Abstract
Free full text

Regulation of Synaptic Transmission and Plasticity by Neuronal Nicotinic Acetylcholine Receptors
Abstract
Nicotinic acetylcholine receptors (nAChRs) are widely expressed throughout the central nervous system and participate in a variety of physiological functions. Recent advances have revealed roles of nAChRs in the regulation of synaptic transmission and synaptic plasticity, particularly in the hippocampus and midbrain dopamine centers. In general, activation of nAChRs causes membrane depolarization and directly and indirectly increases the intracellular calcium concentration. Thus, when nAChRs are expressed on presynaptic membranes their activation generally increases the probability of neurotransmitter release. When expressed on postsynaptic membranes, nAChR-initiated calcium signals and depolarization activate intracellular signaling mechanisms and gene transcription. Together, the presynaptic and postsynaptic effects of nAChRs generate and facilitate the induction of long-term changes in synaptic transmission. The direction of hippocampal nAChR-mediated synaptic plasticity –either potentiation or depression – depends on the timing of nAChR activation relative to coincident presynaptic and postsynaptic electrical activity, and also depends on the location of cholinergic stimulation within the local network. Therapeutic activation of nAChRs may prove efficacious in the treatment of neuropathologies where synaptic transmission is compromised, as in Alzheimer’s or Parkinson’s disease.
Neuronal nicotinic acetylcholine receptors
Receptor structure
Nicotinic acetylcholine receptors (nAChRs) are widely expressed throughout the central nervous system [1]. Each receptor/channel consists of five subunits surrounding a water-filled, cation-permeable pore [2, 3] (Fig. 1). Each nAChR subunit has a similar general linear structure and transmembrane topology. The nAChR subunits have a large extracellular N-terminal domain that contributes to ligand binding, followed by three hydrophobic transmembrane regions (M1 to M3), a large intracellular loop, a fourth transmembrane region (M4), and finally a short extracellular C-terminus (Fig. 1A) [4]. The M2 transmembrane segment in each subunit provides the main lining of the ionic pore with some contribution from M1. The M1, M3 and M4 segments separate the pore-lining region from the hydrophobic membrane [5]. Subunits α2 through α10 have been cloned and are homologous to the α1 subunit identified in muscle [6–9]. The neuronal non-α subunits, β2 through β4, have also been identified [6–9]. Many neuronal nAChRs are assembled as αβ hetero-oligomers with a typical α:β stoichiometry of 2:3. In brain, the most common hetero-oligomeric nAChRs are assembled from α4 and β2 subunits (Fig. 1B) [3, 10, 11]. More complex hetero-oligomeric compositions are also possible, such as the α4α6β2 nAChRs commonly distributed within midbrain dopamine areas. Homo-oligomeric nAChRs are only formed by α7, α8 or α9 subunits, with α7 homo-oligomers being the only ones widely distributed in the brain (Fig. 1B) [10, 12, 13]. Accumulating evidence reveals that α7-containing (α7*) and β2-containing (β2*) nAChRs are expressed at preterminal, axonal, somatic and dendritic locations [10, 14–20].
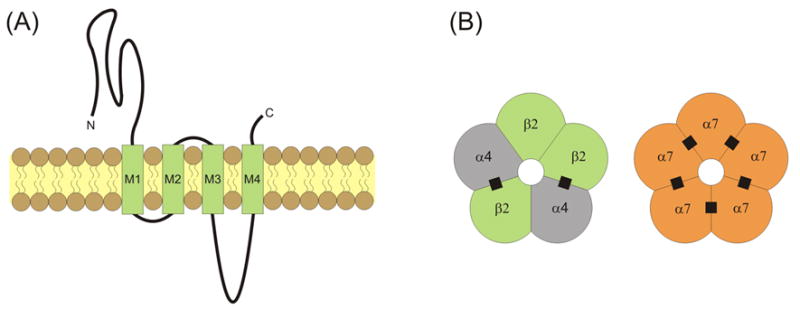
Transmembrane topology and pentameric structure of nAChRs. (A) nAChRs consist of four transmembrane domains (M1 through M4) with extracellular C- and N-termini. (B) Subunits are assembled into pentamers that include a water-filled cation-permeable pore. The most common nAChRs in the brain are hetero-oligomeric α4β2 nAChRs and homo-oligomeric α7 nAChRs. The recognized ACh binding sites are indicated by filled black squares.
Functional characteristics
Nicotinic AChRs transition between three principal states: closed, open and desensitized [21]. In the closed state the channel is non-conducting, but the receptor sites can bind ligands. Upon binding agonist (usually 2) the channel is open and conducts cations. In the desensitized state the channel is non-conducting and the receptor is unresponsive to ligands.
Ionic flow through the open channel, particularly with respect to the proportion of calcium ions, depends on the nAChR subunit composition. For instance, the ratio of Ca2+ to Na+ permeability is α7* > β2* > muscle nAChRs [18, 19, 22–25]. The marked permeability of α7 nAChRs to Ca2+ has significant ramifications for synaptic transmission and plasticity. The α7* and β2* nAChRs are further distinguished by their fast and slow rates of desensitization, fast and slow tau of recovery from desensitization, and their low and high affinity for ACh, respectively [8, 26–28].
Neuronal nicotinic AChR subtypes are activated by nicotine and ACh, and inhibited by mecamylamine (MEC) [7]. The α7* nAChRs are activated by choline, which is produced at signaling concentrations as a metabolite of ACh hydrolysis [29, 30]. Selective inhibitors of the α7* nAChRs include α-bungarotoxin (α-BTX) and low concentrations (≤ 5 nM) of methyllycaconitine (MLA) [19, 31]. MLA at 50 nM begins to inhibit other nAChR subtypes significantly [32]. In electrophysiological studies of rodents, dihydro-β-erythroidine (DHβE) inhibits β2* nAChRs [33], and low concentrations of epibatidine activate those receptors [34]. Epibatidine binding studies indicate a higher affinity for rat β2* than for β4* nAChRs [35], however, binding studies in primate brain tissue indicate that epibatidine displays less selectivity between β2* and β4* nAChRs [36]. The agonist, A-85380, more selectively activates β2* nAChRs [37]. For all activators and inhibitors, selectivity for one subunit over another is restricted to defined concentration ranges [28, 38–40].
