Abstract
Free full text

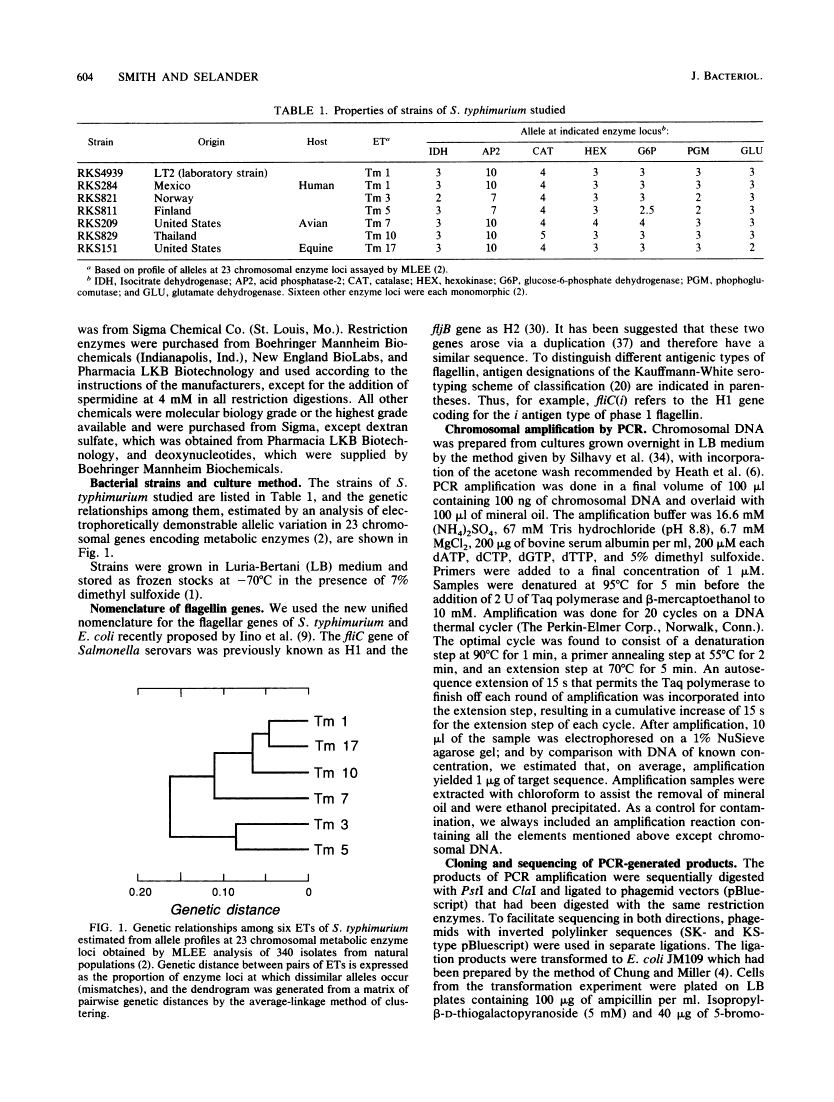
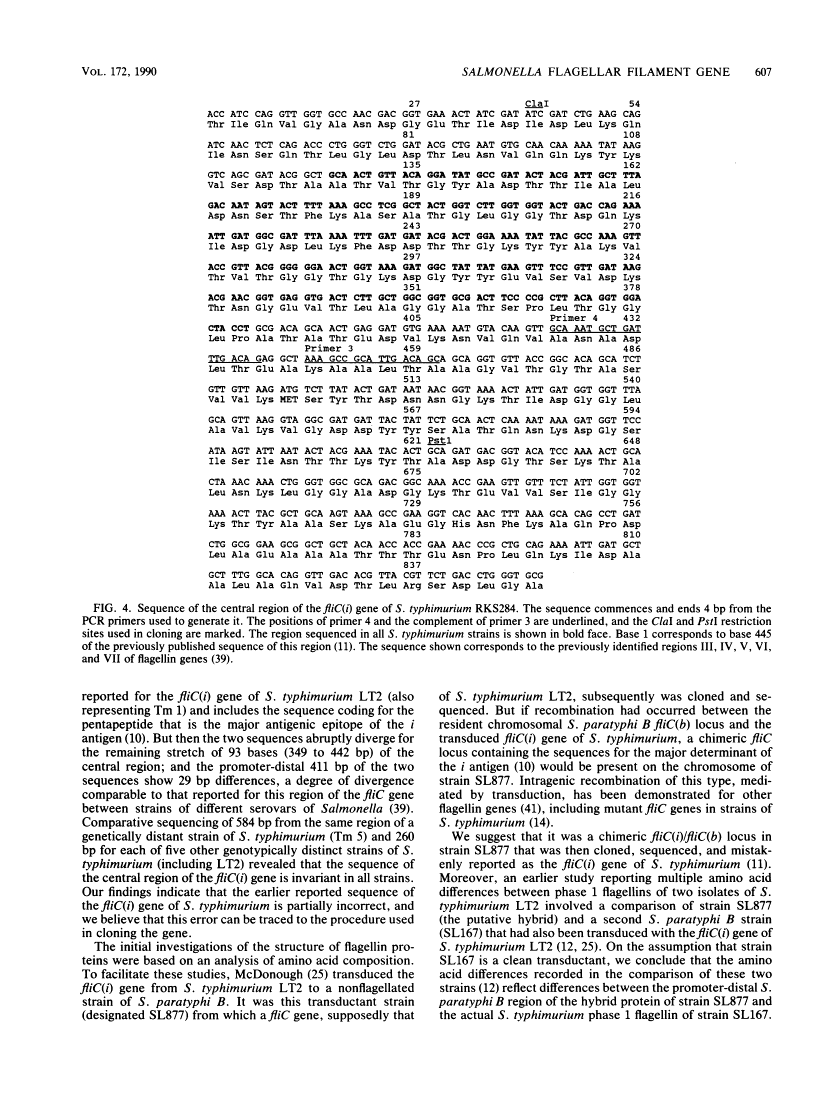
Sequence invariance of the antigen-coding central region of the phase 1 flagellar filament gene (fliC) among strains of Salmonella typhimurium.
Abstract
Previous studies of the phase 1 flagellar filament protein (flagellin) in strains of five serovars of Salmonella indicated that the central region of the fliC gene encoding the antigenic part of the protein is hypervariable both between and within serovars. To explore the possible use of this variation as a source of information on the phylogenetic relationships of closely related strains, we used the polymerase chain reaction technique to sequence part of the central region of the phase 1 flagellar genes of seven strains of Salmonella typhimurium that were known to differ in chromosomal genotype, as indexed by multilocus enzyme electrophoresis. We found that the nucleotide sequences of the central region were identical in all seven strains and determined that both the previously published sequence of the fliC gene in S. typhimurium LT2 and a report of a marked difference in the amino acid sequence of the phase 1 flagellins of two isolates of this serovar are erroneous. Our finding that the fliC gene is not evolving by sequence drift at an unusually rapid rate is compatible with a model that invokes lateral transfer and recombination of the flagellin genes as a major evolutionary process generating new serovars (antigen combinations) of salmonellae.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltran P, Musser JM, Helmuth R, Farmer JJ, 3rd, Frerichs WM, Wachsmuth IK, Ferris K, McWhorter AC, Wells JG, Cravioto A, et al. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7753–7757. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruist MF, Simon MI. Phase variation and the Hin protein: in vivo activity measurements, protein overproduction, and purification. J Bacteriol. 1984 Jul;159(1):71–79. [Europe PMC free article] [Abstract] [Google Scholar]
- Chung CT, Miller RH. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res. 1988 Apr 25;16(8):3580–3580. [Europe PMC free article] [Abstract] [Google Scholar]
- Gyllensten UB, Erlich HA. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. [Europe PMC free article] [Abstract] [Google Scholar]
