Abstract
Free full text

Identification and initial characterization of the eutF locus of Salmonella typhimurium.
Abstract
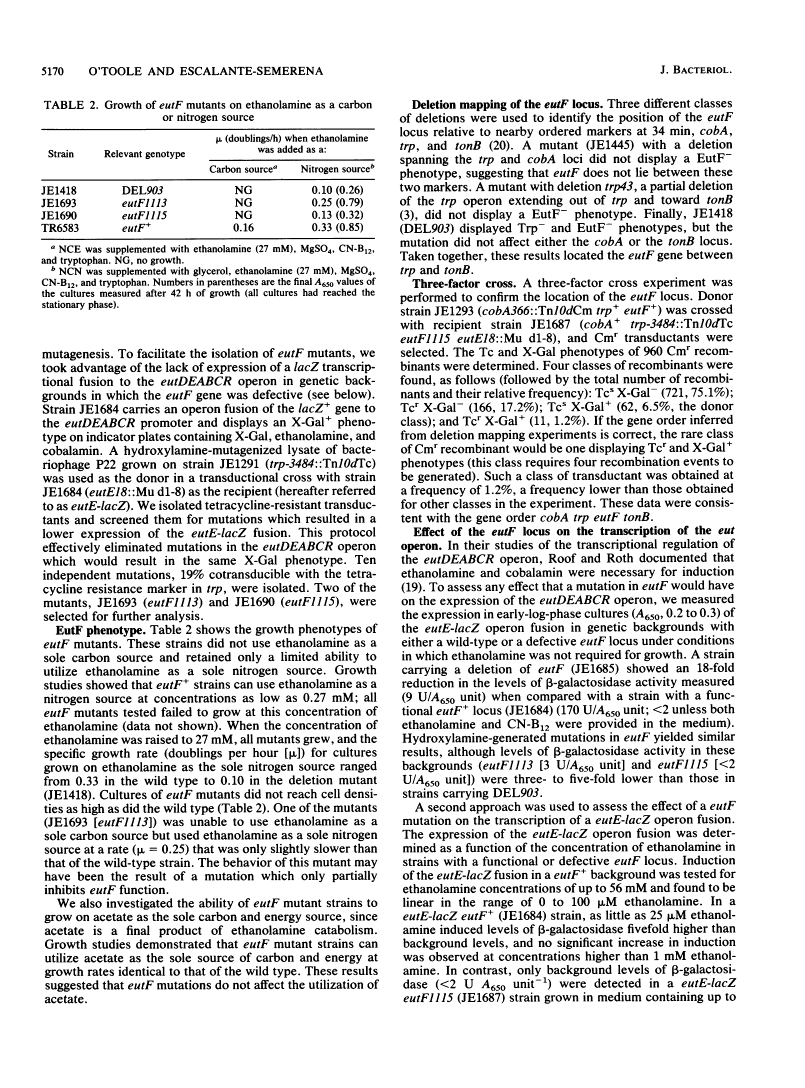
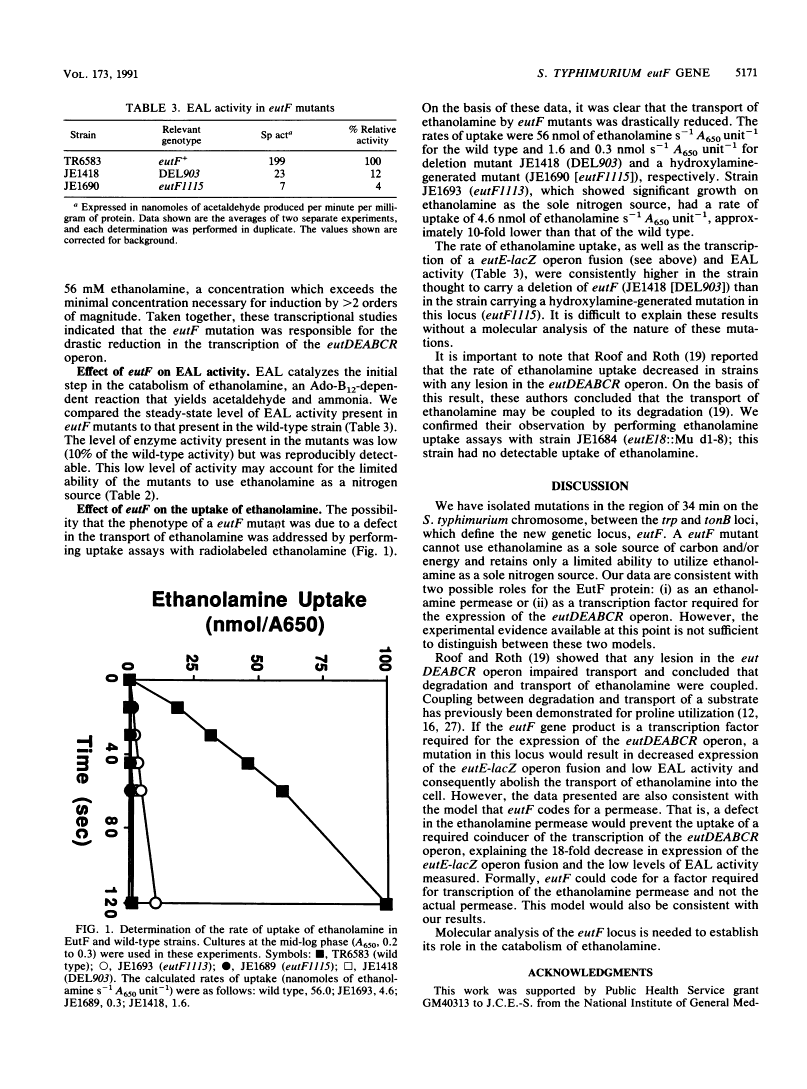
We report the isolation and initial characterization of mutations in the newly described eutF locus of Salmonella typhimurium LT2. Mutations in eutF render a strain unable to utilize ethanolamine as a source of carbon and/or energy and impair growth on ethanolamine as a sole nitrogen source. Strains carrying eutF mutations exhibit a 2-order-of-magnitude decrease in transcription of the unlinked eutDEABCR operon (50 min), which codes for the enzymes needed to catabolize ethanolamine; have only 10% of the ethanolamine ammonia-lyase activity found in the wild type; and show a marked reduction in the rate of ethanolamine uptake. Deletion mapping and three-factor cross analysis results are consistent with the gene order cobA trp eutF tonB at 34 min on the linkage map. We discuss two possible roles for the EutF protein: (i) as an ethanolamine permease or (ii) as a transcription factor required for the expression of the eutDEABCR operon.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford PJ, Jr, kadner RJ. Genetic analysis of components involved in vitamin B12 uptake in Escherichia coli. J Bacteriol. 1977 Dec;132(3):796–805. [Europe PMC free article] [Abstract] [Google Scholar]
- Blackwell CM, Turner JM. Microbial metabolism of amino alcohols. Formation of coenzyme B12-dependent ethanolamine ammonia-lyase and its concerted induction in Escherichia coli. Biochem J. 1978 Dec 15;176(3):751–757. [Europe PMC free article] [Abstract] [Google Scholar]
- Blume AJ, Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. [Europe PMC free article] [Abstract] [Google Scholar]
- Bochner BR, Huang HC, Schieven GL, Ames BN. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. [Europe PMC free article] [Abstract] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. II. Requirement for a cobamide coenzyme by an ethanolamine deaminase. J Biol Chem. 1965 Dec;240(12):4675–4681. [Abstract] [Google Scholar]
- Chang GW, Chang JT. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature. 1975 Mar 13;254(5496):150–151. [Abstract] [Google Scholar]
- Escalante-Semerena JC, Suh SJ, Roth JR. cobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium. J Bacteriol. 1990 Jan;172(1):273–280. [Europe PMC free article] [Abstract] [Google Scholar]