Extensive and complex expression patterns of nAChRs are complemented by the prominent innervations of cholinergic afferents throughout the brain [41]. Fast, synaptic nAChR-mediated EPSPs are relatively rare, but they have been detected in the hippocampus, the supraoptic nucleus, and in the cortex [42–45]. Given the number of neuronal types in which nAChR currents have been measured following local puffs of ACh, this list likely underestimates the extent of synaptic nAChR activity in the brain. Cholinergic signaling at synapses is brief and intense. Within the cleft of the neuromuscular junction, ACh reaches a relatively high concentration (~1 mM for ~1 ms) before being hydrolyzed by acetylcholinesterase [46]. A combination of rapid delivery and then breakdown of ACh minimizes the desensitization of nAChRs. Synaptically released ACh can also diffuse to non-synaptic sites, and evidence suggests that significant cholinergic signaling in the CNS is mediated via such volume transmission [9, 47, 48].
Neuronal nAChRs facilitate presynaptic neurotransmitter release
Neurotransmitters are released from presynaptic terminals, a process that typically requires an action potential to invade and depolarize the terminal. Depolarization activates voltage-gated Ca2+ channels (VGCCs), and the intra-terminal Ca2+ increase triggers neurotransmitter release. Vesicular release can also occur stochastically in an action potential-independent manner. Because nAChRs are expressed presynaptically and because the cationic current through nAChRs can both depolarize membranes and raise intracellular Ca2+ levels, nAChRs influence neurotransmitter release.
Presynaptic nAChRs
Early experiments conducted at mossy fiber–CA3 glutamatergic synapses in the rat hippocampus [49] and at medial habenula–interpeduncular nucleus glutamatergic synapses in the chick brain [50] provided evidence identifying a presynaptic function for nAChRs. First, in the presence of TTX to eliminate action potentials arriving at the presynaptic terminals, nicotine application increased the frequency of spontaneous (miniature) EPSCs. Nicotine did not affect the sensitivity of neurotransmitter detection, as inferred by an absence of change in EPSC amplitudes. Second, the amplitudes of stimulus-evoked responses were increased with a decreased incidence of synaptic failures, suggesting an increase in the probability of vesicle release. Third, the amount of transmitter released by nicotine stimulation was enhanced by increasing the concentration of extracellular Ca2+ and decreased by reducing the concentration of extracellular Ca2+. Under some conditions, Ca2+ signaling initiated by nAChRs was sufficient to trigger neurotransmitter release, as the Ca2+ influx through VGCCs was not obligatory. Later work showed that nAChRs not only mediated a direct Ca2+ signal, they also activated VGCCs and indirectly stimulated release from intracellular stores [51–54]. Fourth, nicotine robustly increased the intra-terminal Ca2+ concentration. Fifth, the effects of nicotine were mediated through multiple nAChR subtypes, often including the α7* nAChRs that have a high Ca2+-permeability. Taken together, those findings provided electrophysiological evidence for a presynaptic function for nAChRs in intact neurons. Those results complement and extend biochemical assays of nicotine-evoked neurotransmitter and ion release completed on synaptosomes prepared from a variety of brain regions [55–57]. In the sections below we focus our attention on the nicotinic mechanisms contributing to synaptic transmission and plasticity as determined by electrophysiological experiments.
Nicotinic AChRs regulate the release of multiple neurotransmitters
Activation of presynaptic nicotinic receptors facilitates the release of a variety of neurotransmitters throughout the brain [9, 58–61]. Nicotinic receptor activity directly and indirectly initiates an intracellular Ca2+ signal that contributes to neurotransmitter release. In many cases the Ca2+ influx through nAChRs (often α7*) is sufficient to evoke neurotransmitter release [49, 62]. However, the depolarization caused by the cation influx through nAChRs also can trigger neurotransmitter release indirectly by activating VGCCs [52, 63–66]. Given that CaMKII blocks facilitation by nicotine of VGCCs, it is reasonable to propose that the Ca2+ influx through nAChRs may regulate VGCCs via CaMKII-dependent, Ca2+-dependent mechanisms [67]. In addition, there are other intracellular events, such as Ca2+-induced Ca2+ release from intracellular stores that can be indirectly activated by nAChR activity [68].
In paired-pulse facilitation experiments, two stimuli of equal size are delivered to presynaptic fibers in rapid succession, and the amount of neurotransmitter released by the second stimulus is greater than the amount released by the first (priming) stimulus. Because the paired stimuli are identical in size, each stimulus provides a comparable depolarization to the terminal. The increase in intracellular Ca2+ from each stimulus has a half-decay time of a few hundred msec and, thus, intra-terminal Ca2+ can accumulate when two stimuli are closely paired [69]. Because the probability of transmitter release varies approximately with the fourth power of the Ca2+ concentration [69], a modest increase in intra-terminal Ca2+ from the second stimulus results in a robust increase in neurotransmitter release (e.g., paired-pulse facilitation). As nicotine, and likely endogenous ACh, acts through presynaptic nAChRs to elevate intra-terminal Ca2+, nAChR activation may thus function as a ‘priming’ stimulus to augment the efficacy of incoming APs.
Nicotinic AChRs regulate hippocampal synaptic plasticity in vitro
The hippocampus is central to learning, memory, and attention mechanisms, and nAChRs contribute to these functions [6, 70–77]. For instance, local application of nAChR antagonists impairs working but not reference memory [76], whereas working memory deficits are reversed by nicotine following cholinergic denervation of the hippocampus [77]. The hippocampus receives cholinergic input from the septum-diagonal band complex, whose fibers project to all regions including the dentate gyrus, CA3 and CA1, and to most cell types, including pyramidal cells, granule cells, interneurons, and hilar neurons [78, 79]. Targeted neurons robustly express nAChRs, in particular α7* nAChRs, but also β2* nAChRs [10, 16, 18–20]. Small nicotinic receptor-mediated currents have been measured in pyramidal neurons and substantially greater nicotinic currents have been measured in interneurons [6, 25, 42, 44, 80–84], with volume transmission also present [47]. In fact, α7* nAChRs are commonly found at both presynaptic and postsynaptic sites in the hippocampus CA1 region as identified with immunogold labeling and electron microscopy [16]. Remarkably, the density of nAChRs is similar to the densities of both NMDARs and AMPARs at these synapses [85].