- Heath LS, Sloan GL, Heath HE. A simple and generally applicable procedure for releasing DNA from bacterial cells. Appl Environ Microbiol. 1986 May;51(5):1138–1140. [Europe PMC free article] [Abstract] [Google Scholar]
- Homma M, Fujita H, Yamaguchi S, Iino T. Regions of Salmonella typhimurium flagellin essential for its polymerization and excretion. J Bacteriol. 1987 Jan;169(1):291–296. [Europe PMC free article] [Abstract] [Google Scholar]
- Homma M, Iino T. Locations of hook-associated proteins in flagellar structures of Salmonella typhimurium. J Bacteriol. 1985 Apr;162(1):183–189. [Europe PMC free article] [Abstract] [Google Scholar]
- Iino T, Komeda Y, Kutsukake K, Macnab RM, Matsumura P, Parkinson JS, Simon MI, Yamaguchi S. New unified nomenclature for the flagellar genes of Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1988 Dec;52(4):533–535. [Europe PMC free article] [Abstract] [Google Scholar]
- Joys TM. Identification of an antibody binding site in the phase-1 flagellar protein of Salmonella typhimurium. Microbios. 1976;15(61-62):221–228. [Abstract] [Google Scholar]
- Joys TM. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985 Dec 15;260(29):15758–15761. [Abstract] [Google Scholar]
- Joys TM, Martin JF, Wilson HL, Rankis V. Differences in the primary structure of the phase-1 flagellins of two strains of Salmonella typhimurium. Biochim Biophys Acta. 1974 Jun 7;351(2):301–305. [Abstract] [Google Scholar]
- JOYS TM, STOCKER BA. Mutation and recombination of flagellar antigen i of Salmonella typhimurium. Nature. 1963 Jan 26;197:413–414. [Abstract] [Google Scholar]
- Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuwajima G. Construction of a minimum-size functional flagellin of Escherichia coli. J Bacteriol. 1988 Jul;170(7):3305–3309. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuwajima G, Asaka J, Fujiwara T, Fujiwara T, Node K, Kondo E. Nucleotide sequence of the hag gene encoding flagellin of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1479–1483. [Europe PMC free article] [Abstract] [Google Scholar]
- Langman RE. The occurrence of antigenic determinants common to flagella of different salmonella strains. Eur J Immunol. 1972 Dec;2(6):582–586. [Abstract] [Google Scholar]
- Martin JH, Savage DC. Cloning, nucleotide sequence, and taxonomic implications of the flagellin gene of Roseburia cecicola. J Bacteriol. 1988 Jun;170(6):2612–2617. [Europe PMC free article] [Abstract] [Google Scholar]
