Abstract
Free full text

Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion
Associated Data
Abstract
Type 2 immunity, which involves coordinated regulation of innate and adaptive immune responses, can protect against helminth parasite infection, but may lead to allergy and asthma after inappropriate activation. We demonstrate that il25−/− mice display inefficient Nippostrongylus brasiliensis expulsion and delayed cytokine production by T helper 2 cells. We further establish a key role for interleukin (IL)-25 in regulating a novel population of IL-4–, IL-5–, IL-13–producing non–B/non–T (NBNT), c-kit+, Fc R1− cells during helminth infection. A deficit in this population in il25−/− mice correlates with inefficient N. brasiliensis expulsion. In contrast, administration of recombinant IL-25 in vivo induces the appearance of NBNT, c-kit+, Fc
R1− cells during helminth infection. A deficit in this population in il25−/− mice correlates with inefficient N. brasiliensis expulsion. In contrast, administration of recombinant IL-25 in vivo induces the appearance of NBNT, c-kit+, Fc R1− cells and leads to rapid worm expulsion that is T and B cell independent, but type 2 cytokine dependent. We demonstrate that these IL-25–regulated cells appear rapidly in the draining lymph nodes, implicating them as a source of type 2 cytokines during initiation of worm expulsion.
R1− cells and leads to rapid worm expulsion that is T and B cell independent, but type 2 cytokine dependent. We demonstrate that these IL-25–regulated cells appear rapidly in the draining lymph nodes, implicating them as a source of type 2 cytokines during initiation of worm expulsion.
IL-25/IL-17E is a member of the structurally related IL-17 cytokine family (1). The cardinal family member is IL-17 (IL-17A), which is a proinflammatory cytokine derived from T cells with specific roles in allergic and humoral responses (2) that has been found to be elevated in diseases such as asthma (3), rheumatoid arthritis (4), multiple sclerosis (5), and systemic lupus erythematosus (6), as well as in transplant rejection (7). Human IL-17A is secreted as a homodimeric glycoprotein. Initial cloning of IL-17A cDNA identified significant sequence homology with an open reading frame of Herpes saimiri (8). More recently, database searches and degenerative RT-PCR strategies have identified five related cytokines (IL-17B, IL-17C, IL-17D, IL-17E/IL-25, and IL-17F) that share between 16 and 50% sequence homology with IL-17A (9, 10).
Although the biological functions of the various IL-17 family members remain to be fully characterized, studies have suggested that IL-17A, IL-17B, and IL-17C have several coincident biological activities typical of type 1 inflammatory responses. In contrast, studies of IL-17E (IL-25) have indicated that its functions are significantly different from the other family members and are associated with type 2 responses. Indeed, transgenic expression of both human IL-25 (11) and mouse IL-25 (12) has been shown to induce type 2 biased responses with increased IL-4, IL-5, IL-13 production, eosinophilia, and elevated IgE. Similarly, administration of recombinant mouse IL-25 (rIL-25) also induced Th2 pathologies that were shown to be dependent on the downstream production of the Th2 cytokines IL-4, IL-5 and IL-13 (13). Native IL-25 expression has been detected from in vitro–differentiated Th2 cells (13) and in vitro–cultured mast cells (14). However, identification of the IL-25–responsive cell populations has proven elusive, although a non–B/non–T (NBNT) cell population expressing high levels of MHC class II and low levels of CD11c has been reported to up-regulate IL-5 and IL-13 production after stimulation with IL-25 (13).
Type 2 responses are characteristic of the beneficial immune responses generated to combat parasitic helminth infection, but also of the inappropriate immune response leading to allergy and asthma. Within this response, the CD4+ Th2 cell subset-producing cytokines that include IL-4, IL-5, IL-9, and IL-13 play a central role in the regulation of effector functions such as stimulating B cell growth and initiating immunoglobulin isotype switching to IgE, and inducing goblet cell hyperplasia and associated mucus production, eosinophilia, mastocytosis, and fibrosis (15). It is also evident that cells of the innate immune system, including eosinophils, basophils, and mast cells, can also produce significant levels of IL-4, IL-5, IL-9, and IL-13 that can alter the magnitude of the type 2 response, both by providing appropriate signals at the initiation of a response, as well as sustaining ongoing type 2 responses (15–17). Indeed, it has been reported recently that polarized type 2 responses, induced after infection with the parasitic helminth Nippostrongylus brasiliensis, develop independently of the adaptive immune response with innate cells representing an important initial source of IL-4 to drive Th2 differentiation (18, 19). Basophils have been shown to be a principal source of IL-4 during N. brasiliensis infection (20) and in response to T-independent antigen challenge (21).
The role of IL-25 in protective immunity is currently undefined. To investigate how IL-25 might regulate type 2 cytokine responses, we generated a novel line of il25
−/− mice and assessed their immune responses to helminth infection. Significantly, in the absence of IL-25, mice fail to expel N. brasiliensis efficiently. This defect correlates with a striking delay in the up-regulation of type 2 cytokine production. This does not appear to be the result of an inability to generate Th2 cells per se, but correlates with a deficit in a population of NBNT, c-kit+, Fc R1−, IL-4–, IL-5–, IL-13–producing cells in the draining lymph nodes of the infected il25
−/− mice after parasite infection.
R1−, IL-4–, IL-5–, IL-13–producing cells in the draining lymph nodes of the infected il25
−/− mice after parasite infection.
RESULTS
il25−/− mice fail to expel N. brasiliensis efficiently and have delayed type 2 cytokine production
Gastrointestinal helminth parasites typically induce a type 2 phenotype (22). N. brasiliensis is a commonly used parasite model and disruption of type 2 cytokines has been shown to compromise efficient parasite clearance (15). To determine a possible role for IL-25 in protective type 2 responses, we generated il25 −/− mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20051615/DC1). We infected il25 −/− mice and wild-type controls with N. brasiliensis and monitored for worm burden and type 2 effector functions. The wild-type and il25 −/− mice displayed very similar worm burdens at day 5 after infection; by day 10 after infection, the wild-type animals had expelled their parasites, whereas the il25 −/− mice retained an extremely high worm burden (Fig. 1 A). Even at 15 d after infection, the il25 −/− mice still harbored significant numbers of intestinal parasites. Furthermore, measurement of N. brasiliensis egg production from the infected groups demonstrated that, before expulsion, the worms were healthy and fecund (Fig. 1 B).

