Abstract
Background
Amyotrophic lateral sclerosis (ALS) is defined as a disease of the motor neurones, although several studies indicate involvement of the sensory nervous system.Aim
To evaluate the sensory nerve conduction studies (NCS) in 88 patients with ALS as part of a European multicentre study.Methods
Seven European clinical neurophysiologists examined consecutive series of ALS patients. The examinations were peer reviewed, and the diagnosis of ALS was confirmed clinically.Results
20 (22.7%) patients with ALS had sensory NCS abnormalities in at least one nerve. Of those, 11 (12.5% of all patients) obtained an additional peer review diagnosis of electrophysiological polyneuropathy. There was no difference between the subgroups of patients with normal versus abnormal sensory NCS findings with respect to age, duration and region of onset.Conclusion
The findings support previous reports of sensory involvement in ALS, and raise the question of whether patients with ALS with sensory nerve abnormalities represent a variant of ALS. ALS associated with generalised sensory system abnormalities may be consistent with degeneration of motor neurones and dorsal root ganglion cells.Free full text

Generalised sensory system abnormalities in amyotrophic lateral sclerosis: a European multicentre study
Abstract
Background
Amyotrophic lateral sclerosis (ALS) is defined as a disease of the motor neurones, although several studies indicate involvement of the sensory nervous system.
Aim
To evaluate the sensory nerve conduction studies (NCS) in 88 patients with ALS as part of a European multicentre study.
Methods
Seven European clinical neurophysiologists examined consecutive series of ALS patients. The examinations were peer reviewed, and the diagnosis of ALS was confirmed clinically.
Results
20 (22.7%) patients with ALS had sensory NCS abnormalities in at least one nerve. Of those, 11 (12.5% of all patients) obtained an additional peer review diagnosis of electrophysiological polyneuropathy. There was no difference between the subgroups of patients with normal versus abnormal sensory NCS findings with respect to age, duration and region of onset.
Conclusion
The findings support previous reports of sensory involvement in ALS, and raise the question of whether patients with ALS with sensory nerve abnormalities represent a variant of ALS. ALS associated with generalised sensory system abnormalities may be consistent with degeneration of motor neurones and dorsal root ganglion cells.
Sporadic amyotrophic lateral sclerosis (ALS) is defined as a progressive degeneration of upper motor neurones (UMNs) and lower motor neurones (LMNs). Normal electrophysiological studies on sensory nerves are generally required for the diagnosis of ALS.1 Nevertheless, several neurological, clinical neurophysiological and neuropathological studies have suggested that ALS is a more generalised neurodegenerative disorder.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16
The aim of this study was to determine the incidence of patients with ALS with electrophysiological sensory nerve abnormalities and to examine the possible differences between patients with ALS with normal versus abnormal sensory nerve conduction studies (NCS). The study was carried out on the basis of electrodiagnostic examinations of 88 patients with ALS included in the European multicentre project ESTEEM (European Standardised Telematic tool to Evaluate Electrodiagnostic Methods).17
Methods
Patient collection
Seven clinical neurophysiologists from six different European countries prospectively collected 159 anterior horn cell disorder examinations from 1995 to 2003. Three doctors submitted 24 consecutive examinations, whereas the rest submitted 23, 22, 21 and 21 each. All doctors were experienced consultants, who examined the patients following regular electrophysiological examination routines. Skin temperature was maintained at between 32 and 35°C for all NCS.
All information from the examinations was transferred to an electronic data format, including standardised data from electrophysiological recordings and free text clinical information. The examinations were peer reviewed by the regular ESTEEM procedure, including discussions at workshop meetings, with the primary goal of reaching a consensus on the diagnosis.17 Firstly, an electrodiagnostic consensus diagnosis based solely on the electrophysiological data was sought. Secondly, to reach a final consensus diagnosis, all clinical neurological, biochemical and radiological data, in addition to the electrophysiological data, were reviewed. Information on quantitative sensory testing, as well as the nutritional status of the patients, was not systematically recorded. However, severe weight loss, vitamin B12 deficiency or any other medical condition was noted.