Synaptic plasticity is a cellular correlate of memory and is modulated by nAChR activation
A cellular correlate to learning and memory in the hippocampus and elsewhere is a change in synaptic efficacy between neurons, such as STP, LTP and LTD [86–88]. These types of changes can be evoked by pairing presynaptic stimulation at specific frequencies with coincident postsynaptic depolarization. Changes in synaptic strength are interpreted from the change in amplitude or slope of the EPSP or EPSC, or amplitude of the population spike (a synchronized discharge of multiple neurons measured with extracellular ‘field’ recordings). For instance, if the ratio of EPSP amplitude (post-pairing protocol)/EPSP amplitude (pre-pairing protocol) is greater than 1, then synaptic potentiation has occurred.
Numerous studies have now shown that nAChR activation facilitates the induction of LTP in vitro. Such facilitation may be operationally defined as nicotine boosting STP to LTP, and facilitation occurs in acute brain slices prepared from naïve animals, animals chronically-treated with nicotine, and even slices from aged animals in which LTP is difficult to induce [81, 89–92]. Furthermore, after establishing the maximum possible LTP via high-frequency stimulation, subsequent application of nicotine can increase the amplitude of LTP to a new maximum unattainable by stimulation alone [93].
Timing of nAChR activation regulates hippocampal synaptic plasticity
In one set of experiments, STP was induced at Schaffer collateral–CA1 synapses in acutely prepared hippocampal slices while varying the timing of nicotine application relative to afferent stimulation (Fig. 2A–F) [92]. STP was induced by 1 sec of 100 Hz stimulation paired with 100 pA depolarization of CA1 pyramidal neurons and persisted for ~20 min. Brief (0.5 to 1 sec) puffs of ACh (1 mM, applied in the presence of atropine to block muscarinic AChRs) were delivered to the pyramidal cells and evoked action potential (AP) discharge. One of three outcomes was obtained that depended on the timing of the last ACh-induced AP relative to afferent stimulation [92]. First, if the last ACh-induced AP preceded HFS by more than 5 sec (Fig. 2B, F), or trailed HFS (Fig. 2E, F), then STP was unaltered. Second, if the last ACh-induced AP preceded HFS by 1–5 sec, LTP was produced that persisted for at least one hour (Fig. 2C, F). Third, if the last ACh-induced AP occurred <1 sec prior to HFS, then LTD resulted (Fig. 2D, F). Mimicking ACh’s postsynaptic depolarization with direct current injection failed to boost STP to LTP, revealing an obligatory requirement for nAChR activation.
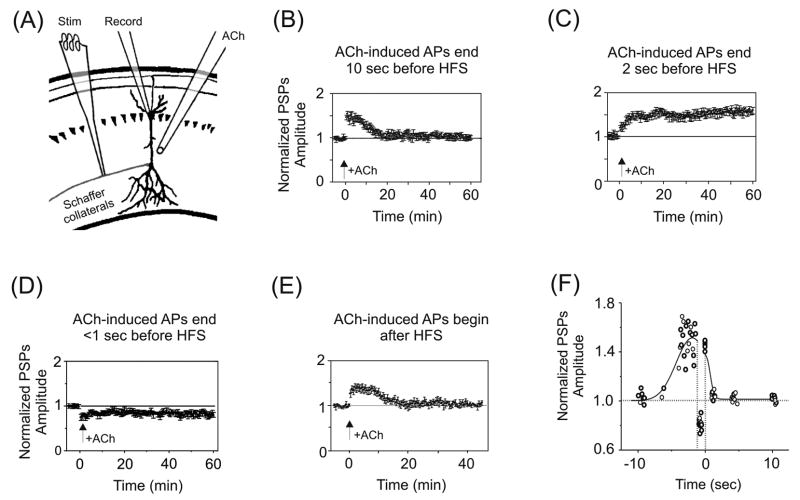
Temporal-dependence of nAChR activation for hippocampal synaptic plasticity. (A) Illustration of the experimental setup for experiments shown in panels (B) through (E). Whole-cell patch recordings were obtained from CA1 pyramidal somata, with activation of Schaffer collateral afferents in stratum radiatum. A puffer pipette delivered brief pulses of ACh (in the presence of atropine) to the pyramidal neuron’s dendrites. All postsynaptic potentials (PSPs) were normalized to baseline. (B) ACh-induced APs that preceded HFS of the Schaffer collaterals by ≥ 10 sec did not affect STP. (C) In contrast, ACh-induced APs that terminated 1–5 sec prior to HFS boosted STP to LTP. (D) If the APs terminated < 1 sec before HFS, then LTD resulted. (E) If the ACh-evoked APs followed HFS then there was no effect on STP. (F) Summary of the findings in (B) through (E). The normalized PSPs from the last 15 min of post-HFS recording were averaged and then plotted against the time between the end of ACh application and the onset of HFS. Negative time values correspond to the interval between the last ACh-induced AP and the onset of HFS. Positive time values correspond to the interval between the end of HFS and the onset of the first ACh-induced AP. The curve drawn through the data points indicates the general trend of the data. Arrows indicate timing of HFS for all panels. Adapted with permission from [92] (copyright 2005 by the Society for Neuroscience).
The temporal profile revealed in Figure 2F differs substantially from reports of spike timing dependent plasticity (STDP), which have identified intervals on the order of a few msec over which single EPSPs paired with single APs can change synaptic weights [94, 95]. Cholinergic regulation of plasticity, which emphasizes pairing intervals on the order of seconds, provides a broad window over which the outcome of presynaptic information can be transmitted and influence synaptic strength.
