm.org
http://aem.asm.org/cgi/content/abstract/54/9/2287
Free after 4 months at aem.asm.org
http://aem.asm.org/cgi/reprint/54/9/2287
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aem.54.9.2287-2292.1988
Article citations
A unique self-truncation of bacterial GH5 endoglucanases leads to enhanced activity and thermostability.
BMC Biol, 20(1):137, 09 Jun 2022
Cited by: 1 article | PMID: 35681203 | PMCID: PMC9185962
A comparative study of β-1, 4-endoglucanase (possessing β-1, 4-exoglucanase activity) from Bacillus subtilis LH expressed in Pichia pastoris GS115 and Escherichia coli Rosetta (DE3).
Bioresour Technol, 110:539-545, 02 Jan 2012
Cited by: 16 articles | PMID: 22336741
Enhancing functional expression of Heterologous Burkholderia lipase in Escherichia coli.
Mol Biotechnol, 47(2):130-143, 01 Feb 2011
Cited by: 6 articles | PMID: 20730511
Improved catalytic efficiency of endo-beta-1,4-glucanase from Bacillus subtilis BME-15 by directed evolution.
Appl Microbiol Biotechnol, 82(4):671-679, 03 Dec 2008
Cited by: 30 articles | PMID: 19050861
Purification and characterization of an arabinofuranosidase from Bacillus polymyxa expressed in Bacillus subtilis.
Appl Microbiol Biotechnol, 44(1-2):112-117, 01 Dec 1995
Cited by: 10 articles | PMID: 8579824
Go to all (17) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cloning and expression of β-1, 4-endoglucanase gene from Bacillus subtilis isolated from soil long term irrigated with effluents of paper and pulp mill.
Microbiol Res, 169(9-10):693-698, 25 Feb 2014
Cited by: 11 articles | PMID: 24636744
Nucleotide sequence of the gene for an alkaline endoglucanase from an alkalophilic Bacillus and its expression in Escherichia coli and Bacillus subtilis.
Biosci Biotechnol Biochem, 56(6):872-877, 01 Jun 1992
Cited by: 15 articles | PMID: 1368251
Construction, purification, and properties of a truncated alkaline endoglucanase from Bacillus sp. KSM-635.
Biosci Biotechnol Biochem, 59(9):1613-1618, 01 Sep 1995
Cited by: 4 articles | PMID: 8520106
Recombinant expression and characterization of a novel endoglucanase from Bacillus subtilis in Escherichia coli.
Mol Biol Rep, 41(5):3295-3302, 04 Feb 2014
Cited by: 13 articles | PMID: 24493451