- Hong JS, Ames BN. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. [Europe PMC free article] [Abstract] [Google Scholar]
- Jeter RM, Olivera BM, Roth JR. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones PW, Turner JM. A model for the common control of enzymes of ethanolamine catabolism in Escherichia coli. J Gen Microbiol. 1984 Apr;130(4):849–860. [Abstract] [Google Scholar]
- Maloy SR, Nunn WD. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. [Europe PMC free article] [Abstract] [Google Scholar]
- Myers RS, Maloy SR. Mutations of putP that alter the lithium sensitivity of Salmonella typhimurium. Mol Microbiol. 1988 Nov;2(6):749–755. [Abstract] [Google Scholar]
- Ratzkin B, Grabnar M, Roth J. Regulation of the major proline permease gene of Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):737–743. [Europe PMC free article] [Abstract] [Google Scholar]
- Ratzkin B, Roth J. Cluster of genes controlling proline degradation in Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):744–754. [Europe PMC free article] [Abstract] [Google Scholar]
- Roof DM, Roth JR. Ethanolamine utilization in Salmonella typhimurium. J Bacteriol. 1988 Sep;170(9):3855–3863. [Europe PMC free article] [Abstract] [Google Scholar]
- Roof DM, Roth JR. Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium. J Bacteriol. 1989 Jun;171(6):3316–3323. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanderson KE, Roth JR. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. [Europe PMC free article] [Abstract] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. [Abstract] [Google Scholar]
- Schmieger H, Backhaus H. The origin of DNA in transducing particles in P22-mutants with increased transduction-frequencies (HT-mutants). Mol Gen Genet. 1973 Jan 24;120(2):181–190. [Abstract] [Google Scholar]
- Stocker BA, Nurminen M, Mäkelä PH. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. [Europe PMC free article] [Abstract] [Google Scholar]
- VOGEL HJ, BONNER DM. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [Abstract] [Google Scholar]
- Way JC, Davis MA, Morisato D, Roberts DE, Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. [Abstract] [Google Scholar]
- Wood JM, Zadworny D. Characterization of an inducible porter required for L-proline catabolism by Escherichia coli K12. Can J Biochem. 1979 Oct;57(10):1191–1199. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.173.16.5168-5172.1991
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc208209?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/173/16/5168
