Abstract
Free full text

Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci.
Abstract
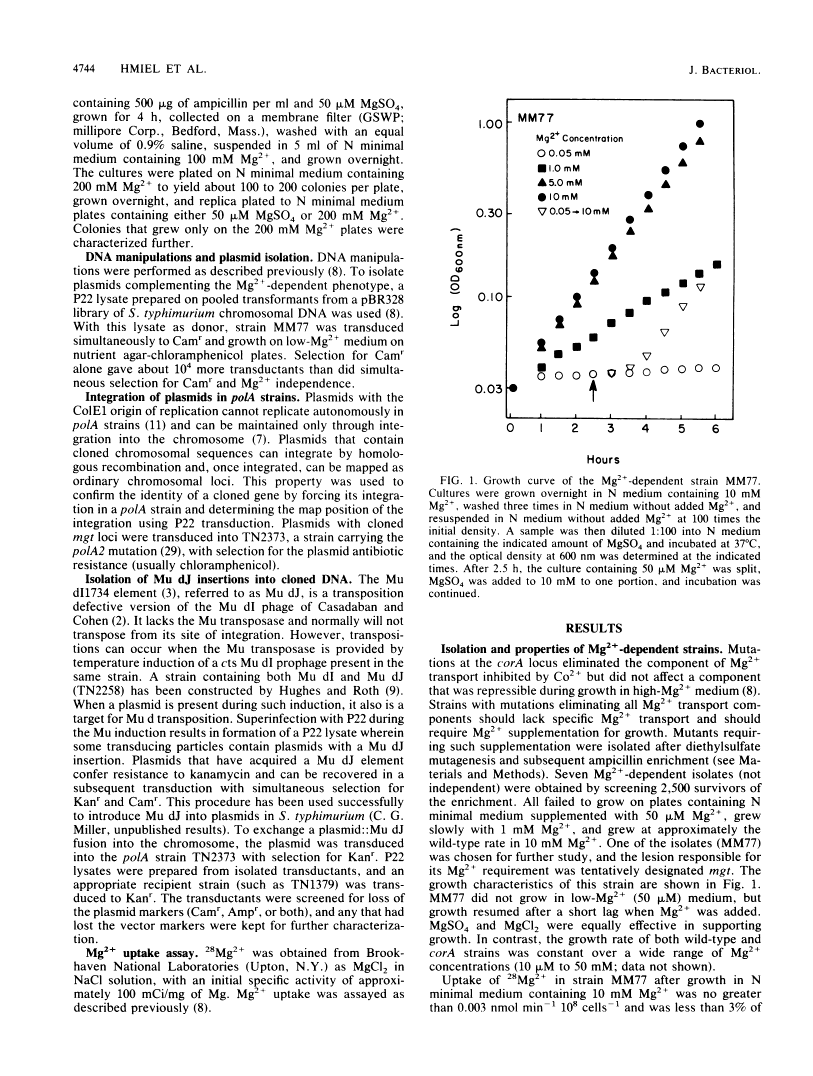
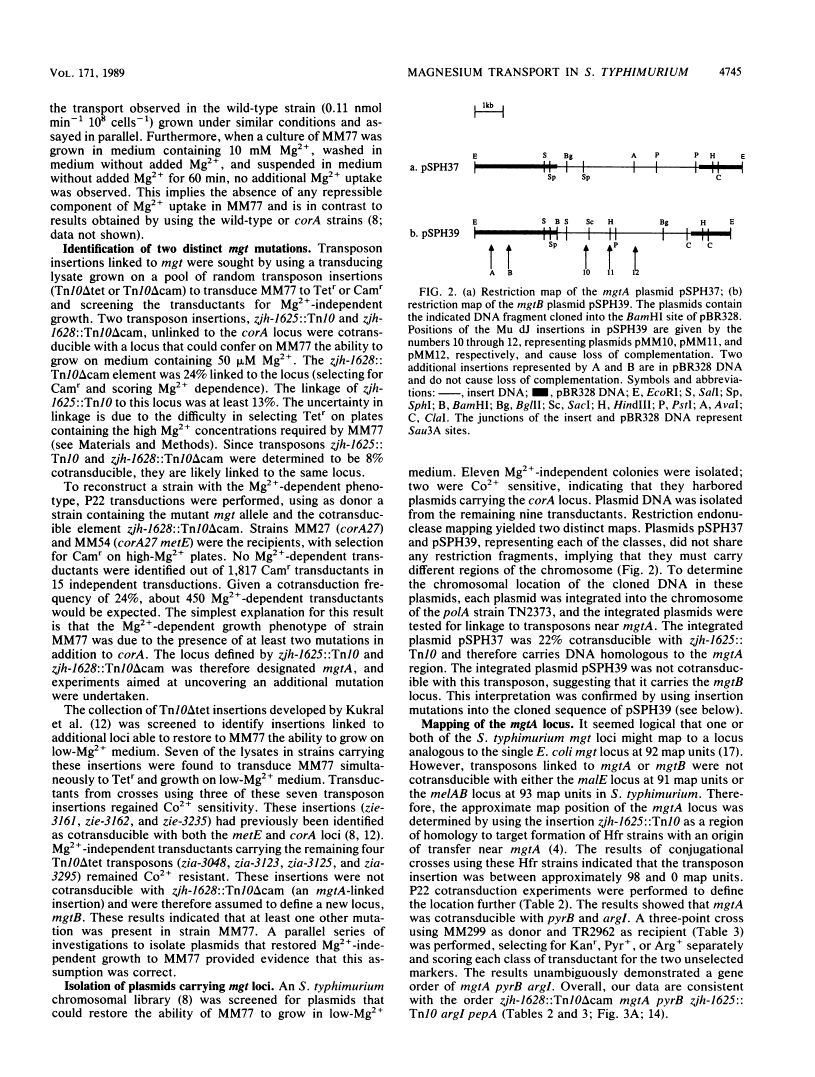
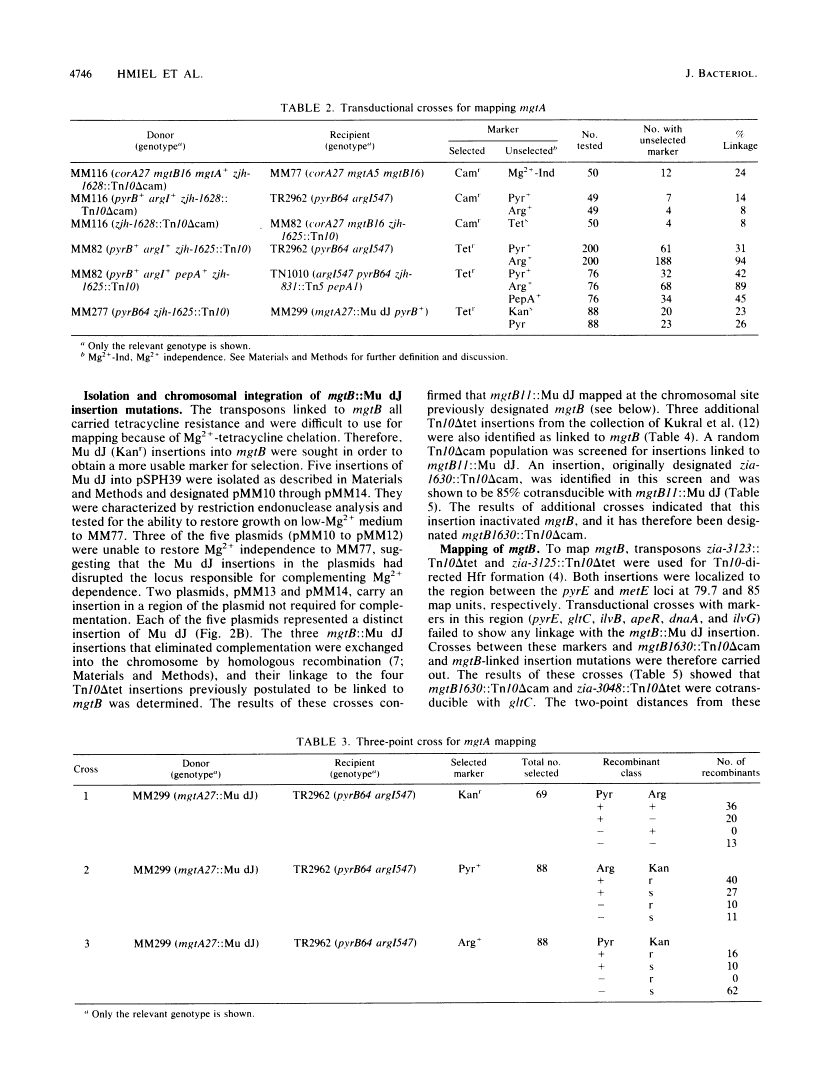
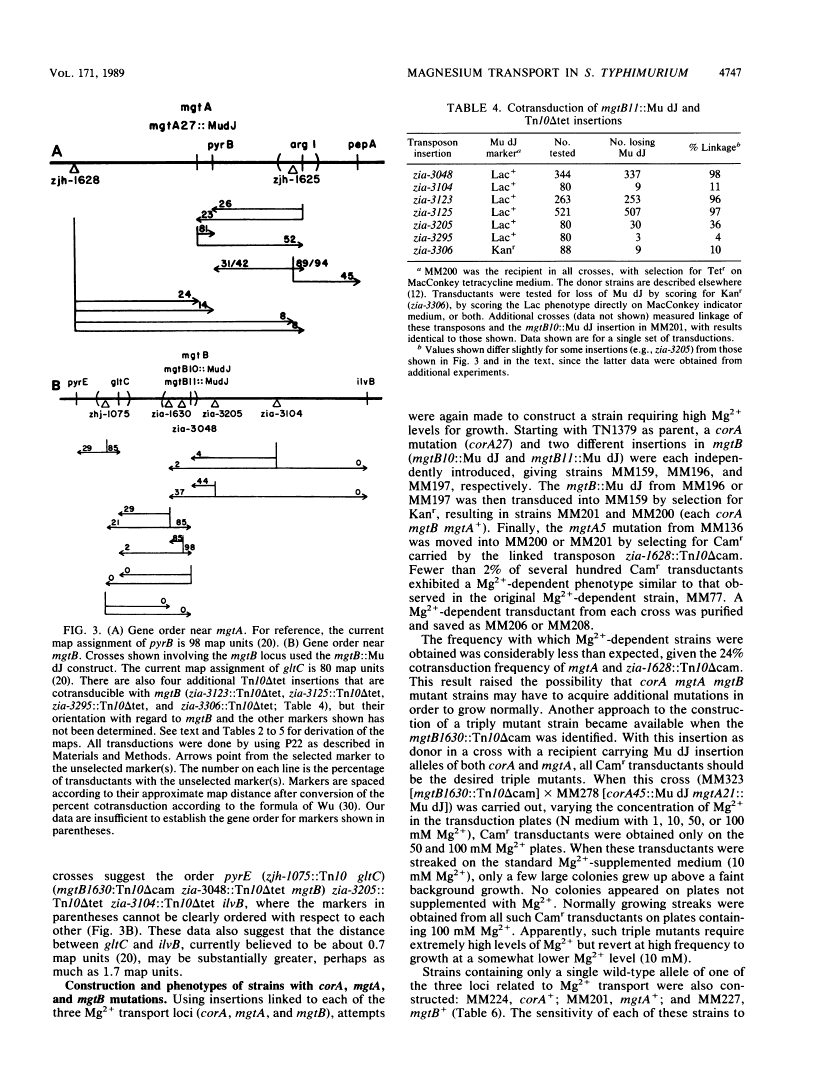
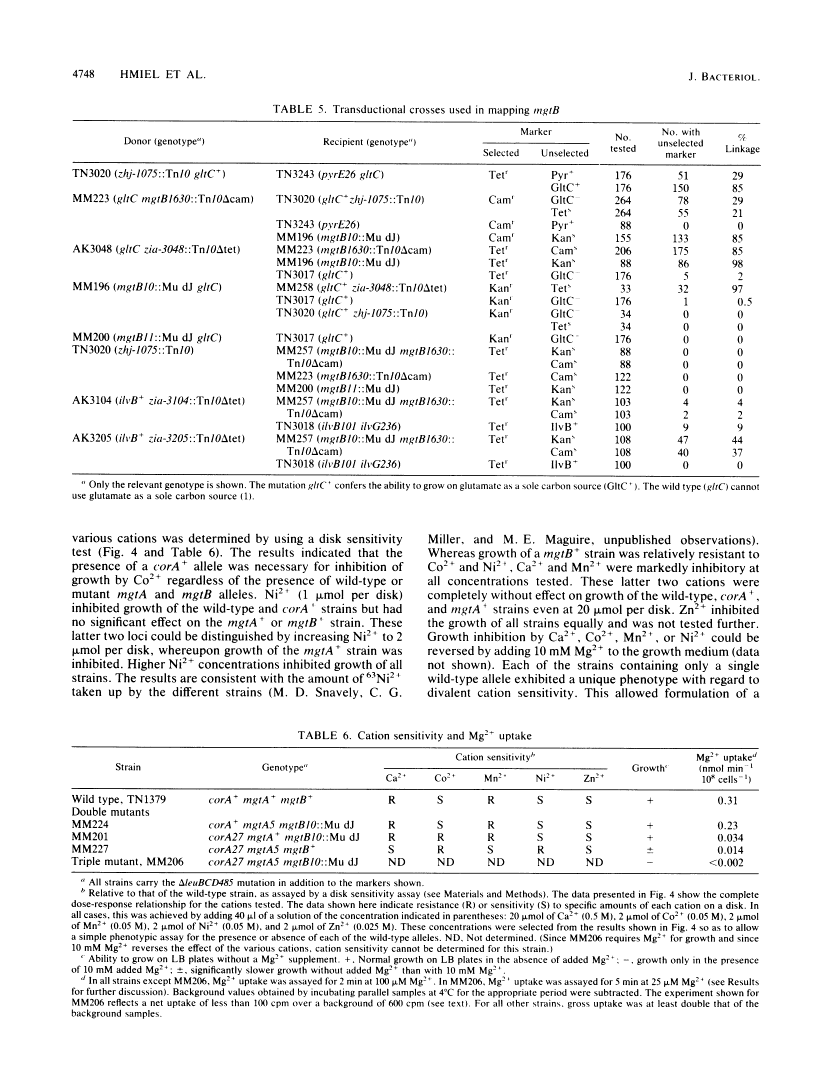
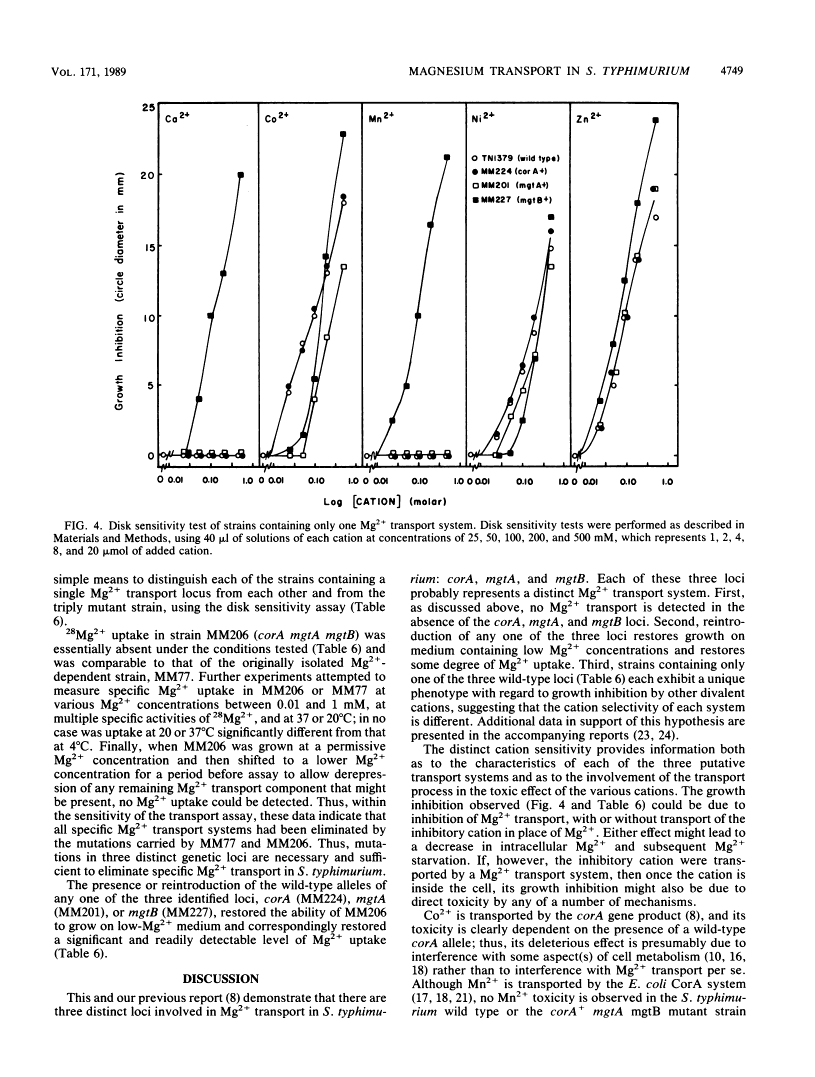
Salmonella typhimurium strains lacking the CorA Mg2+ transport system retain Mg2+ transport and the ability to grow in medium containing a low concentration of Mg2+. Mutagenesis of a corA strain followed by ampicillin selection allowed isolation of a strain that required Mg2+-supplemented media for growth. This strain contained mutations in at least two loci in addition to corA, designated mgtA and mgtB (for magnesium transport). Strains with mutations at all three loci (corA, mgtA, and mgtB) exhibited no detectable Mg2+ uptake and required 10 mM Mg2+ in the medium for growth at the wild-type rate. A wild-type allele at any one of the three loci was sufficient to restore both Mg2+ transport and growth on 50 microM Mg2+. P22 transduction was used to map the mgt loci. The mgtA mutation was located to approximately 98 map units (cotransducible with pyrB), and mgtB mapped at about 80.5 map units (near gltC). A chromosomal library from S. typhimurium was screened for clones