Abstract
Free full text

Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems.
Abstract
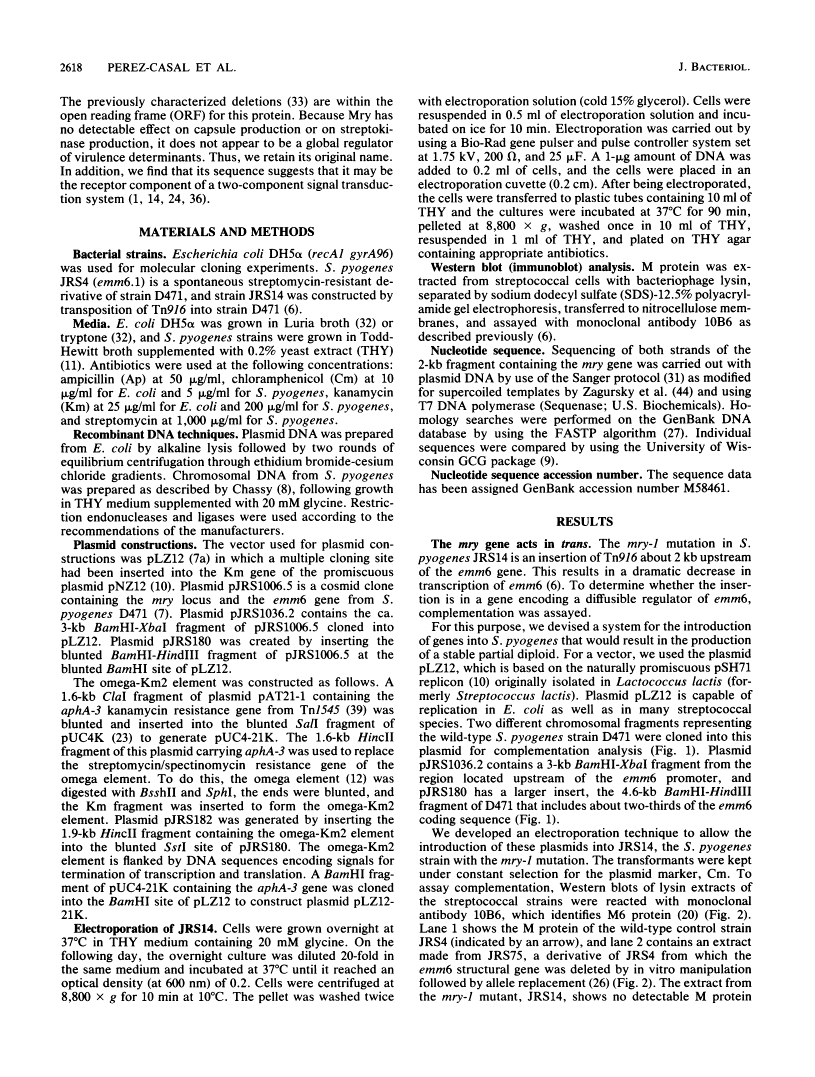
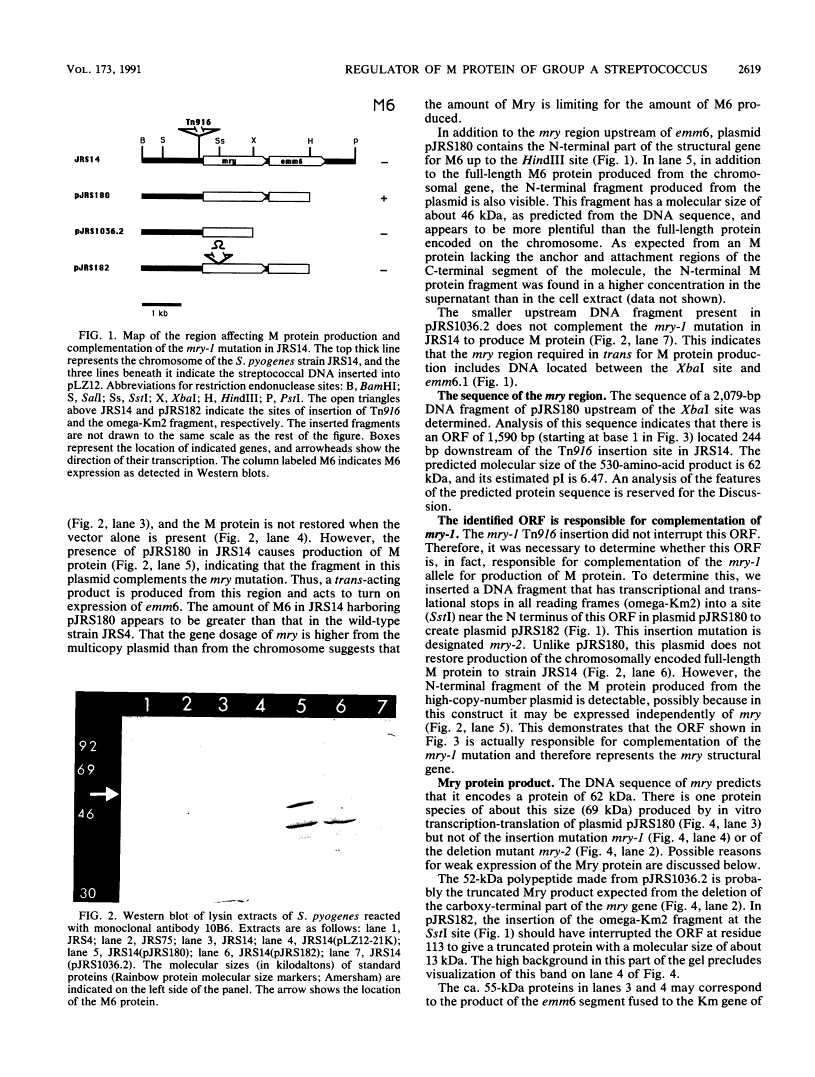
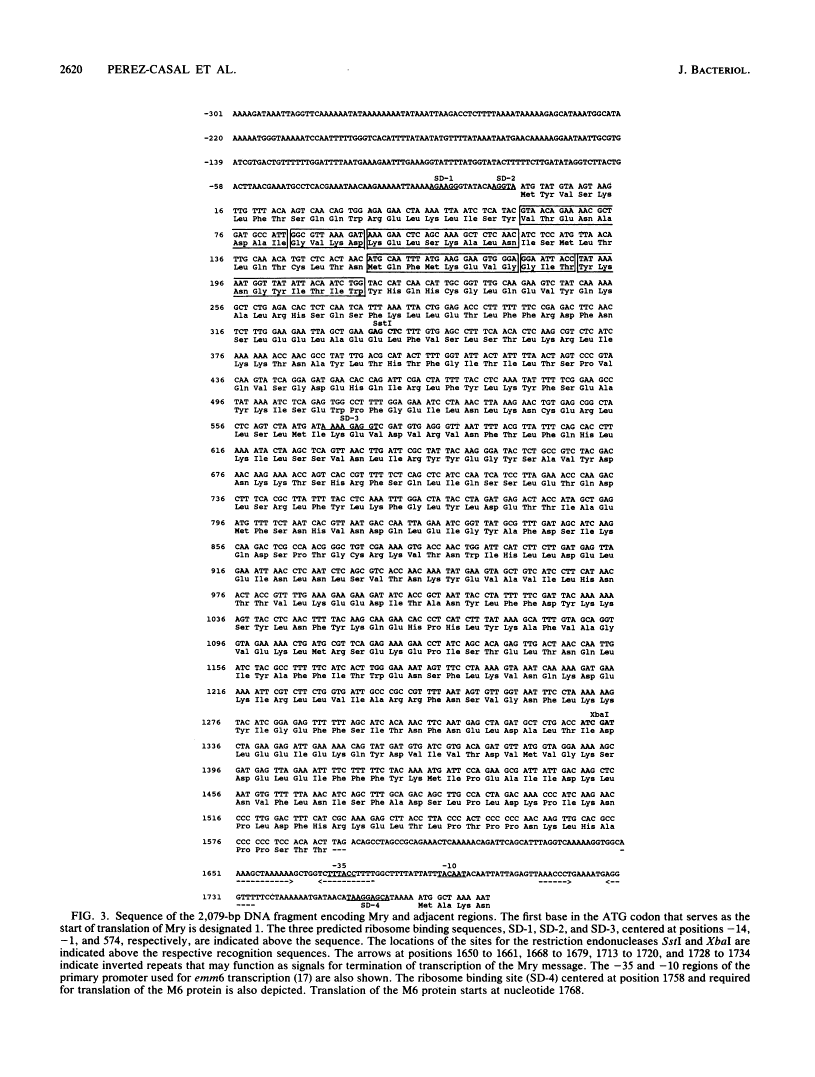
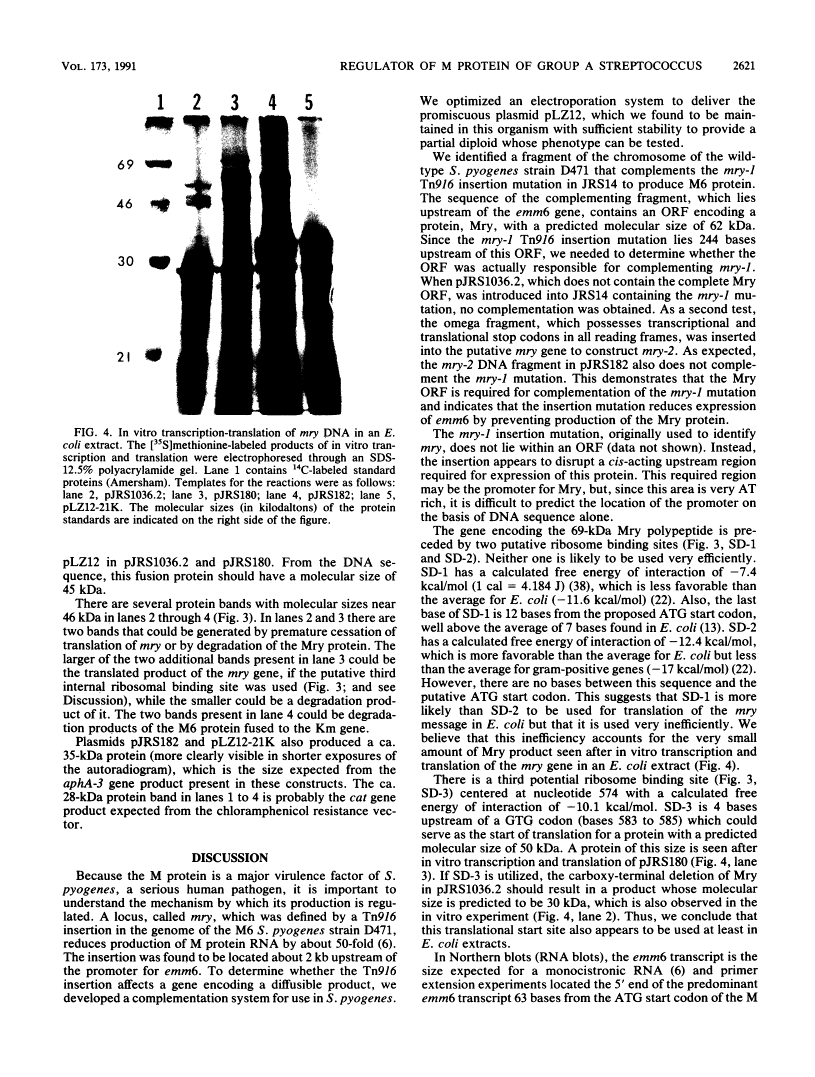
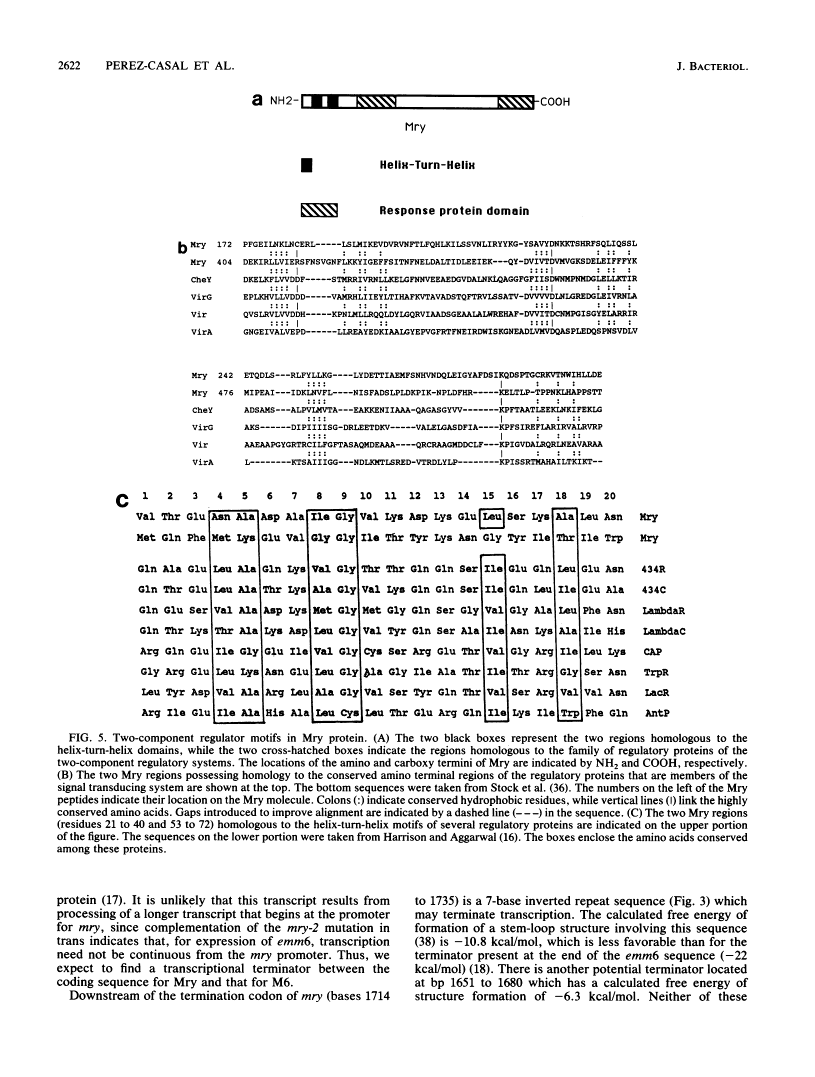
In the Streptococcus pyogenes M6 strain D471, an insertion of the conjugative transposon Tn916 into a region 2 kb upstream of the promoter of emm6 (the structural gene for the M protein) rendered the strain M negative (M. G. Caparon and J. R. Scott, Proc. Natl. Acad. Sci. USA 84:8677-8681, 1987). In the present work, we show that this insertion mutation, mry-1, is 244 bp upstream of an open reading frame encoding a protein we call Mry. This protein is visible on a gel after transcription and translation in vitro. We have developed a technique for complementation analysis in S. pyogenes and have used it to show that the wild-type mry gene is dominant to two mutant alleles. This dominance indicates that Mry acts in trans as a positive regulator of the emm6 gene. The translated DNA sequence of mry has two regions of similarity to the motif common to the receptor protein of two-component regulatory systems. In addition, the N terminus of Mry has two regions resembling a helix-turn-helix motif. Mry does not appear to be a global regulator of virulence determinants in the group A streptococcus because there is no effect of the mry-1 mutation on production of the hyaluronic acid capsule or streptokinase.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright LM, Huala E, Ausubel FM. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. [Abstract] [Google Scholar]