Boosting STP to LTP requires the activation of mainly postsynaptic α7* nAChRs (with some β2* nAChRs) in the rat hippocampus [81, 89, 90, 92, 93, 96]. Despite the fast desensitization of α7 nAChRs, brief activation of these receptors in rapid succession (e.g., <200 msec inter-stimulus intervals) actually potentiates their currents [97]. During this imposed protocols, activation of α7* nAChRs is only required for the induction of LTP, and not its expression or maintenance, as blocking α7 nAChRs has no effect once LTP is established [98].
Location of nAChR activation regulates hippocampal synaptic plasticity
In another set of experiments, STP was induced at Schaffer collateral–CA1 synapses, while varying the location of agonist application (Fig. 3A–C) [81]. For these experiments a more intense stimulation protocol (3 × 1 sec, 100Hz stimulus trains) was used to produce robust LTP [81]. When nAChRs were activated by puffing ACh onto neighboring GABAergic interneurons, which have been shown to inhibit CA1 pyramidal neurons directly [81, 84, 99, 100], this protocol produced only STP. Thus nicotinic effects in the hippocampus on synaptic transmission are mediated to a significant extent by the direct activation of inhibitory GABAergic interneurons, which serves to decrease the net output of pyramidal cells, or negate plasticity mechanisms in pyramidal cells. Interestingly, for the cellular combination of interneuron-interneuron-pyramidal cell, nicotinic activation of the first GABAergic neuron can inhibit the second interneuron, which then disinhibits the pyramidal cell, thereby potentially increasing pyramidal cell output [84]. In situ the nicotinic influences via GABAergic networks are generally stronger than direct action at glutamatergic pyramidal neurons because nAChRs are more densely expressed on the GABAergic interneurons [84, 99, 101, 102].
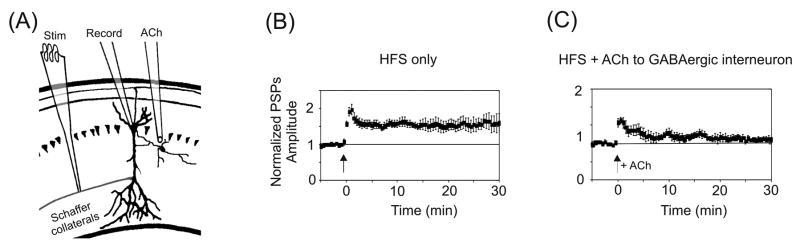
Spatial-dependence of nAChR activation for hippocampal synaptic plasticity. (A) Illustration of the experimental setup for experiments shown in panels (B) and (C). The puffer pipette applies ACh to a GABAergic interneuron neighboring the pyramidal neuron. All PSPs were normalized to baseline. (B, C) HFS-evoked LTP (B) was prevented by activating inhibitory interneurons with ACh (C). Arrows indicate timing of HFS for all panels. Adapted with permission from [81] (copyright 2001 by Cell Press).
nAChR-mediated synaptic plasticity and membrane properties
Synaptic plasticity can be modulated by active membrane events like action potentials (APs), but also by passive membrane properties. Na+-dependent APs are usually initiated in the axon initial segment or first node of Ranvier and the APs propagate down the axon [103–105]. For many cell types, including CA1 pyramidal cells, APs also propagate backwards into the dendrites where they can contribute to synaptic plasticity [106, 107]. Back-propagating APs depolarize dendrites, which removes the Mg2+ block of NMDARs and also inactivates low threshold A-type K+ channels. Together, these processes make synaptic integration and plasticity more permissive [108]. Back-propagation of APs is also important for nAChR-mediated synaptic plasticity, as plasticity is blocked by loading CA1 pyramidal neurons with the Na+ channel blocker QX-314 [92]. By virtue of their depolarizing influence, nAChRs may also directly regulate the availability of voltage-dependent ion channels, decreasing the availability of the aforementioned A-type K+ channels via inactivation, and increasing the activation of ion channels linked with propagating excitation like Na+ and Ca2+ channels. In some cells, due to the expression of Ca2+-activated K+ channels, the Ca2+ influx through nAChRs could alternatively contribute to the stabilization or hyperpolarization of the membrane potential.
Nicotinic AChRs may also modify the passive (electrotonic) characteristics of neurons. For instance, opening nAChR channels provides an effective shunt by increasing membrane conductance (decreasing impedance). This shunt will change the membrane time and length constants. Such changes may affect the electrotonic filtering of the amplitudes and time courses of synaptic inputs. Overall these nicotinic properties could alter the likelihood that synaptic potentials can integrate, conduct to the soma, depolarize the neuron to AP threshold, or trigger synaptic plasticity [9, 21].
Intracellular signaling mechanisms in nAChR-mediated synaptic plasticity
The mechanisms through which nAChRs modulate plasticity resemble known mechanisms for non-cholinergic regulation of synaptic plasticity [92]. In particular, there is evidence for a requirement for NMDA receptors in nAChR-mediated boosting of STP to LTP [93, 96, 98]. Because nAChRs have a voltage-dependent-rectification [24, 109], they are suited to supply a substantial Ca2+ influx to neurons at hyperpolarized membrane potentials, where the driving force on Ca2+ ions is high. Thus, when considering that NMDARs readily pass Ca2+ ions only at depolarized voltages, the Ca2+ influx through nAChRs may extend the voltage range over which Ca2+-dependent intracellular processes are initiated. Interestingly, in cultured hippocampal neurons, nicotinic simulation may in fact reduce some NMDAR currents in a calmodulin-dependent manner. This modulation may provide a cholinergic mechanism under which some synapses can convey information via AMPARs without any long-term change in synaptic efficacy [110]. Additionally, loading neurons with a high affinity Ca2+ chelator prevented the STP to LTP transition, revealing the importance of intracellular Ca2+ signaling to ACh-induced plasticity. In this regard, it has been shown that Ca2+ influx through nAChR can further increase the concentration of intracellular Ca2+ by activating Ca2+-induced Ca2+ release from internal stores [68, 111], which may also contribute to synaptic plasticity.