- MCDONOUGH MW. AMINO ACID COMPOSITION OF ANTIGENICALLY DISTINCT SALMONELLA FLAGELLAR PROTEINS. J Mol Biol. 1965 Jun;12:342–355. [Abstract] [Google Scholar]
- Milkman R, Crawford IP. Clustered third-base substitutions among wild strains of Escherichia coli. Science. 1983 Jul 22;221(4608):378–380. [Abstract] [Google Scholar]
- Milkman R, Stoltzfus A. Molecular evolution of the Escherichia coli chromosome. II. Clonal segments. Genetics. 1988 Oct;120(2):359–366. [Europe PMC free article] [Abstract] [Google Scholar]
- Newton SM, Jacob CO, Stocker BA. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. [Abstract] [Google Scholar]
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. [Abstract] [Google Scholar]
- Sharp PM, Li WH. The rate of synonymous substitution in enterobacterial genes is inversely related to codon usage bias. Mol Biol Evol. 1987 May;4(3):222–230. [Abstract] [Google Scholar]
- Sharp PM, Li WH. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987 Feb 11;15(3):1281–1295. [Europe PMC free article] [Abstract] [Google Scholar]
- Shyamala V, Ames GF. Amplification of bacterial genomic DNA by the polymerase chain reaction and direct sequencing after asymmetric amplification: application to the study of periplasmic permeases. J Bacteriol. 1989 Mar;171(3):1602–1608. [Europe PMC free article] [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Stoltzfus A, Leslie JF, Milkman R. Molecular evolution of the Escherichia coli chromosome. I. Analysis of structure and natural variation in a previously uncharacterized region between trp and tonB. Genetics. 1988 Oct;120(2):345–358. [Europe PMC free article] [Abstract] [Google Scholar]
- Szekely E, Simon M. DNA sequence adjacent to flagellar genes and evolution of flagellar-phase variation. J Bacteriol. 1983 Jul;155(1):74–81. [Europe PMC free article] [Abstract] [Google Scholar]
- Trachtenberg S, DeRosier DJ. Three-dimensional reconstruction of the flagellar filament of Caulobacter crescentus. A flagellin lacking the outer domain and its amino acid sequence lacking an internal segment. J Mol Biol. 1988 Aug 20;202(4):787–808. [Abstract] [Google Scholar]
- Wei LN, Joys TM. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985 Dec 20;186(4):791–803. [Abstract] [Google Scholar]