- Aricó B, Miller JF, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. [Europe PMC free article] [Abstract] [Google Scholar]
- Bartter T, Dascal A, Carroll K, Curley FJ. 'Toxic strep syndrome'. A manifestation of group A streptococcal infection. Arch Intern Med. 1988 Jun;148(6):1421–1424. [Abstract] [Google Scholar]
- Björck L, Akesson P, Bohus M, Trojnar J, Abrahamson M, Olafsson I, Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989 Jan 26;337(6205):385–386. [Abstract] [Google Scholar]
- Caparon MG, Scott JR. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989 Dec 22;59(6):1027–1034. [Abstract] [Google Scholar]
- Chassy BM. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. [Abstract] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. [Europe PMC free article] [Abstract] [Google Scholar]
- Fischetti VA, Jones KF, Scott JR. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. [Europe PMC free article] [Abstract] [Google Scholar]
- Frey J, Krisch HM. Omega mutagenesis in gram-negative bacteria: a selectable interposon which is strongly polar in a wide range of bacterial species. Gene. 1985;36(1-2):143–150. [Abstract] [Google Scholar]
- Gold L, Pribnow D, Schneider T, Shinedling S, Singer BS, Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. [Abstract] [Google Scholar]
- Gross R, Aricò B, Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989 Nov;3(11):1661–1667. [Abstract] [Google Scholar]