In a recent study, field recordings obtained from the dentate gyrus in acute slices revealed that, consistent with previous studies, nicotine enhances stimulation-induced LTP in an α7* nAChR- and NMDAR-dependent manner [98]. However, unlike the LTP induced by high-frequency stimulation (HFS) alone, nAChR-augmented LTP required activation of mGluR5, Ca2+ influx through L-type VGCCs, and Ca2+-induced Ca2+ release from ryanodine-sensitive intracellular Ca2+ stores. Interestingly, following two weeks of twice-daily nicotine injections, HFS elicited a robust LTP in acute slices prepared from these animals. The magnitude of LTP measured in these slices was comparable to that obtained for the acute combination of HFS and bath application of nicotine. Blocking mGluR5 or ryanodine-sensitive Ca2+ stores reduced this LTP to the level normally expected for the HFS protocol alone, revealing that acute in vitro or chronic in vivo nicotine administration may function through similar pathways.
Modification of transcription by nAChR activation
In addition to second messenger pathways, long-term changes in neuronal function often require regulation at the gene level, and nicotine, at least in differentiated PC12 cells, activates transcription [112]. In addition, it has been shown that 1 μM nicotine applied for 25 min to cultured hippocampal neurons activates the transcription factor CREB, which in turn activates the immediate early gene c-fos. This transcriptional activation requires both CaMKII/IV and MAP kinases, as well as Ca2+ release from internal stores [113]. This combination of events immediately increases phosphorylated CREB (pCREB), whose levels remain elevated for at least one hour. Interestingly, pCREB levels are reduced by ~1/2 if glutamate receptors are blocked. Thus, nAChR-mediated facilitation of presynaptic glutamate release may act in tandem with postsynaptic nAChR-mediated gene transcription to effect long-term changes in neural function. CREB, and in particular pCREB, have been implicated in various forms of learning and memory [114, 115]. The results suggest that changes in CREB activation via nAChRs represent a means by which nicotine may modify neural function over very long periods.
Nicotinic AChRs regulate hippocampal synaptic plasticity in vivo
Our understanding of nAChR regulation of synaptic transmission and plasticity has for the most part been gleaned from in vitro studies, which offer a high level of experimental control to probe mechanistic questions. However, potential drawbacks, such as the severing or remodeling of network connections and changes in cellular physiology that may impact plasticity [116], suggest the need for systems-level approaches with in vivo strategies.
Systemic administration of very high doses of nicotine has been shown to evoke LTP at perforant path–dentate gyrus synapses in anesthetized mice [34, 117]. In the dentate gyrus, chemical LTP evoked by 3 mg/kg nicotine gradually develops over the first ~10 min post-injection and reaches a stable >2-fold increase over baseline that persists for at least 2 hours [117]. In contrast to in vitro cases, where nicotine boosts STP to LTP, in the intact anesthetized animal very high doses of nicotine actually induce LTP in the absence of the tetanization of afferent inputs. Interestingly, nicotine-induced LTP and perforant path HFS-induced LTP are similar in time course and amplitude, and both are blocked by pre-administration of mecamylamine, suggesting that tetanus-induced LTP requires the endogenous activation of cholinergic inputs and nAChRs. Neither nicotine-induced nor HFS-induced LTP is blocked by mecamylamine delivered after nicotine injection or following HFS. As seen in vitro, this result indicates that the induction, but not the expression or maintenance of LTP, is dependent on nAChRs. This same study also showed that the α7* nAChR agonist choline elicited a dose-dependent LTP in vivo, and that the maximum LTP evoked by choline could be further increased by nicotine [117]. In a complementary experiment, these authors showed that epibatidine, a somewhat specific β2* agonist, also produced chemical LTP [118]. Like choline-induced LTP, epibatidine-induced LTP was sub-maximal and was increased to maximum values by nicotine. The amount of potentiation evoked by choline or epibatidine individually reveals that α7* and β2* receptors, respectively, each contribute about 50% to the LTP evoked by nicotine [117, 118]. At Schaffer collateral–CA1 synapses in anesthetized rats, α7* nAChRs contribute approximately one-third of the HFS-induced LTP [119].
Although these results are intriguing, there are a number of issues to consider. The antagonist mecamylamine in wake rodents also weakly inhibits NMDARs [120] and inhibits ACh synthesis [121]. Thus, work with more specific blockers that exclude alternative conclusions will be necessary. In addition, the rodents were anesthetized and LTP was evoked by very high concentrations of agonists: concentrations of nicotine that would induce seizures in wake mice [122]. The use of anesthesia further confounds the nicotinic role because some anesthetics inhibit nAChRs [123]. In addition, nAChRs often influence circuit events by a predominant action upon GABAergic inhibitory interneurons [84, 101], and GABAergic signaling is important during the induction of nicotine-evoked plasticity [81, 124]. Given the propensity for anesthetics to modulate GABAergic activity, GABARs, and nAChRs [123, 125], additional studies using freely-moving animals and physiological concentrations of nicotine are going to be extremely important to understand the biological implications of nicotine influences over plasticity in vivo.
Nicotinic AChRs contribute to hippocampal plasticity during early postnatal development
During the first few weeks of postnatal life, the rodent brain undergoes extraordinary development. As noted for adult animals, nAChR-mediated plasticity appears to play important roles during these times as well. In early (<1 week) postnatal rats, α7* nAChRs regulate the frequency of GABA-mediated giant depolarizing potentials (GDPs) in CA3 [126]. As pairing GDPs with mossy fiber input is known to strengthen mossy fiber–CA3 synapses [127], early nicotinic regulation may contribute to the maturation of these neuronal connections.