Selection criteria
The group's consensus diagnosis of ALS was based on the following criteria1:
Clinical and electromyographic evidence of progressive LMN degeneration
Clinical evidence of UMN degeneration
LMN and UMN signs in at least two of the four central nervous system regions: brain stem, cervical, thoracic or lumbosacral spinal cord
Clear progression of the disease.
Enrolment in this study further required:
No evidence of diabetes mellitus, malignancy, paraproteinaemia, nutritional deficiency or other known potential causes of polyneuropathy
No family history of ALS
Being an ambulatory patient
Confirmation of ALS diagnosis by follow‐up.
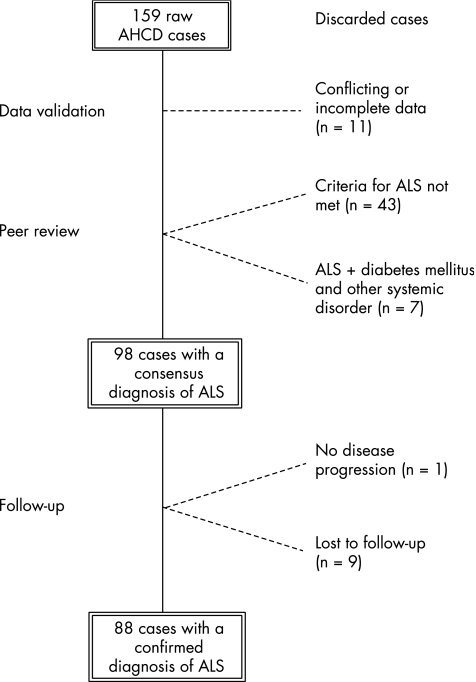
Figure 1 Outline of the group of patients with ALS. Of the 159 patients with anterior horn cell disorder (AHCD) sampled, 61 were excluded: 11 at the data validation level because of incomplete data submission (n
Outline of the group of patients with ALS. Of the 159 patients with anterior horn cell disorder (AHCD) sampled, 61 were excluded: 11 at the data validation level because of incomplete data submission (n =
= 3) or because of a consensus diagnosis other than anterior horn cell disorder (n
3) or because of a consensus diagnosis other than anterior horn cell disorder (n =
= 8); 43 at the peer review level as they did not meet the established criteria for ALS (23 primarily due to absence of upper motor neurone signs, 17 primarily due to lower motor neurone signs in one central nervous system region only or due to unconvincing electromyographic findings, 2 with Kennedy's disease and 1 with spinal muscular atrophy), 6 with diabetes mellitus and 1 with other systemic disorder. At follow‐up, 10 patients with an ALS consensus diagnosis were excluded: 9 patients were lost to follow‐up and 1 patient was excluded as there had been no disease progression over 5 years.
8); 43 at the peer review level as they did not meet the established criteria for ALS (23 primarily due to absence of upper motor neurone signs, 17 primarily due to lower motor neurone signs in one central nervous system region only or due to unconvincing electromyographic findings, 2 with Kennedy's disease and 1 with spinal muscular atrophy), 6 with diabetes mellitus and 1 with other systemic disorder. At follow‐up, 10 patients with an ALS consensus diagnosis were excluded: 9 patients were lost to follow‐up and 1 patient was excluded as there had been no disease progression over 5 years.
Patients with ALS
In all, 88 patients (48 women and 40 men) were included in the study (fig 1). The mean age was 63.6 years (range 36–85) and the mean duration from onset of symptoms to electrophysiological examination was 10.2 months (range 2–48). Limb onset was reported in 61 patients and bulbar onset in 23, and a combination of limb and bulbar onset was reported in 4 patients.
The patients were divided into three subgroups according to the consensus diagnosis and the sensory NCS findings, with nerve abnormality defined as a decrease in sensory nerve conduction velocity (SNCV) or a decrease in amplitude of the sensory nerve action potential (SNAP) of minimum 2 standard deviations from the mean of controls according to the locally used normative values.18 The groups were defined as follows:
ALS: Patients with normal sensory NCS findings.