il25−/− mice fail to expel N. brasiliensis efficiently and have delayed type 2 cytokine production. (A) Infected mice were killed at the times indicated to obtain intestinal worm counts. (B) Fecal egg counts were determined at the times indicated. Epg, eggs per gram of feces. (C) Cytokine production after infection. MLN cells from wild-type and il25
−/− animals after infection with N. brasiliensis were stimulated with N. brasiliensis antigen. Cytokines were analyzed by ELISA. (D) Total serum IgE expression from wild-type and il25
−/− animals after infection. (E) Mouse mast cell protease-1 (MMCP-1) activity was measured from sera. (F) Determination of goblet cell number and enumeration of PAS positive goblet cells per villus crypt unit. (G) Blood basophils were enumerated after FACS analysis of NBNT, c-kit−, Fc R1+, SSClow cells. (H) Blood eosinophils were enumerated after FACS analysis of NBNT, CCR3+, SSChigh. White bars, wild type; black bars, il25
−/−. Cohorts of five to six infected mice were used per group. Data are presented as means plus standard error and are representative of two to three experiments. ND, not detected.
R1+, SSClow cells. (H) Blood eosinophils were enumerated after FACS analysis of NBNT, CCR3+, SSChigh. White bars, wild type; black bars, il25
−/−. Cohorts of five to six infected mice were used per group. Data are presented as means plus standard error and are representative of two to three experiments. ND, not detected.
We assessed antigen-specific cytokine production from the mesenteric lymph node (MLN) cells of the infected il25 −/− and wild-type mice. As expected, wild-type animals produced elevated levels of IL-5 and IL-13 in response to infection as determined by N. brasiliensis antigen restimulation of MLN cells isolated at day 5 after infection (Fig. 1 C). In contrast, cytokine production from the il25 −/− mice at the same time point revealed IL-5 and IL-13 levels approximately fivefold lower than wild-type controls (Fig. 1 C). By day 10 after infection, when the wild-type animals had already expelled their parasite burden, the levels of IL-5 and IL-13 had fallen relative to day 5 levels. These data contrast with the highly up-regulated IL-4, IL-5, and IL-13 secretion detected at day 10 after infection in il25 −/− mice, at a time when viable egg-laying worms remained in the intestine. Such elevated levels of type 2 cytokines are indicative of compensatory cytokine production arising from the sustained antigenic stimulation as a result of the temporal delay in the il25 −/− mice expelling their parasite burden (Fig. 1 C). With the decreasing parasitic load observed at days 15 and 20 after infection, the levels of type 2 cytokines declined substantially (Fig. 1 C).
Type 2 cytokines induce a spectrum of effector functions that have been demonstrated to play central roles in the regulation of helminth infections, including eosinophilia, immunoglobulin isotype switching to IgE and IgG1, mastocytosis, goblet cell hyperplasia, and fibrosis (15, 22). Serum samples collected from wild-type and il25
−/− mice at various time points after infection were analyzed for the presence of total serum IgE. At days 0 and 5, comparable levels of IgE were detected in the serum of wild-type and il25
−/− mice; however, after day 10 of infection more highly elevated IgE production was detected in the serum of il25
−/− mice (Fig. 1 D). This correlates with the increased expression of IL-4, a key switch factor for IgE production in the mouse (15). A similar overexpression was also detected for mast cell protease-1, used as a measure of mast cells, in the serum from il25
−/− mice 10–20 d after infection (Fig. 1 E). Surprisingly, given the central role for mucus production in worm expulsion and the importance of IL-13 in inducing this effector function, we were unable to detect any significant delay in the onset of goblet cell hyperplasia (Fig. 1 F). There was also no difference between wild-type and il25
−/− mice in the marked elevation in blood basophils (NBNT, c-kit−, Fc R1+, SSClow; Fig. 1 G) that occurs during N. brasiliensis infection (16, 23, 24). However, using flow cytometric analysis of the blood isolated from animals after infection, we observed a deficit in the number of circulating eosinophils (NBNT, CCR3+, SSChigh) in il25
−/− mice at day 10, but not at day 5 after infection (Fig. 1 H). These data indicate that, with the exception of eosinophils, the main type 2 effector functions in the il25
−/− mice are either normal or elevated at day 10 after infection with N. brasiliensis and that this up-regulation correlates with the continued presence of worms in these animals at this time point. However, given the deficit in Th2 cytokines observed at day 5 in the il25
−/− mice, it is probable that a combination of subtle changes in the onset of IL-4–, IL-5–, and IL-13–induced effector functions may explain the initial failure to expel the intestinal worms effectively. Equally, it is also possible that IL-25 could alter worm expulsion by a Th2-cytokine–independent pathway such as altering intestinal muscle contractility as has been demonstrated for IL-13 (25).
R1+, SSClow; Fig. 1 G) that occurs during N. brasiliensis infection (16, 23, 24). However, using flow cytometric analysis of the blood isolated from animals after infection, we observed a deficit in the number of circulating eosinophils (NBNT, CCR3+, SSChigh) in il25
−/− mice at day 10, but not at day 5 after infection (Fig. 1 H). These data indicate that, with the exception of eosinophils, the main type 2 effector functions in the il25
−/− mice are either normal or elevated at day 10 after infection with N. brasiliensis and that this up-regulation correlates with the continued presence of worms in these animals at this time point. However, given the deficit in Th2 cytokines observed at day 5 in the il25
−/− mice, it is probable that a combination of subtle changes in the onset of IL-4–, IL-5–, and IL-13–induced effector functions may explain the initial failure to expel the intestinal worms effectively. Equally, it is also possible that IL-25 could alter worm expulsion by a Th2-cytokine–independent pathway such as altering intestinal muscle contractility as has been demonstrated for IL-13 (25).
Administration of rIL-25 induces rapid worm expulsion that is T and B cell independent but type-2 cytokine dependent
To confirm a role for IL-25 in parasite worm expulsion, N. brasiliensis–infected wild-type and il25 −/− mice were treated with exogenous rIL-25. rIL-25 induced a potent inflammatory response in the intestines of all treated mice, independent of helminth infection (unpublished data). Significantly, the intraperitoneal administration of rIL-25 caused extremely rapid N. brasiliensis expulsion from both experimental groups, with no worms present in the intestines of treated mice even at the 5 d time point (Fig. 2 A). Control animals injected with vehicle solution contained worm numbers consistent with successful infection (Fig. 2 A). To confirm that, after IL-25 treatment, the N. brasiliensis larvae were not retained abnormally in the lungs and were able to migrate to the intestine, worm counts were made in these tissues from days 1 to 5. Worm counts in the lungs and intestine confirmed normal migration and infection levels in both the treated and untreated animals before the more rapid expulsion of the worms from the IL-25–treated mice on day 5 (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20051615/DC1). Thus, rIL-25 can accelerate worm expulsion and reverse the phenotype of the il25 −/− mice.