that complemented the Mg2+ requirement of a corA mgtA mgtB mutant. The three classes of plasmids obtained could each independently restore Mg2+ transport to this strain and corresponded to the corA, mgtA, and mgtB loci. Whereas the corA locus of S. typhimurium is analogous to the corA locus previously described for Escherichia coli, neither of the mgt loci described in this report appears analogous to the single mgt locus described in E. coli. Our data in this and the accompanying papers (M. D. Snavely, J. B. Florer, C. G. Miller, and M. E. Maguire, J. Bacteriol. 171:4752-4760, 4761-4766, 1989) indicate that the corA, mgtA, and mgtB loci of S. typhimurium represent three distinct systems that transport Mg2+.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner BR, Savageau MA. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl Environ Microbiol. 1977 Feb;33(2):434–444. [Europe PMC free article] [Abstract] [Google Scholar]
- Casadaban MJ, Cohen SN. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. [Europe PMC free article] [Abstract] [Google Scholar]
- Castilho BA, Olfson P, Casadaban MJ. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. [Europe PMC free article] [Abstract] [Google Scholar]
- Chumley FG, Menzel R, Roth JR. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6(3):113–127. [Abstract] [Google Scholar]
- Gutterson NI, Koshland DE., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. [Europe PMC free article] [Abstract] [Google Scholar]
- Hmiel SP, Snavely MD, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986 Dec;168(3):1444–1450. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughes KT, Roth JR. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. [Europe PMC free article] [Abstract] [Google Scholar]
- Kingsbury DT, Helinski DR. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. [Abstract] [Google Scholar]
- Kukral AM, Strauch KL, Maurer RA, Miller CG. Genetic analysis in Salmonella typhimurium with a small collection of randomly spaced insertions of transposon Tn10 delta 16 delta 17. J Bacteriol. 1987 May;169(5):1787–1793. [Europe PMC free article] [Abstract] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller CG. Gentic mapping of Salmonella typhimurium peptidase mutations. J Bacteriol. 1975 Apr;122(1):171–176. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson DL, Kennedy EP. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971 May 10;246(9):3042–3049. [Abstract] [Google Scholar]
- Nelson DL, Kennedy EP. Transport of magnesium by a repressible and a nonrepressible system in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1091–1093. [Europe PMC free article] [Abstract] [Google Scholar]