- Haanes-Fritz E, Kraus W, Burdett V, Dale JB, Beachey EH, Cleary P. Comparison of the leader sequences of four group A streptococcal M protein genes. Nucleic Acids Res. 1988 May 25;16(10):4667–4677. [Europe PMC free article] [Abstract] [Google Scholar]
- Harrison SC, Aggarwal AK. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. [Abstract] [Google Scholar]
- Hollingshead SK, Fischetti VA, Scott JR. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [Abstract] [Google Scholar]
- Jin S, Roitsch T, Ankenbauer RG, Gordon MP, Nester EW. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones KF, Manjula BN, Johnston KH, Hollingshead SK, Scott JR, Fischetti VA. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med. 1985 Mar 1;161(3):623–628. [Europe PMC free article] [Abstract] [Google Scholar]
- Malke H, Ferretti JJ. Streptokinase: cloning, expression, and excretion by Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3557–3561. [Europe PMC free article] [Abstract] [Google Scholar]
- McLaughlin JR, Murray CL, Rabinowitz JC. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [Abstract] [Google Scholar]
- Messing J, Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. [Abstract] [Google Scholar]
- Miller JF, Mekalanos JJ, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. [Abstract] [Google Scholar]
- Miller VL, Taylor RK, Mekalanos JJ. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. [Abstract] [Google Scholar]