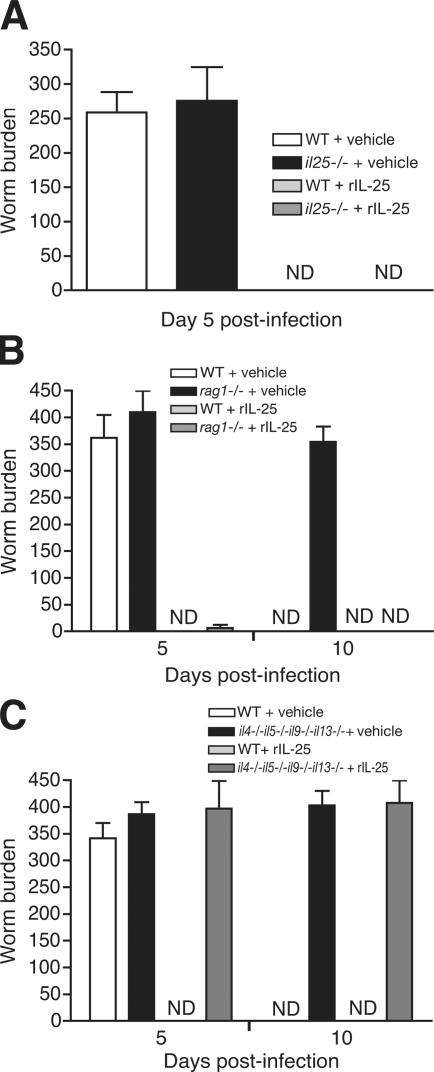
Administration of rIL-25 induces rapid worm expulsion that is independent of T and B cells, but dependent on type 2 cytokine production. Cohorts of mice (n = 3–5 per group) were infected with N. brasiliensis in the presence or absence of rIL-25 and killed on days 5 or 10 to obtain intestinal worm counts. Data are presented as means plus standard error and are representative of two to three experiments. ND, not detected. (A) Wild-type and il25 −/− mice. (B) Wild-type and rag1 −/− mice. (C) Wild-type and il4 −/− il5 −/− il9 −/− il13 −/− mice.
Although we had failed to find a critical requirement for IL-25 in T cell differentiation to Th2 cells in vitro (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20051615/DC1), in agreement with a report that treatment of T cells with IL-25 does not alter Th2 cytokine secretion (13), it remained possible that the IL-25 was mediating worm expulsion by directly or indirectly activating T cells. We therefore infected rag1 −/− mice with N. brasiliensis to determine whether this expulsion was mediated via NBNT cells and administered them with rIL-25 or vehicle. As expected, rag1 −/− failed to expel worms even 10 d after infection (Fig. 2 B). In contrast, treatment of rag1 −/− mice with rIL-25 resulted in highly accelerated worm expulsion with only a few worms surviving in the intestine at day 5 after infection.
We next assessed whether helminth expulsion was mediated directly by IL-25 or by the downstream induction of the classical type 2 cytokines (IL-4, IL-5, IL-9, and IL-13) and their subsequent initiation of type 2 effector functions. We have shown previously that il4 −/− il5 −/− il9 −/− il13 −/− mice exhibit a severe delay in worm expulsion as a result of their inability to initiate type 2 effector functions. We infected il4 −/− il5 −/− il9 −/− il13 −/− mice with N. brasiliensis, treated them with either rIL-25 or vehicle, and assessed worm burden. In complete contrast with wild-type mice treated with rIL-25, the intestines of il4 −/− il5 −/− il9 −/− il13 −/− mice treated with rIL-25 and infected with N. brasiliensis appeared macroscopically normal (unpublished data) and contained high numbers of worms even 10 d after infection (Fig. 2 C). These results demonstrate that IL-25 mediates its effects by regulating the production of at least one of the classical type 2 cytokines, leading to helminth expulsion.
Collectively, these data demonstrate that T cells, or indeed B cells, are not obligatory for N. brasiliensis expulsion after rIL-25 treatment and suggest that IL-25 can induce a potent type 2 cytokine response from NBNT cells capable of eliminating parasite infection even in the absence of T cell help.
Infection with N. brasiliensis induces a novel population of NBNT, c-kit+, Fc R1− cells that is dependent on IL-25
R1− cells that is dependent on IL-25
Recent reports have indicated that NBNT cells (initially identified as eosinophils, and subsequently as basophils and putative mast cell/basophil precursors) in the lungs of N. brasiliensis–infected mice play key roles in innate immunity to helminth infection by secreting type 2 cytokines, including IL-4 and IL-5 (18, 19). Furthermore, Fort et al. reported that IL-25 induces cytokine production by a NBNT population, although they were unable to identify the specific cell population involved (13).
We therefore used flow cytometry to determine the composition of NBNT cells in the draining MLN, which is the site of differential cytokine activity in infected mice (Fig. 1 C), of wild-type and il25
−/− mice 5 and 10 d after infection. In wild-type mice, infection stimulates an increase in CD19−, CD4−, CD8− (NBNT), c-kit+, Fc R1− cells in the MLN (Fig. 3 A). In contrast, no increase in this cell population was detected in MLN from il25
−/− mice 5 d after infection (Fig. 3 A). Furthermore, this deficiency was specific to the MLN, as we were unable to detect differences in any of these populations in blood or cell suspensions derived from infected lungs and spleen isolated from infected wild-type or il25
−/− mice 5 and 10 d after infection (unpublished data). Uninfected il25
−/− mice had similar frequencies to these NBNT, c-kit+ Fc
R1− cells in the MLN (Fig. 3 A). In contrast, no increase in this cell population was detected in MLN from il25
−/− mice 5 d after infection (Fig. 3 A). Furthermore, this deficiency was specific to the MLN, as we were unable to detect differences in any of these populations in blood or cell suspensions derived from infected lungs and spleen isolated from infected wild-type or il25
−/− mice 5 and 10 d after infection (unpublished data). Uninfected il25
−/− mice had similar frequencies to these NBNT, c-kit+ Fc R1− cells as wild-type mice (Fig. 3 A). These results indicate that, in the absence of IL-25, a population of NBNT, c-kit+, Fc
R1− cells as wild-type mice (Fig. 3 A). These results indicate that, in the absence of IL-25, a population of NBNT, c-kit+, Fc R1− cells either fails to develop normally in the lymph node or fails to migrate efficiently to this site during the innate response to worm infection.