- Park MH, Wong BB, Lusk JE. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol. 1976 Jun;126(3):1096–1103. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanderson KE, Roth JR. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. [Europe PMC free article] [Abstract] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. [Europe PMC free article] [Abstract] [Google Scholar]
- Silver S, Johnseine P, Whitney E, Clark D. Manganese-resistant mutants of Escherichia coli: physiological and genetic studies. J Bacteriol. 1972 Apr;110(1):186–195. [Europe PMC free article] [Abstract] [Google Scholar]
- Snavely MD, Florer JB, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems. J Bacteriol. 1989 Sep;171(9):4752–4760. [Europe PMC free article] [Abstract] [Google Scholar]
- Snavely MD, Florer JB, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol. 1989 Sep;171(9):4761–4766. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker GM. Magnesium and cell cycle control: an update. Magnesium. 1986;5(1):9–23. [Abstract] [Google Scholar]
- Way JC, Davis MA, Morisato D, Roberts DE, Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. [Abstract] [Google Scholar]
- Webb M. Interrelationships between the utilization of magnesium and the uptake of other bivalent cations by bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):428–439. [Abstract] [Google Scholar]
- Whitfield HJ, Levine G. Isolation and characterization of a mutant of Salmonella typhimurium deficient in a major deoxyribonucleic acid polymerase activity. J Bacteriol. 1973 Oct;116(1):54–58. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu TT. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.171.9.4742-4751.1989
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/171/9/4742.full.pdf
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/171/9/4742
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/171/9/4742
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Functional genomics and taxonomic insights into heavy metal tolerant novel bacterium Brevibacterium metallidurans sp. nov. NCCP-602<sup>T</sup> isolated from tannery effluent in Pakistan.
Antonie Van Leeuwenhoek, 117(1):111, 05 Aug 2024
Cited by: 0 articles | PMID: 39103503
Klebsiella pneumoniae DedA family proteins have redundant roles in divalent cation homeostasis and resistance to phagocytosis.
Microbiol Spectr, 12(2):e0380723, 12 Jan 2024
Cited by: 0 articles | PMID: 38214522 | PMCID: PMC10846249
P-type ATPases: Many more enigmas left to solve.
J Biol Chem, 299(11):105352, 12 Oct 2023