ALS+GSSA: Patients with generalised sensory system abnormalities (GSSA). In addition to the main diagnosis of ALS, these patients obtained a peer review diagnosis of electrophysiological polyneuropathy, including abnormal NCS findings in at least two sensory nerves. The pathophysiological changes in these patients are, however, referred to as generalised sensory system abnormalities, because peripheral neuropathy and, for example, sensory neuronopathy cannot be distinguished by electrophysiological data.
ALS+possible GSSA: Patients with abnormal sensory NCS findings in at least one nerve.
Control group
A control group comprising 100 consecutive age‐matched patients (mean age 55.8 years; range 36–87) from the ESTEEM database with a main diagnosis other than polyneuropathy or anterior horn cell disorder was used to assess the frequency of an additional diagnosis of polyneuropathy by this particular group of doctors.
Statistical analysis
Continuous variables were compared among all three groups using the Kruskal–Wallis non‐parametric analysis of variance, and between the ALS+GSSA and ALS groups using the Mann–Whitney U test for independent samples. Proportions were compared using the χ2 statistics. p Values <0.05 were considered significant.
Results
Of the 88 patients with ALS, 68 (77.3%) patients were entered into the ALS group, 11 (12.5%) patients into the ALS+GSSA group and 9 (10.3%) patients into the ALS+possible GSSA group. The incidence of patients with additional consensus diagnosis of polyneuropathy was significantly higher in the ALS group than in the control group (12.5% v 2.0%; p<0.01).
Comparison of sex, disease duration and region of onset among the three subgroups of patients with ALS did not show any differences. The mean age in the three groups was ALS: 62.7 years (range 36–85); ALS GSSA: 69.3 years (range 50–81); and ALS+possible GSSA: 62.8 years (range 52–77). The mean duration to examination in the three groups was ALS: 10.4 months (range 2–48); ALS+GSSA: 10 months (range 2–24); and ALS+possible GSSA: 8.9 months (range 3–30). The frequency of patients with bulbar onset was ALS: 26%; ALS+GSSA: 27%; and ALS+possible GSSA: 22%.
When comparing the ALS+GSSA and ALS groups separately, the average number of sensory nerves examined per patient was higher in the ALS+GSSA group (3.7 v 2.8; p =
= 0.04). Furthermore, there was a tendency (p
0.04). Furthermore, there was a tendency (p =
= 0.09) towards older age of the patients in the ALS+GSSA group.
0.09) towards older age of the patients in the ALS+GSSA group.
No sensory deficits on neurological examination were reported in any of the patients in the ALS+GSSA or ALS+possible GSSA groups.
Electrophysiological findings
Of 41 sensory nerves examined in the ALS+GSSA group, 32 (78%) nerves had abnormal SNC parameters: 6 had decreased SNCV and decreased SNAP amplitude, 10 had decreased SNCV, 14 had decreased SNAP amplitude and 2 nerves did not elicit any response (table 11).). Of the 41 sensory nerves examined in the ALS+possible GSSA group, 15 (37%) nerves had electrophysiological deficits: 5 had decreased SNCV and SNAP amplitude, 7 had decreased SNAP amplitude, 2 had decreased SNCV and 1 nerve did not yield any sensory response. None of the abnormalities in any of the patients in the two groups was due to nerve entrapment.