R1− cells either fails to develop normally in the lymph node or fails to migrate efficiently to this site during the innate response to worm infection.
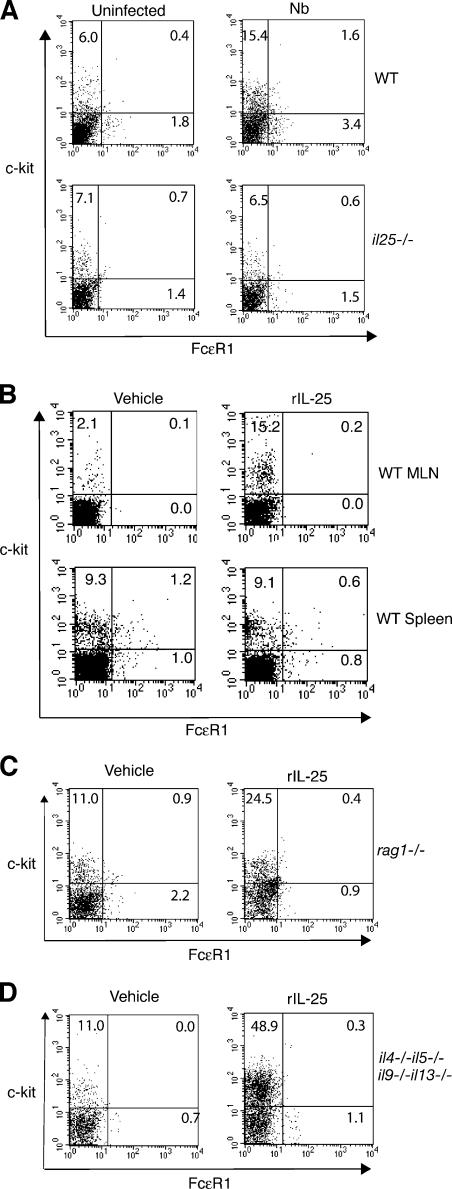
IL-25 induces NBNT, c-kit+, Fc R1− cell population in the MLN. (A) NBNT, c-kit+, Fc
R1− cell population in the MLN. (A) NBNT, c-kit+, Fc R1− cells are deficient in the MLN of il25
−/− animals 5 d after infection with N. brasiliensis (Nb). Cohorts of wild-type and il25
−/−-infected mice (n = 5–6) were killed and the NBNT, c-kit+, Fc
R1− cells are deficient in the MLN of il25
−/− animals 5 d after infection with N. brasiliensis (Nb). Cohorts of wild-type and il25
−/−-infected mice (n = 5–6) were killed and the NBNT, c-kit+, Fc R1− cell population assessed by flow cytometry. (B) BALB/c mice (n = 3–5) were administered with rIL-25 and flow cytometry was used to analyze NBNT, c-kit+, Fc
R1− cell population assessed by flow cytometry. (B) BALB/c mice (n = 3–5) were administered with rIL-25 and flow cytometry was used to analyze NBNT, c-kit+, Fc R1− cells in the MLN and spleen 4 d later. (C) Cohorts of wild-type and rag1
−/− mice (n = 3–5) were administered with rIL-25 and flow cytometry used to analyze NBNT, c-kit+, Fc
R1− cells in the MLN and spleen 4 d later. (C) Cohorts of wild-type and rag1
−/− mice (n = 3–5) were administered with rIL-25 and flow cytometry used to analyze NBNT, c-kit+, Fc R1− cells in the MLN 4 d later. (D) Cohorts of wild-type and il4
−/−
il5
−/−
il9
−/−
il13
−/− mice (n = 3–5) were administered with rIL-25 and flow cytometry used to analyze NBNT, c-kit+, Fc
R1− cells in the MLN 4 d later. (D) Cohorts of wild-type and il4
−/−
il5
−/−
il9
−/−
il13
−/− mice (n = 3–5) were administered with rIL-25 and flow cytometry used to analyze NBNT, c-kit+, Fc R1− cells in the MLN 4 d later. Data are representative of two to three experiments.
R1− cells in the MLN 4 d later. Data are representative of two to three experiments.
To confirm that the NBNT, c-kit+, Fc R1− cell population was induced by IL-25, we administered rIL-25 to wild-type mice and used flow cytometry to determine the composition of NBNT cell populations in the MLN and spleen 4 d afterward. Treatment with rIL-25 resulted in an increase in the population of NBNT, c-kit+, Fc
R1− cell population was induced by IL-25, we administered rIL-25 to wild-type mice and used flow cytometry to determine the composition of NBNT cell populations in the MLN and spleen 4 d afterward. Treatment with rIL-25 resulted in an increase in the population of NBNT, c-kit+, Fc R1− cells in MLN, but not in the spleen (Fig. 3 B). We also confirmed that, using rag1
−/− animals, the NBNT, c-kit+, Fc
R1− cells in MLN, but not in the spleen (Fig. 3 B). We also confirmed that, using rag1
−/− animals, the NBNT, c-kit+, Fc R1− cells do not require T or B cells for their induction in the MLN after rIL-25 administration (Fig. 3 C). Furthermore, administration of rIL-25 continued to induce the appearance of NBNT, c-kit+, Fc
R1− cells do not require T or B cells for their induction in the MLN after rIL-25 administration (Fig. 3 C). Furthermore, administration of rIL-25 continued to induce the appearance of NBNT, c-kit+, Fc R1− cells in the MLN of il4
−/−
il5
−/−
il9
−/−
il13
−/− mice (Fig. 3 D). Indeed, we observed substantially greater numbers of these cells in the il4
−/−
il5
−/−
il9
−/−
il13
−/− mice, possibly indicating a role for the type 2 cytokines in trafficking or differentiation of these precursors. These data indicate that rIL-25 treatment induces NBNT, c-kit+, Fc
R1− cells in the MLN of il4
−/−
il5
−/−
il9
−/−
il13
−/− mice (Fig. 3 D). Indeed, we observed substantially greater numbers of these cells in the il4
−/−
il5
−/−
il9
−/−
il13
−/− mice, possibly indicating a role for the type 2 cytokines in trafficking or differentiation of these precursors. These data indicate that rIL-25 treatment induces NBNT, c-kit+, Fc R1− cells independently of T and B cells and the type 2 cytokines.