In the developing hippocampus, nAChRs provide a powerful control over glutamate release probability, converting low probability synapses to high probability synapses [128] and vice versa [129]. The effect is sufficiently dramatic that nicotine can convert ‘silent’ synapses to functional synapses [128]. In the adult, ‘silent’ synapses do not express AMPARs in the postsynaptic membrane and, thus, cannot detect glutamate release at hyperpolarized potentials [130]. In contrast, in the early postnatal brain, ‘silent’ synapses express postsynaptic glutamate receptors, but the probability of presynaptic neurotransmitter release is near zero. As noted in adult neurons, nicotine application to juvenile neurons markedly increases the probability of release from Schaffer collateral fibers onto CA1 pyramidal neurons in an α7* nAChR-dependent manner. Presynaptic nAChR activity increases the frequency, but not the amplitude, of spontaneous synaptic currents. Importantly, stimulating cholinergic fibers within juvenile slices mimics the effect of nicotine, suggesting that the switch from low to high probability synapse is mediated by endogenous ACh. For those rare cases when synapses with a high probability of release were identified, nicotine application decreased release probability, an effect that required both α7* and β2* nAChRs [129]. Taken together, these results suggest that activation of nAChRs may function as a bidirectional switch on release probability in developing hippocampal neurons.
Interestingly, the transition of GABA as an excitatory neurotransmitter in the juvenile brain to an inhibitory neurotransmitter in the adult brain is also regulated by nAChR signaling [131]. In juvenile neurons, expression of the Cl− transporter NKCC1 maintains a high intracellular Cl− concentration, and thus GABA-mediated channel openings favor an outward flow of negative current that is depolarizing. In adult neurons the Cl− transporter KCC2 is more robustly expressed, and it maintains a high extracellular Cl− concentration that favors hyperpolarization when the Cl− channels are opened [132, 133]. During the second postnatal week NKCC1 expression is reduced and KCC2 expression is increased, rendering GABA inhibitory. These events were recently shown to involve α7* nAChR-mediated signaling, albeit through an unidentified transduction mechanism [131].
Nicotinic AChRs regulate synaptic plasticity in midbrain dopaminergic neurons
Nicotinic receptors are known to regulate synaptic plasticity in areas that play a critical role in reward and addiction, such as the neurons of the ventral midbrain. Of particular significance are the dopaminergic neurons of the ventral tegmental area (VTA), which respond to rewarding stimuli or reward-predicting stimuli [134]. VTA neurons broadcast reward and salience information to areas of the brain underlying emotion and decision making, including the prefrontal cortex, amygdala, striatum, and nucleus accumbens [41]. The VTA dopaminergic neurons receive excitatory input from numerous sources, including the prefrontal cortex, and inhibitory inputs from afferent projections and from local GABAergic interneurons. Cholinergic inputs arrive from the laterodorsal and the pedunculopontine tegmental nuclei and project to both dopaminergic and GABAergic cells in this region. The ventral midbrain neurons express many nAChR subunits, with α7* and β2* nAChRs predominating [135, 136]. Nicotine robustly activates this system, resulting in the acquisition of behaviors that are reinforced by drug use [137–139]. In fact, a single exposure to nicotine elevates dopamine levels in the nucleus accumbens for more than two hours in rats (Fig. 4A) [140, 141]. The question of how a drug such as nicotine triggers long-term changes in the midbrain dopamine system has been explored from the hypothesis that nicotine, acting through nAChRs, modulates short- and long-term plasticity.
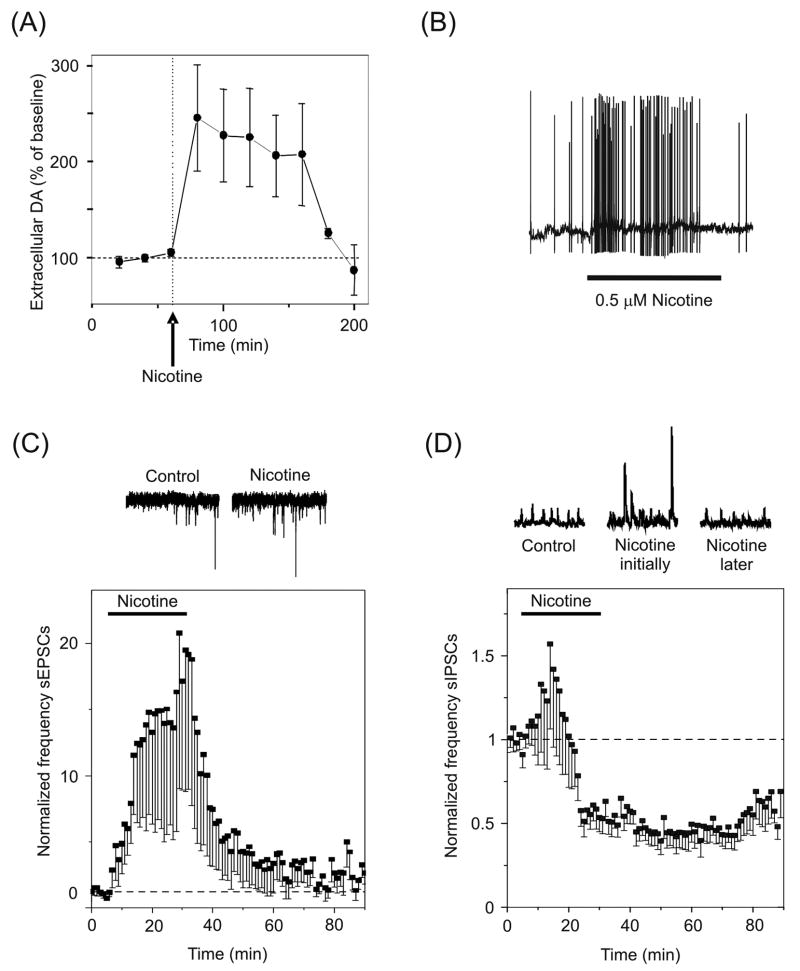
Synaptic plasticity in the VTA. (A) Dopamine levels are elevated in the nucleus accumbens of rats for more than two hours following a single i.p. injection of 0.6 mg/kg nicotine. (B) Bath applied nicotine increases the frequency of AP discharge in dopaminergic neurons mainly via β2* nAChRs. (C) Bath applied nicotine increases the frequency of spontaneous EPSCs (sEPSCs) mainly via presynaptic α7* nAChRs. The coincidence of postsynaptic and presynaptic activation by nicotine is sufficient to induce LTP of the glutamatergic synapses. (D) The frequency of spontaneous IPSCs (sIPSCs) first increases, and then decreases below baseline following desensitization of β2* nAChRs on the somata of GABAergic neurons. The decrease in IPSC frequency indirectly enhances the glutamatergic excitation of dopamine neurons. Adapted with permission from [140, 144] (copyright 1997 by Nature Publishing Group [144] and copyright 2004 by Cold Spring Harbor Laboratory Press [140]).