Cited by: 8 articles | PMID: 37838176 | PMCID: PMC10654040
Review Free full text in Europe PMC
Complete genome assembly of Hawai'i environmental nontuberculous mycobacteria reveals unexpected co-isolation with methylobacteria.
PLoS One, 18(9):e0291072, 13 Sep 2023
Cited by: 1 article | PMID: 37703253 | PMCID: PMC10499228
Membrane-Bound Protease FtsH Protects PhoP from the Proteolysis by Cytoplasmic ClpAP Protease in Salmonella Typhimurium.
J Microbiol Biotechnol, 33(9):1130-1140, 17 Jun 2023
Cited by: 0 articles | PMID: 37330414 | PMCID: PMC10580885
Go to all (94) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Magnesium transport in Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems.
J Bacteriol, 171(9):4752-4760, 01 Sep 1989
Cited by: 33 articles | PMID: 2548999 | PMCID: PMC210276
Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems.
J Bacteriol, 171(9):4761-4766, 01 Sep 1989
Cited by: 110 articles | PMID: 2670893 | PMCID: PMC210277
Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression.
J Biol Chem, 266(2):824-829, 01 Jan 1991
Cited by: 114 articles | PMID: 1898738
MgtA and MgtB: prokaryotic P-type ATPases that mediate Mg2+ influx.
J Bioenerg Biomembr, 24(3):319-328, 01 Jun 1992
Cited by: 31 articles | PMID: 1328179
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: T32-GM07250
Grant ID: GM26340