R1− cells independently of T and B cells and the type 2 cytokines.
NBNT, c-kit+, Fc R1− cells induced by N. brasiliensis infection or administration of rIL-25 produce IL-4, IL-5, and IL-13
R1− cells induced by N. brasiliensis infection or administration of rIL-25 produce IL-4, IL-5, and IL-13
Our data suggest the requirement for a type 2 cytokine–secreting NBNT cell in mediating N. brasiliensis expulsion. We therefore purified the NBNT, c-kit+, Fc R1− cells using flow cytometry from mice either infected with N. brasiliensis or treated with rIL-25 and used PCR analysis to determine their cytokine expression. Significantly, the NBNT, c-kit+, Fc
R1− cells using flow cytometry from mice either infected with N. brasiliensis or treated with rIL-25 and used PCR analysis to determine their cytokine expression. Significantly, the NBNT, c-kit+, Fc R1− cells purified from MLN 4 d after rIL-25 treatment expressed mRNA encoding the classical type 2 cytokines IL-4, IL-5, and IL-13 (Fig. 4 A). A similar profile was also detected 5 d after infection with N. brasiliensis (Fig. 4 B).
R1− cells purified from MLN 4 d after rIL-25 treatment expressed mRNA encoding the classical type 2 cytokines IL-4, IL-5, and IL-13 (Fig. 4 A). A similar profile was also detected 5 d after infection with N. brasiliensis (Fig. 4 B).
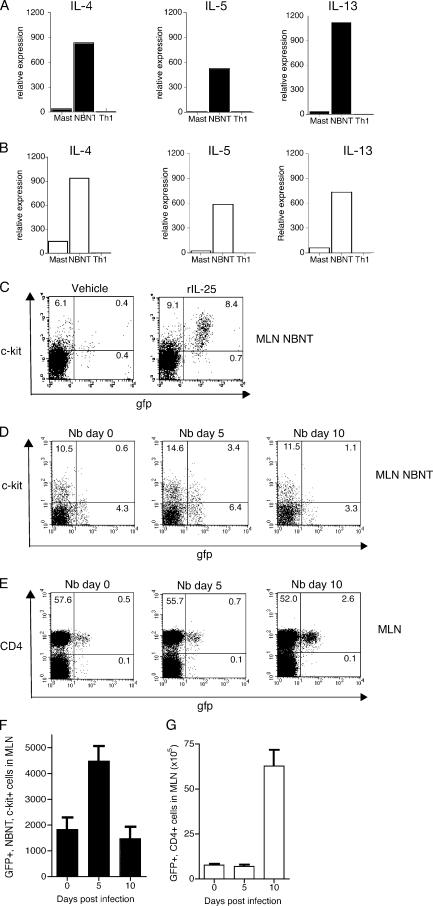
NBNT, c-kit+, Fc R1− cells induced by IL-25 in vivo produce IL-4, IL-5, and IL-13. (A) After administration of IL-25, NBNT, c-kit+, Fc
R1− cells induced by IL-25 in vivo produce IL-4, IL-5, and IL-13. (A) After administration of IL-25, NBNT, c-kit+, Fc R1− cells were purified using flow cytometry and the expression of il4, il5, and il13 determined using Taqman real-time PCR. Mast, PMA/ionomycin-activated, bone marrow–derived mast cells. NBNT, c-kit+, Fc
R1− cells were purified using flow cytometry and the expression of il4, il5, and il13 determined using Taqman real-time PCR. Mast, PMA/ionomycin-activated, bone marrow–derived mast cells. NBNT, c-kit+, Fc R1− sorted cells. Th1, splenocytes stimulated under Th1 cell differentiation conditions for 48 h. (B) After N. brasiliensis infection NBNT, c-kit+ cells were purified using flow cytometry and the expression of il4, il5, and il13 was determined using Taqman real-time PCR. Mast, PMA/ionomycin-activated, bone marrow–derived mast cells. NBNT, NBNT, c-kit+ sorted cells. Th1, splenocytes stimulated under Th1 cell differentiation conditions for 48 h. (C) 4get mice were administered with rIL-25 and the frequency of GFP+ NBNT, c-kit+, Fc
R1− sorted cells. Th1, splenocytes stimulated under Th1 cell differentiation conditions for 48 h. (B) After N. brasiliensis infection NBNT, c-kit+ cells were purified using flow cytometry and the expression of il4, il5, and il13 was determined using Taqman real-time PCR. Mast, PMA/ionomycin-activated, bone marrow–derived mast cells. NBNT, NBNT, c-kit+ sorted cells. Th1, splenocytes stimulated under Th1 cell differentiation conditions for 48 h. (C) 4get mice were administered with rIL-25 and the frequency of GFP+ NBNT, c-kit+, Fc R1− cells was analyzed by flow cytometry. (D) 4get mice were infected with N. brasiliensis (Nb) and the frequency of GFP+ NBNT, c-kit+ cells was analyzed by flow cytometry at the time points indicated. (E) 4get mice were infected with N. brasiliensis and the frequency of GFP+ CD4+ cells was analyzed by flow cytometry at the time points indicated. (F) Numbers of GFP+, NBNT, c-kit+, Fc
R1− cells was analyzed by flow cytometry. (D) 4get mice were infected with N. brasiliensis (Nb) and the frequency of GFP+ NBNT, c-kit+ cells was analyzed by flow cytometry at the time points indicated. (E) 4get mice were infected with N. brasiliensis and the frequency of GFP+ CD4+ cells was analyzed by flow cytometry at the time points indicated. (F) Numbers of GFP+, NBNT, c-kit+, Fc R1− cells in MLN after infection. (G) Numbers of GFP+, CD4+ cells in MLN after infection are shown.
R1− cells in MLN after infection. (G) Numbers of GFP+, CD4+ cells in MLN after infection are shown.