- Norgren M, Caparon MG, Scott JR. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989 Dec;57(12):3846–3850. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. [Europe PMC free article] [Abstract] [Google Scholar]
- Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. [Europe PMC free article] [Abstract] [Google Scholar]
- Powell BS, Rogowsky PM, Kado CI. virG of Agrobacterium tumefaciens plasmid pTiC58 encodes a DNA-binding protein. Mol Microbiol. 1989 Mar;3(3):411–419. [Abstract] [Google Scholar]
- Robbins JC, Spanier JG, Jones SJ, Simpson WJ, Cleary PP. Streptococcus pyogenes type 12 M protein gene regulation by upstream sequences. J Bacteriol. 1987 Dec;169(12):5633–5640. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Scott JR. A turbid plaque-forming mutant of phage P1 that cannot lysogenize Escherichia coli. Virology. 1974 Dec;62(2):344–349. [Abstract] [Google Scholar]
- Simpson WJ, LaPenta D, Chen C, Cleary PP. Coregulation of type 12 M protein and streptococcal C5a peptidase genes in group A streptococci: evidence for a virulence regulon controlled by the virR locus. J Bacteriol. 1990 Feb;172(2):696–700. [Europe PMC free article] [Abstract] [Google Scholar]
- Spanier JG, Jones SJ, Cleary P. Small DNA deletions creating avirulence in Streptococcus pyogenes. Science. 1984 Aug 31;225(4665):935–938. [Abstract] [Google Scholar]
- Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989 Jul 6;321(1):1–7. [Abstract] [Google Scholar]
- Stock JB, Ninfa AJ, Stock AM. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. [Europe PMC free article] [Abstract] [Google Scholar]