- Wei LN, Joys TM. The nucleotide sequence of the H-1r gene of Salmonella rubislaw. Nucleic Acids Res. 1986 Oct 24;14(20):8227–8227. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamaguchi S, Iino T. Genetic determination of the antigenic specificity of flagellar protein in salmonella. J Gen Microbiol. 1969 Jan;55(1):59–74. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.172.2.603-609.1990
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc208483?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/172/2/603
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/172/2/603
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jb.172.2.603-609.1990
Article citations
Bending stiffness characterization of Bacillus subtilis' flagellar filament.
Biophys J, 121(11):1975-1985, 12 May 2022
Cited by: 0 articles | PMID: 35550881 | PMCID: PMC9247555
Phase-variable expression of pdcB, a phosphodiesterase, influences sporulation in Clostridioides difficile.
Mol Microbiol, 116(5):1347-1360, 18 Oct 2021
Cited by: 13 articles | PMID: 34606654 | PMCID: PMC8876291
The Remarkable Dual-Level Diversity of Prokaryotic Flagellins.
mSystems, 5(1):e00705-19, 11 Feb 2020
Cited by: 7 articles | PMID: 32047063 | PMCID: PMC7018530
Salmonella Virulence and Immune Escape.
Microorganisms, 8(3):E407, 13 Mar 2020
Cited by: 49 articles | PMID: 32183199 | PMCID: PMC7143636
Review Free full text in Europe PMC
Salmonella in Dairy Cattle.
Vet Clin North Am Food Anim Pract, 34(1):133-154, 08 Dec 2017
Cited by: 49 articles | PMID: 29224803 | PMCID: PMC7135009
Review Free full text in Europe PMC
Go to all (46) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recombination of Salmonella phase 1 flagellin genes generates new serovars.
J Bacteriol, 172(5):2209-2216, 01 May 1990
Cited by: 62 articles | PMID: 2129534 | PMCID: PMC208845
Development of a PCR system for the characterisation of Salmonella flagellin genes.
Acta Vet Hung, 53(2):163-172, 01 Jan 2005
Cited by: 9 articles | PMID: 15959975
Conversion of the Salmonella phase 1 flagellin gene fliC to the phase 2 gene fljB on the Escherichia coli K-12 chromosome.
J Bacteriol, 175(3):758-766, 01 Feb 1993
Cited by: 17 articles | PMID: 8423149 | PMCID: PMC196215
Sequence analysis of operator mutants of the phase-1 flagellin-encoding gene, fliC, in Salmonella typhimurium.
Gene, 85(1):221-226, 01 Dec 1989
Cited by: 21 articles | PMID: 2695399