To complement the expression data obtained from cells purified by flow cytometry, we also used 4get mice, in which IL-4 expression is linked to egfp (26), to determine the frequency of IL-4–producing NBNT, c-kit+, Fc R1− cells in the MLN of mice administrated with rIL-25. Using this approach, we confirmed that the NBNT, c-kit+, Fc
R1− cells in the MLN of mice administrated with rIL-25. Using this approach, we confirmed that the NBNT, c-kit+, Fc R1− cell subpopulation induced by rIL-25 was primed as an IL-4–producing cell type (Fig. 4 C). We also determined the frequency of IL-4–producing NBNT, c-kit+, Fc
R1− cell subpopulation induced by rIL-25 was primed as an IL-4–producing cell type (Fig. 4 C). We also determined the frequency of IL-4–producing NBNT, c-kit+, Fc R1− cells in the MLN of uninfected 4get mice and 4get animals infected for 5 and 10 d with N. brasiliensis. N. brasiliensis infection evoked a substantial induction of IL-4–producing NBNT, c-kit+, Fc
R1− cells in the MLN of uninfected 4get mice and 4get animals infected for 5 and 10 d with N. brasiliensis. N. brasiliensis infection evoked a substantial induction of IL-4–producing NBNT, c-kit+, Fc R1− cells in the MLN by day 5 of infection (Fig. 4 D).
R1− cells in the MLN by day 5 of infection (Fig. 4 D).
The 4get mice also allowed us to assess the relative proportions of IL-4–producing CD4+ T cells and IL-4–producing NBNT, c-kit+, Fc R1− cells in the MLN at 5 and 10 d after infection. It is noteworthy that the onset of the IL-4–producing NBNT, c-kit+, Fc
R1− cells in the MLN at 5 and 10 d after infection. It is noteworthy that the onset of the IL-4–producing NBNT, c-kit+, Fc R1− cells precedes the major expansion of IL-4–producing CD4+ T cells (Fig. 4 E). IL-4–producing NBNT, c-kit+, Fc
R1− cells precedes the major expansion of IL-4–producing CD4+ T cells (Fig. 4 E). IL-4–producing NBNT, c-kit+, Fc R1− cells increase approximately two- to threefold in number, rising to 4–5 × 103 per MLN 5 d after infection (Fig. 4 F), making up almost 30% of the NBNT, c-kit+, Fc
R1− cells increase approximately two- to threefold in number, rising to 4–5 × 103 per MLN 5 d after infection (Fig. 4 F), making up almost 30% of the NBNT, c-kit+, Fc R1− population (not depicted). In contrast, we detected no change in the number of IL-4–producing CD4+ T cells at day 5 after infection (Fig. 4 G), although by day 10 of infection, IL-4–producing CD4+ T cells had increased dramatically.
R1− population (not depicted). In contrast, we detected no change in the number of IL-4–producing CD4+ T cells at day 5 after infection (Fig. 4 G), although by day 10 of infection, IL-4–producing CD4+ T cells had increased dramatically.
Several studies have evaluated the functional importance of eosinophils, basophils, and mast cells in providing an early source of type 2 cytokines. Histological analysis of cytospins of FACS-purified NBNT, c-kit+, Fc R1− cells showed them to be small agranular cells with limited cytoplasm (Fig. 5 A). Flow cytometric analysis using several lineage markers was undertaken to further define the NBNT, c-kit+, Fc
R1− cells showed them to be small agranular cells with limited cytoplasm (Fig. 5 A). Flow cytometric analysis using several lineage markers was undertaken to further define the NBNT, c-kit+, Fc R1− cells. The cell surface marker analysis of the NBNT, c-kit+, Fc
R1− cells. The cell surface marker analysis of the NBNT, c-kit+, Fc R1− cells precluded their definition as mature eosinophils, basophils, or mast cells (Fig. 5 B). Furthermore, cell surface phenotyping also precluded their definition as NK cells (Fig. 5 B). Similar staining profiles were also obtained with NBNT, c-kit+, Fc
R1− cells precluded their definition as mature eosinophils, basophils, or mast cells (Fig. 5 B). Furthermore, cell surface phenotyping also precluded their definition as NK cells (Fig. 5 B). Similar staining profiles were also obtained with NBNT, c-kit+, Fc R1− cells from N. brasiliensis–infected mice (unpublished data). To further define their lineage, mRNA was prepared from NBNT, c-kit+, Fc
R1− cells from N. brasiliensis–infected mice (unpublished data). To further define their lineage, mRNA was prepared from NBNT, c-kit+, Fc R1− cells purified from animals that were administered rIL-25 and subjected to gene expression analysis using a panel of eosinophil, basophil, and mast cell genes (Fig. 5 C). Our data demonstrate that these cells do not express cell surface markers or lineage-specific genes that are characteristic of mature eosinophils, mast cells, or basophils. Furthermore, preliminary microarray data indicate an absence of eosinophil, basophil, mast cell, neutrophil, and NK-specific genes in the mRNA isolated from the NBNT, c-kit+, Fc
R1− cells purified from animals that were administered rIL-25 and subjected to gene expression analysis using a panel of eosinophil, basophil, and mast cell genes (Fig. 5 C). Our data demonstrate that these cells do not express cell surface markers or lineage-specific genes that are characteristic of mature eosinophils, mast cells, or basophils. Furthermore, preliminary microarray data indicate an absence of eosinophil, basophil, mast cell, neutrophil, and NK-specific genes in the mRNA isolated from the NBNT, c-kit+, Fc R1− cells (unpublished data). This suggests that the NBNT, c-kit+, Fc
R1− cells (unpublished data). This suggests that the NBNT, c-kit+, Fc R1− cells represent a novel cell population and may represent a precursor population.
R1− cells represent a novel cell population and may represent a precursor population.

Analysis of purified NBNT, c-kit+, Fc R1− cells from IL-25–treated wild-type mice. (A) NBNT, c-kit+, Fc
R1− cells from IL-25–treated wild-type mice. (A) NBNT, c-kit+, Fc R1− cells were isolated by cell sorting and cytospin preparations of sorted cells stained with Giemsa or Toludine. (B) Flow cytometry of cell surface marker expression. (C) Analysis of gene expression. neg, no template control; mast, bone marrow–derived mast cells; c-kit+, NBNT, c-kit+, Fc
R1− cells were isolated by cell sorting and cytospin preparations of sorted cells stained with Giemsa or Toludine. (B) Flow cytometry of cell surface marker expression. (C) Analysis of gene expression. neg, no template control; mast, bone marrow–derived mast cells; c-kit+, NBNT, c-kit+, Fc R1− cells; Th2, splenocytes stimulated under Th2 cell differentiation conditions for 48 h; pos, image clone used as positive control; CCR, chemokine receptor; MCP, mast cell protease; EPO, eosinophil peroxidase. EPO positive control was IMAGE clone 30044595. CCR1 positive control was IMAGE clone 3992755.