 Electrophysiological findings in the ALS+GSSA and ALS+possible GSSA groups
Electrophysiological findings in the ALS+GSSA and ALS+possible GSSA groups| Group | Patient | No of examined sensory nerves | Percentage of abnormal sensory nerves | Abnormal nerve(s) | SNCV | SNAP amplitude |
|---|---|---|---|---|---|---|
| ALS+GSSA | 1 | 5 | 100 | Radial | ↓ | ↓ |
| Median | ↓ | ↓ | ||||
| Ulnar | ↓ | ↓ | ||||
| Sural | No response | |||||
| Peroneal | No response | |||||
| 2 | 3 | 67 | Ulnar | ↓ | ||
| Sural | ↓ | ↓ | ||||
| 3 | 2 | 100 | Ulnar | ↓ | ||
| Peroneal | ↓ | |||||
| 4 | 4 | 50 | Median | ↓ | ||
| Median | ↓ | |||||
| 5 | 3 | 100 | Median | ↓ | ||
| Ulnar | ↓ | |||||
| Sural | ↓ | |||||
| 6 | 4 | 75 | Radial | ↓ | ||
| Median | ↓ | |||||
| Sural | ↓ | |||||
| 7 | 3 | 67 | Median | ↓ | ||
| Ulnar | ↓ | |||||
| 8 | 4 | 50 | Median | ↓ | ||
| Sural | ↓ | |||||
| 9 | 2 | 100 | Median | ↓ | ||
| Ulnar | ↓ | |||||
| 10 | 8 | 75 | Median | ↓ | ↓ | |
| Median | ↓ | ↓ | ||||
| Ulnar | ↓ | |||||
| Sural | ↓ | |||||
| Peroneal | ↓ | |||||
| Peroneal | ↓ | |||||
| 11 | 3 | 100 | Median | ↓ | ||
| Sural | ↓ | |||||
| Sural | ↓ | |||||
| ALS+possible GSSA | 12 | 4 | 25 | Sural | ↓ | |
| 13 | 5 | 20 | Median | ↓ | ||
| 14 | 5 | 20 | Ulnar | ↓ | ||
| 15 | 3 | 67 | Ulnar | ↓ | ||
| Ulnar | ↓ | |||||
| 16 | 5 | 40 | Median | ↓ | ↓ | |
| Ulnar | No response | |||||
| 17 | 3 | 33 | Median | ↓ | ||
| 18 | 2 | 50 | Median | ↓ | ↓ | |
| 19 | 8 | 25 | Peroneal Peroneal | ↓ | ↓ | |
| ↓ | ↓ | |||||
| 20 | 6 | 33 | Peroneal Peroneal | ↓ | ||
ALS, amyotrophic lateral sclerosis; GSSA, generalised sensory system abnormalities; SNAP, sensory nerve action potential; SNCV, sensory nerve conduction velocity; ↓, decrease.
Discussion
In this study, 20 (22.7%) patients from a series of 88 patients with ALS sampled by an international group of seven experienced neurophysiologists showed abnormalities in sensory NCS parameters. The doctors, serving as a peer review group, furthermore diagnosed 11 (12.5%) patients with additional electrophysiological polyneuropathy. This proportion clearly exceeds the reported frequencies of polyneuropathy in the background population by 0.04–0.1%, and the 1.6% prevalence of polyneuropathy in patients >55 years with no recognised exposure to diseases or neurotoxic agents found in an Italian multicentre study.19,20,21 These rates may be underestimated, as they were based on clinical assessment; higher frequencies are expected when electrodiagnostic tests are applied.22 However, the frequency of additional polyneuropathy in our age‐matched control group comprising patients with various disorders examined in the involved laboratories was 2%.
The significantly greater proportion of patients with normal sensory NCS findings in this study indicates that sensory nerves are not generally affected in patients with ALS. Rather, patients with sensory nerve dysfunction may represent a variant of ALS. The similar disease duration of <11 months in the three ALS subgroups indicates that the sensory nerve involvement is part of the primary disease, which is supported by the non‐progressive subclinical findings of the sensory system in patients with ALS by Theys et al,7 and the recent quantitative demonstration of a marked degeneration of sensory axons in the dorsal roots of the SOD1G93A mouse model of ALS, which appeared to be non‐progressive with advancing disease.23
Although the patients in this study were diagnosed with electrophysiological peripheral polyneuropathy, the sensory nerve abnormalities can be generated in the dorsal root ganglion, as a peripheral sensory axonal neuropathy and a ganglionopathy cannot be distinguished using electrodiagnostic methods. Morphological findings of sensory nerve biopsies from patients with ALS have shown axonal degeneration, segmental demyelination and a reduced number of large neurones of the L5 dorsal root ganglion.8,9,10,12 These findings and the findings of disparate electrophysiological abnormalities in this study, not consistently indicative of either axonal loss or demyelination, are in keeping with the suggestion by Heads et al10 that the primary pathology in the sensory peripheral nerve in ALS is dorsal root ganglion neuronopathy, resulting initially in progressive axonal atrophy followed by secondary de‐remyelination and ultimately by axonal loss.