- Svensson M, Christensen P, Schalén D. Monoclonal opsonic mouse antibodies specific for streptococcal IgG Fc-receptor. J Med Microbiol. 1986 Nov;22(3):251–256. [Abstract] [Google Scholar]
- Tinoco I, Jr, Borer PN, Dengler B, Levin MD, Uhlenbeck OC, Crothers DM, Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. [Abstract] [Google Scholar]
- Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. [Abstract] [Google Scholar]
- Wexler DE, Nelson RD, Cleary PP. Human neutrophil chemotactic response to group A streptococci: bacteria-mediated interference with complement-derived chemotactic factors. Infect Immun. 1983 Jan;39(1):239–246. [Europe PMC free article] [Abstract] [Google Scholar]
- Whitnack E, Bisno AL, Beachey EH. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect Immun. 1981 Mar;31(3):985–991. [Europe PMC free article] [Abstract] [Google Scholar]
- WILSON AT. The relative importance of the capsule and the M-antigen in determining colony form of group A streptococci. J Exp Med. 1959 Mar 1;109(3):257–270. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.173.8.2617-2624.1991
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc207828?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/173/8/2617
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/173/8/2617
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Cas9 interaction with the tracrRNA nexus modulates the repression of type II-A CRISPR-cas genes.
Nucleic Acids Res, 52(17):10595-10606, 01 Sep 2024
Cited by: 0 articles | PMID: 38994567 | PMCID: PMC11417352
Enterococcal quorum-controlled protease alters phage infection.