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/173/16/5168
Citations & impact
Impact metrics
Citations of article over time
Article citations
A pH-sensitive function and phenotype: evidence that EutH facilitates diffusion of uncharged ethanolamine in Salmonella enterica.
J Bacteriol, 186(20):6885-6890, 01 Oct 2004
Cited by: 24 articles | PMID: 15466042 | PMCID: PMC522209
The 17-gene ethanolamine (eut) operon of Salmonella typhimurium encodes five homologues of carboxysome shell proteins.
J Bacteriol, 181(17):5317-5329, 01 Sep 1999
Cited by: 184 articles | PMID: 10464203 | PMCID: PMC94038
Molecular characterization of eutF mutants of Salmonella typhimurium LT2 identifies eutF lesions as partial-loss-of-function tonB alleles.
J Bacteriol, 181(2):368-374, 01 Jan 1999
Cited by: 5 articles | PMID: 9882647 | PMCID: PMC93387
Glutathione is required for maximal transcription of the cobalamin biosynthetic and 1,2-propanediol utilization (cob/pdu) regulon and for the catabolism of ethanolamine, 1,2-propanediol, and propionate in Salmonella typhimurium LT2.
J Bacteriol, 177(19):5434-5439, 01 Oct 1995
Cited by: 44 articles | PMID: 7559326 | PMCID: PMC177348
Genetic map of Salmonella typhimurium, edition VIII.
Microbiol Rev, 59(2):241-303, 01 Jun 1995
Cited by: 75 articles | PMID: 7603411 | PMCID: PMC239362
Review Free full text in Europe PMC
Go to all (6) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular characterization of eutF mutants of Salmonella typhimurium LT2 identifies eutF lesions as partial-loss-of-function tonB alleles.
J Bacteriol, 181(2):368-374, 01 Jan 1999
Cited by: 5 articles | PMID: 9882647 | PMCID: PMC93387
Glutathione is required for maximal transcription of the cobalamin biosynthetic and 1,2-propanediol utilization (cob/pdu) regulon and for the catabolism of ethanolamine, 1,2-propanediol, and propionate in Salmonella typhimurium LT2.
J Bacteriol, 177(19):5434-5439, 01 Oct 1995
Cited by: 44 articles | PMID: 7559326 | PMCID: PMC177348
Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium.
J Bacteriol, 171(6):3316-3323, 01 Jun 1989
Cited by: 71 articles | PMID: 2656649 | PMCID: PMC210052
Ethanolamine utilization in Salmonella typhimurium.
J Bacteriol, 170(9):3855-3863, 01 Sep 1988
Cited by: 117 articles | PMID: 3045078 | PMCID: PMC211381
Funding
Funders who supported this work.
NIGMS NIH HHS (3)
Grant ID: 1 T32 GM08349
Grant ID: GM40313
Grant ID: R01 GM040313