R1− cells; Th2, splenocytes stimulated under Th2 cell differentiation conditions for 48 h; pos, image clone used as positive control; CCR, chemokine receptor; MCP, mast cell protease; EPO, eosinophil peroxidase. EPO positive control was IMAGE clone 30044595. CCR1 positive control was IMAGE clone 3992755.
IL-25 induces type 2 cytokine production from c-kit+ MLN cells in vitro
It has been shown previously that IL-25 can induce production of IL-5 and IL-13 in vitro, primarily from an NBNT subset (13). We wished to determine if NBNT, c-kit+, Fc R1− cells isolated from naive animals and stimulated in vitro with rIL-25 could be induced to express type 2 cytokines. Using flow cytometry, we purified NBNT, c-kit+, Fc
R1− cells isolated from naive animals and stimulated in vitro with rIL-25 could be induced to express type 2 cytokines. Using flow cytometry, we purified NBNT, c-kit+, Fc R1− cells from the MLN and spleen of naive mice. The cells were treated in vitro with rIL-25 for 4 d and cytokine expression was analyzed. We found that the NBNT, c-kit+, Fc
R1− cells from the MLN and spleen of naive mice. The cells were treated in vitro with rIL-25 for 4 d and cytokine expression was analyzed. We found that the NBNT, c-kit+, Fc R1− cells isolated from the MLN, but not the spleen, produced highly elevated levels of IL-5 and IL-13 after stimulation with IL-25 (Fig. 6, A and B). We were unable to detect IL-5 or IL-13 expression after IL-25 stimulation of NBNT cells purified from the bone marrow of naive mice (Fig. 6 C). Thus, the NBNT, c-kit+, Fc
R1− cells isolated from the MLN, but not the spleen, produced highly elevated levels of IL-5 and IL-13 after stimulation with IL-25 (Fig. 6, A and B). We were unable to detect IL-5 or IL-13 expression after IL-25 stimulation of NBNT cells purified from the bone marrow of naive mice (Fig. 6 C). Thus, the NBNT, c-kit+, Fc R1− MLN cell population that arises in response to IL-25 represents a potent source of type 2 cytokines that are initiated early in the immune response to intestinal parasite infection. It is noteworthy that IL-4 protein was below the levels of detection using ELISA and ELISPOT (unpublished data) in these cultures, although we cannot preclude that it is secreted, but is being used by the cells and removed from the medium.
R1− MLN cell population that arises in response to IL-25 represents a potent source of type 2 cytokines that are initiated early in the immune response to intestinal parasite infection. It is noteworthy that IL-4 protein was below the levels of detection using ELISA and ELISPOT (unpublished data) in these cultures, although we cannot preclude that it is secreted, but is being used by the cells and removed from the medium.

IL-25 induces type-2 cytokine production from NBNT, c-kit+, Fc R1− MLN cells in vitro. (A) NBNT, c-kit+, Fc
R1− MLN cells in vitro. (A) NBNT, c-kit+, Fc R1− cells from the MLN of naive mice were purified by flow cytometry and treated in vitro with rIL-25 for 4 d and cytokine expression was analyzed by ELISA. (B) NBNT, c-kit+, Fc
R1− cells from the MLN of naive mice were purified by flow cytometry and treated in vitro with rIL-25 for 4 d and cytokine expression was analyzed by ELISA. (B) NBNT, c-kit+, Fc R1− cells from the spleens of naive mice were purified by flow cytometry and treated in vitro with rIL-25 for 4 d and cytokine expression was analyzed by ELISA. (C) NBNT cells were purified from bone marrow and treated in vitro with rIL-25 for 4 d and cytokine expression analyzed by ELISA. ND, not detected. (D and E) NBNT, c-kit+, Fc
R1− cells from the spleens of naive mice were purified by flow cytometry and treated in vitro with rIL-25 for 4 d and cytokine expression was analyzed by ELISA. (C) NBNT cells were purified from bone marrow and treated in vitro with rIL-25 for 4 d and cytokine expression analyzed by ELISA. ND, not detected. (D and E) NBNT, c-kit+, Fc R1− cells were purified from rIL-25–treated il13
−/− mice and cultured in triplicate with highly purified wild-type CD3+ cells, on anti-CD3–coated plates, in the presence or absence of IL-25. D and E are two independent experiments. Differences in IL-13 production between untreated and IL-25–treated cells were analyzed using Student's t test.
R1− cells were purified from rIL-25–treated il13
−/− mice and cultured in triplicate with highly purified wild-type CD3+ cells, on anti-CD3–coated plates, in the presence or absence of IL-25. D and E are two independent experiments. Differences in IL-13 production between untreated and IL-25–treated cells were analyzed using Student's t test.
To assess whether NBNT, c-kit+, Fc R1− MLN cells could enhance type 2 cytokine production from activated T cells, we cocultured NBNT, c-kit+, Fc
R1− MLN cells could enhance type 2 cytokine production from activated T cells, we cocultured NBNT, c-kit+, Fc R1− MLN cells purified by flow cytometry from rIL-25–treated il13
−/− mice with highly purified T cells from untreated wild-type mice in the presence or absence of exogenous IL-25. In this way, we could determine whether coculture increased IL-13 expression from the wild-type T cells in the absence of background IL-13 production from the il13
−/− NBNT, c-kit+, Fc
R1− MLN cells purified by flow cytometry from rIL-25–treated il13
−/− mice with highly purified T cells from untreated wild-type mice in the presence or absence of exogenous IL-25. In this way, we could determine whether coculture increased IL-13 expression from the wild-type T cells in the absence of background IL-13 production from the il13
−/− NBNT, c-kit+, Fc R1− MLN cells. In repeat experiments, we observed a modest increase in the ability of T cells cocultured with NBNT, c-kit+, Fc
R1− MLN cells. In repeat experiments, we observed a modest increase in the ability of T cells cocultured with NBNT, c-kit+, Fc R1− MLN cells in the presence of IL-25 to produce IL-13 when IL-25 was added (Fig. 6, D and E). It is noteworthy that NBNT, c-kit−, Fc
R1− MLN cells in the presence of IL-25 to produce IL-13 when IL-25 was added (Fig. 6, D and E). It is noteworthy that NBNT, c-kit−, Fc R1− MLN cells also induced elevated levels of IL-13 production from T cells after addition of IL-25, and can still produce type 2 cytokines when purified from wild-type mice (unpublished data). These data indicate that the NBNT population contains more than one IL-25-responsive cell type that we have yet to identify, or that c-kit is transiently expressed on the IL-25–responsive population. However, we did not observe an increase in the proportions of NBNT, c-kit−, Fc
R1− MLN cells also induced elevated levels of IL-13 production from T cells after addition of IL-25, and can still produce type 2 cytokines when purified from wild-type mice (unpublished data). These data indicate that the NBNT population contains more than one IL-25-responsive cell type that we have yet to identify, or that c-kit is transiently expressed on the IL-25–responsive population. However, we did not observe an increase in the proportions of NBNT, c-kit−, Fc R1− MLN cells in response to either N. brasiliensis infection or treatment of mice with rIL-25 in vivo. Future studies will be required to elucidate the role of these additional IL-25 responsive cells.