FEMS Microbes, 5:xtae022, 26 Jul 2024
Cited by: 0 articles | PMID: 39156124 | PMCID: PMC11328733
Genetic modification of Streptococcus dysgalactiae by natural transformation.
mSphere, 9(7):e0021424, 21 Jun 2024
Cited by: 0 articles | PMID: 38904369 | PMCID: PMC11288034
Functional Analysis of the Major Pilin Proteins of Type IV Pili in Streptococcus sanguinis CGMH010.
Int J Mol Sci, 25(10):5402, 15 May 2024
Cited by: 0 articles | PMID: 38791440 | PMCID: PMC11121087
Enterococcus faecalis Strains with Compromised CRISPR-Cas Defense Emerge under Antibiotic Selection for a CRISPR-Targeted Plasmid.
Appl Environ Microbiol, 89(6):e0012423, 06 Jun 2023
Cited by: 3 articles | PMID: 37278656 | PMCID: PMC10304774
Go to all (242) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Positive transcriptional control of mry regulates virulence in the group A streptococcus.
Mol Microbiol, 7(6):893-903, 01 Mar 1993
Cited by: 87 articles | PMID: 8483419
VirR and Mry are homologous trans-acting regulators of M protein and C5a peptidase expression in group A streptococci.
Mol Gen Genet, 241(5-6):685-693, 01 Dec 1993
Cited by: 34 articles | PMID: 7505389
The identification of rofA, a positive-acting regulatory component of prtF expression: use of an m gamma delta-based shuttle mutagenesis strategy in Streptococcus pyogenes.
Mol Microbiol, 11(4):671-684, 01 Feb 1994
Cited by: 71 articles | PMID: 8196542
Molecular analysis of Streptococcus pyogenes adhesion.
Methods Enzymol, 253:269-305, 01 Jan 1995
Cited by: 11 articles | PMID: 7476392
Review