The revised El Escorial (World Federation of Neurology) criteria for the diagnosis of ALS allow abnormal sensory NCS only in the presence of entrapment syndrome or coexisting peripheral nerve disease.1 Normal sensory NCS have been shown in a series of patients with ALS.24,25 Other neurophysiological studies have reported frequencies of patients with ALS with abnormal findings of the peripheral nervous system ranging between 13% and 22%.3,5,6,7 In this study, in a few patients only two sensory nerves were studied or reduced SNCV was the sole finding. Clearly, caution should be taken in these cases; however, broad criteria that include the presence of some electrophysiological sensory abnormality may be appropriate. Further prospective studies are needed to determine how much abnormality is allowable in the diagnosis of ALS.
Abbreviations
ALS - amyotrophic lateral sclerosis
ESTEEM - European Standardised Telematic Tool to Evaluate Electrodiagnostic Methods
GSSA - generalised sensory system abnormalities
LMN - lower motor neurone
NCS - nerve conduction studies
SNAP - sensory nerve action potential
SNCV - sensory nerve conduction velocity
UMN - upper motor neurone
Footnotes
Competing interests: None declared.
References
Articles from Journal of Neurology, Neurosurgery, and Psychiatry are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/jnnp.2006.098533
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2117695?pdf=render
Citations & impact
Impact metrics
Article citations
Analysis and occurrence of biallelic pathogenic repeat expansions in RFC1 in a German cohort of patients with a main clinical phenotype of motor neuron disease.
J Neurol, 271(9):5804-5812, 25 Jun 2024
Cited by: 0 articles | PMID: 38916676 | PMCID: PMC11377604
Shotgun Proteomics Links Proteoglycan-4+ Extracellular Vesicles to Cognitive Protection in Amyotrophic Lateral Sclerosis.
Biomolecules, 14(6):727, 19 Jun 2024
Cited by: 0 articles | PMID: 38927130
Non-motor symptoms in patients with amyotrophic lateral sclerosis: current state and future directions.
J Neurol, 271(7):3953-3977, 28 May 2024
Cited by: 0 articles | PMID: 38805053 | PMCID: PMC11233299
Review Free full text in Europe PMC
Non-Pharmacological Interventions on Pain in Amyotrophic Lateral Sclerosis Patients: A Systematic Review and Meta-Analysis.
Healthcare (Basel), 12(7):770, 01 Apr 2024
Cited by: 0 articles | PMID: 38610192 | PMCID: PMC11011838
Review Free full text in Europe PMC
Amyotrophic Lateral Sclerosis and Pain: A Narrative Review from Pain Assessment to Therapy.
Behav Neurol, 2024:1228194, 16 Mar 2024
Cited by: 1 article | PMID: 38524401 | PMCID: PMC10960655
Review Free full text in Europe PMC
Go to all (73) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Quantitative thermal sensory testing in patients with amyotrophic lateral sclerosis using reaction time exclusive method of levels (MLE).
Electromyogr Clin Neurophysiol, 46(3):145-148, 01 May 2006
Cited by: 4 articles | PMID: 16918198
Autosomal dominant juvenile amyotrophic lateral sclerosis.
Brain, 122 ( Pt 8):1539-1550, 01 Aug 1999
Cited by: 54 articles | PMID: 10430837
Involvement of distal sensory nerves in amyotrophic lateral sclerosis.
Muscle Nerve, 54(6):1086-1092, 07 Oct 2016
Cited by: 14 articles | PMID: 27104485
Sensory nerve disturbance in amyotrophic lateral sclerosis.
Life Sci, 203:242-245, 27 Apr 2018
Cited by: 18 articles | PMID: 29709651
Review