nAChRs facilitate glutamate release and evoke postsynaptic depolarization to initiate VTA LTP
Low concentrations of nicotine that approximate the concentration delivered by smoking a cigarette [142] produce LTP in dopaminergic neurons [137, 143]. Bath application of nicotine produces a substantial postsynaptic depolarization that markedly increases the frequency of AP output from VTA dopaminergic neurons via (mainly) β2* nAChRs (Fig. 4B) [143–146]. Nicotine further acts via presynaptic (mainly) α7* nAChRs to increase the frequency of spontaneous EPSCs (Fig. 4C) [140, 146]. This presynaptic facilitation persists during the nicotine application (e.g., 25 min), revealing that the α7* nAChRs are available for activation as long as nicotine is present [136, 140]. Furthermore, the amplitude of synaptically-evoked events is significantly increased by nicotine, collectively suggesting an increase in the probability of glutamate release [140, 143, 146]. The increased delivery of glutamate activates postsynaptic AMPARs, which in combination with the nAChR-mediated postsynaptic depolarization, provides a robust voltage change reducing the Mg2+ blockade on NMDARs. Together the effects of nicotine on presynaptic and postsynaptic membranes produce LTP. This process is in contrast to the hippocampus, where nAChR activation in vitro must be combined with coincident presynaptic tetanization and postsynaptic depolarization by the recording electrode to boost STP to LTP.
nAChRs inhibit interneuron output to strengthen LTP
Occurring simultaneously with the strengthening of excitatory connections onto VTA dopaminergic neurons is a decrease in the strength of inhibitory connections. Application of nicotine to midbrain slices initially results in an increase in the frequency and amplitude of inhibitory events due to the activation of presumably preterminal or somatic (mainly) β2* nAChRs (Fig. 4D) [140, 147, 148]. However, the β2* nAChRs rapidly desensitize [136], and the frequency of inhibitory events falls to below pre-nicotine levels, suggesting a tonic cholinergic excitatory drive onto these neurons. This reduction in inhibitory tone disinhibits the dopaminergic neurons, further strengthening the nicotine-induced enhancement of excitatory inputs.
α7* and β2* nAChRs differentially regulate dopamine neuron AP output in vivo
Midbrain dopamine neurons in vivo generate APs in regular- and burst-firing patterns [149, 150]. Both the frequency of regular firing, and the incidence of bursting, can be increased by nicotine administration [151]. In an effort to identify the specific nAChR subunits that mediate the effects of nicotine on dopamine neuron output, in one study AP output was first subdivided into four principal modes: 1) low frequency regular-firing, low-bursting (LFLB), 2) low frequency regular-firing, high-bursting (LFHB), 3) high frequency regular-firing, low-bursting (HFLB), and 4) high frequency regular-firing, high-bursting (HFHB) [152]. Using an elegant combination of molecular and genetic tools, these authors showed that activation of β2* nAChRs switch dopamine neurons from the ‘resting’ mode (LFLB) to any of the ‘excited’ modes [152]. Once β2* nAChRs shift the neuron into an excited mode, activation of α7* nAChRs fine tunes the pattern of AP output by switching the neuron to other excited output modes (e.g., from HFHB to LFHB or from HFHB to HFLB). Interestingly, selective re-expression of β2* nAChRs into midbrain dopamine neurons of β2 knockout mice is sufficient to restore nicotine-evoked changes in electrophysiological properties, nicotine-evoked dopamine release and nicotine self-administration [153]. These thoughtful experiments revealed fundamental roles for β2* nAChRs in midbrain dopamine neurons.
Comparison of nAChR LTP mechanisms between hippocampus and VTA
The contributions of nAChRs to synaptic plasticity in the hippocampus can be summarized as follows: 1) nAChRs increase presynaptic glutamate release, 2) nAChRs contribute a small postsynaptic membrane depolarization, which presumably increases the open probability of NMDARs via the voltage-dependent relief of Mg2+ blockade, 3) nAChRs directly and indirectly contribute an intracellular Ca2+ signal in the postsynaptic cell that may activate plasticity-evoking Ca2+-dependent signaling and gene transcription, 4) presynaptic activity and nAChR activation may be temporally coordinated to influence synaptic transmission and plasticity, 5) nAChR activation may be spatially coordinated to enhance GABAergic interneuron signaling to block LTP induction on pyramidal neurons, and 6) nAChR signaling can awaken or silence synapses during early postnatal development and control the transition of GABA from an excitatory to an inhibitory neurotransmitter. Taken together, the data support the view that nAChRs exert a temporally- and spatially-dependent bidirectional control over hippocampal synaptic plasticity, both in vitro and in vivo.
In contrast to the facilitory (modulatory) role for nAChRs during LTP induction in the in vitro hippocampal slice, nAChR activity induces LTP in the midbrain slice containing the VTA. Within the VTA, nicotine contributes to LTP of glutamatergic synapses onto dopamine neurons via three major mechanisms: 1) transient activation of postsynaptic β2* nAChRs to depolarize the dopaminergic neurons, 2) sustained activation of presynaptic α7* nAChRs to augment glutamate transmission onto dopaminergic neurons, and 3) activation and desensitization of β2* receptors on GABAergic interneurons to decrease inhibition onto dopaminergic neurons. Within the VTA, nicotinic cholinergic input could conceivably act as a coincidence mechanism, simultaneously recruiting both presynaptic and postsynaptic elements. The fact that nAChR activation in the hippocampus in vivo produces de novo LTP in the absence of afferent tetanization suggests that the hippocampal slice preparation may lack all the long-range connections and the network properties of the in vivo situation. Thus, nAChRs modulate, regulate and likely induce synaptic plasticity in both the hippocampus and the VTA.
Potential therapeutic roles for nAChRs in pathological states
The importance of nAChRs to synaptic transmission and synaptic plasticity throughout the brain is evident from pharmacological studies and investigations with nAChR-subunit knockout mice [152, 154–57]}. Any changes to the expression levels of these receptors, or changes to the afferents that supply these receptors with ACh, could influence the physiology and behavior. A corollary is that some forms of pathology that include compromised synaptic transmission or plasticity could potentially benefit from exogenous stimulation of nAChRs to boost transmission or plasticity capabilities closer to normal levels.
Cholinergic signaling and nAChRs are implicated in many pathological changes to the brain, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, and addiction [137, 158–160]. For instance, in Alzheimer’s disease, the numbers of nAChRs decrease, particularly in the hippocampus and cortex [158, 161]. Evidence indicates that augmenting nAChR activity allosterically or inhibiting acetylcholinesterase may improve attention and rates of learning [75, 162–164], and these effects also may slow neurodegeneration in models of Alzheimer’s disease [165, 166]. Other evidence has shown that, in rats and nonhuman primates, nicotine is neuroprotective against loss of nigrostriatal dopamine neurons [167]. Given the deficit of dopaminergic neurotransmission in Parkinson’s disease, therapeutic activation of nAChRs to facilitate dopamine release onto its target cells may benefit those patients as well. For example, Alzheimer’s drugs, such as donepezil and galantamine, were found to enhance nicotinic function and indirectly influence dopamine release in the striatum [168].
Improved treatments will require the development of new nAChR agonists and antagonists that target nAChRs locally in some cases and broadly in other cases [169, 170]. In addition, the development of new allosteric modulators [171] – those compounds that do not directly activate the receptor but augment function of the receptor when the ligand is present –will also be valuable. Because allosteric modulators do not themselves activate receptors, they will operate with the same spatial and temporal profile as endogenous cholinergic signals. The development of novel compounds, as well as new molecular tools like siRNA and conditional/inducible knock-out or knock-in mice, will also be a benefit to basic research. Presently the nicotinic field has not extensively benefited from the combination of molecular tools and powerful, high-resolution in vivo recordings from freely-moving animals. Those techniques will open up the black-box observations of behavioral studies. The application of these powerful approaches will provide new kinds of data and open additional avenues for research and provide new targets for drug development. These combinations of tools and approaches could be used to determine exactly how and when specific nAChR subunits contribute to processes such as synaptic transmission, synaptic plasticity, normal development, and dysfunction as well as higher order functions such as cognition and consciousness.
Acknowledgments
Work from the laboratory is supported by the NIH’s NINDS and NIDA. BEM is supported by fellowships from the Natural Sciences and Engineering Research Council of Canada, and the Alberta Heritage Foundation for Medical Research. ANP is supported by a training fellowship from the NINDS.
Abbreviations
- ACh
- acetylcholine
- AP
- action potential
- AMPAR
- α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- CNS
- central nervous system
- CREB
- cAMP response element binding protein
- EPSC
- excitatory postsynaptic current
- EPSP
- excitatory postsynaptic potential
- GABA
- gamma-amino butyric acid
- GDP
- giant depolarizing potential
- HFS
- high frequency stimulation
- IPSC
- inhibitory postsynaptic current
- LTD
- long-term depression
- LTP
- long-term potentiation
- MAP kinase
- mitogen activated protein kinase
- mGluR
- metabotropic glutamate receptor
- nAChR
- nicotinic acetylcholine receptor
- NMDAR
- N-methyl-D-aspartate receptor
- PSP
- postsynaptic potential
- sEPSC
- spontaneous EPSC
- sIPSC
- spontaneous IPSC
- STDP
- spike timing dependent plasticity
- STP
- short-term potentiation
- VGCC
- voltage-gated Ca2+ channel
- VTA
- ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bcp.2007.07.001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2047292?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Smoking-informed methylation and expression QTLs in human brain and colocalization with smoking-associated genetic loci.
Neuropsychopharmacology, 49(11):1749-1757, 04 Jun 2024
Cited by: 0 articles | PMID: 38830989 | PMCID: PMC11399277
Identifiability of equilibrium constants for receptors with two to five binding sites.
J Gen Physiol, 155(12):e202313423, 26 Oct 2023
Cited by: 0 articles | PMID: 37882789 | PMCID: PMC10602793
The cellular mechanisms associated with the anesthetic and neuroprotective properties of xenon: a systematic review of the preclinical literature.
Front Neurosci, 17:1225191, 14 Jul 2023
Cited by: 0 articles | PMID: 37521706 | PMCID: PMC10380949
Review Free full text in Europe PMC
Nicotinic acetylcholine receptors and learning and memory deficits in Neuroinflammatory diseases.
Front Neurosci, 17:1179611, 15 May 2023
Cited by: 3 articles | PMID: 37255751 | PMCID: PMC10225599
Review Free full text in Europe PMC
Basal forebrain cholinergic signalling: development, connectivity and roles in cognition.
Nat Rev Neurosci, 24(4):233-251, 23 Feb 2023
Cited by: 21 articles | PMID: 36823458 | PMCID: PMC10439770
Review Free full text in Europe PMC
Go to all (87) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
α6-Containing nicotinic acetylcholine receptors in midbrain dopamine neurons are poised to govern dopamine-mediated behaviors and synaptic plasticity.
Neuroscience, 304:161-175, 23 Jul 2015
Cited by: 19 articles | PMID: 26210579 | PMCID: PMC4547869
Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation.
J Neurosci, 25(26):6084-6091, 01 Jun 2005
Cited by: 81 articles | PMID: 15987938 | PMCID: PMC6725070
Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons.
J Physiol, 579(pt 3):753-763, 04 Jan 2007
Cited by: 49 articles | PMID: 17204496
Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system.
Brain Res Brain Res Rev, 30(3):219-235, 01 Nov 1999
Cited by: 160 articles | PMID: 10567725
Review
Funding
Funders who supported this work.
NINDS NIH HHS (4)
Grant ID: R37 NS021229-21
Grant ID: R01 NS021229
Grant ID: T32 NS043124
Grant ID: R37 NS021229