R1− MLN cells in response to either N. brasiliensis infection or treatment of mice with rIL-25 in vivo. Future studies will be required to elucidate the role of these additional IL-25 responsive cells.
DISCUSSION
Our studies have highlighted the importance of IL-25 in the rapid and robust generation of the type 2 response required for the expulsion of N. brasiliensis from the intestine. Although type 2 responses are essential for the clearance of N. brasiliensis, only IL-13 has been shown to be critical for expulsion (27, 28). However, the analysis of compound type 2 cytokine-deficient mice has uncovered redundant functions of IL-4, IL-5, IL-9, and IL-13 in the process of worm expulsion (15). In the absence of IL-25, we found that antigen-driven Th2 cytokine production was impaired 5 d after N. brasiliensis infection, with reduced levels of IL-5 and IL-13, and this correlated with the inability to expel the parasite. Surprisingly, given this deficit in type 2 cytokines, we did not detect any reduction in goblet cells, mast cells, or IgE in the il25 −/− animals after infection. However, we did observe a significant delay in eosinophilia in the il25 −/− mice. Although eosinophils alone have not been shown to be essential for N. brasiliensis expulsion, they are highly up-regulated after infection and have been reported to play roles in protection against helminth infection (15, 22, 29). We believe that it is likely that the significant reduction in eosinophils probably occurs alongside more minor deficits in the other type 2 effector functions, which we were unable to detect, leading to the observed phenotype.
Our results also indicate the striking potency of IL-25 in inducing worm expulsion, even in the absence of T cells. Thus, although T cells are normally required for worm expulsion, treatment with exogenous IL-25 is able to substitute for these cells. The mechanism of worm clearance is clearly dependent on type 2 cytokines and likely results from the highly accentuated type 2 effector functions that are observed after administration of IL-25, including inflammation and mucus production (references 13, 30 and unpublished data). Interestingly, given the potent biological effects of IL-25, we have found that mRNA-encoding IL-25 is expressed at very low levels in the intestine as reported previously (30). Further studies will be necessary to identify the mechanism of cellular activation and the cell sources of IL-25 production after infection.
Despite the pronounced effect of il25 disruption on the production of Th2 cytokines, we were unable to detect any role for IL-25 in the direct regulation of Th2 cell differentiation or cytokine production in vitro (Fig. S3). This is in agreement with the findings of Fort et al., who, despite observing increased expression of type 2 cytokines after administration of IL-25, were unable to uncover any direct role for IL-25 for the growth or differentiation of T helper cells (13). However, a possible role for IL-25 in regulating type 2 cytokine production by NBNT cells has been proposed (13). We identified a reproducible decrease in the frequency of NBNT, c-kit+, Fc R1− cells in MLN isolated from il25
−/− mice 5 d after infection. Significantly, administration of rIL-25 reversed this deficit and induced an elevation in the NBNT, c-kit+, Fc
R1− cells in MLN isolated from il25
−/− mice 5 d after infection. Significantly, administration of rIL-25 reversed this deficit and induced an elevation in the NBNT, c-kit+, Fc R1− cell population in the MLN that exceeded that observed even after N. brasiliensis infection. The expansion of the NBNT, c-kit+, Fc
R1− cell population in the MLN that exceeded that observed even after N. brasiliensis infection. The expansion of the NBNT, c-kit+, Fc R1− cytokine-producing cells after infection was clearly distinguishable from elevations previously described in basophils (NBNT, c-kit−, Fc
R1− cytokine-producing cells after infection was clearly distinguishable from elevations previously described in basophils (NBNT, c-kit−, Fc R1+) and eosinophils (NBNT, CCR3+, SSChigh) (16, 18–21, 23, 24).
R1+) and eosinophils (NBNT, CCR3+, SSChigh) (16, 18–21, 23, 24).
The expression of type 2 cytokines from cells of the innate immune system, including eosinophils, basophils, and mast cells, has also been implicated in the onset of immune responses to N. brasiliensis. Basophils (NBNT, c-kit−, Fc R1+) are known to be significant IL-4 producers in response to N. brasiliensis infection (16, 24). We determined that the NBNT, c-kit+, Fc
R1+) are known to be significant IL-4 producers in response to N. brasiliensis infection (16, 24). We determined that the NBNT, c-kit+, Fc R1− cell population represented an early potential source of IL-4, IL-5, and IL-13. IL-4 is a key cytokine in the regulation of Th2 cell differentiation, and cellular sources of early IL-4 production are believed to play critical roles in controlling the efficiency of the type 2 response (19, 31, 32). Furthermore, both IL-5 and IL-13 are known to alter the magnitude of the type 2 response, as well as sustaining ongoing type 2 responses, through their induction of type 2 effector functions (15). Our results are consistent with a role for an NBNT, c-kit+, Fc
R1− cell population represented an early potential source of IL-4, IL-5, and IL-13. IL-4 is a key cytokine in the regulation of Th2 cell differentiation, and cellular sources of early IL-4 production are believed to play critical roles in controlling the efficiency of the type 2 response (19, 31, 32). Furthermore, both IL-5 and IL-13 are known to alter the magnitude of the type 2 response, as well as sustaining ongoing type 2 responses, through their induction of type 2 effector functions (15). Our results are consistent with a role for an NBNT, c-kit+, Fc R1− cell population in enhancing Th2 polarization, but we have been unable to detect IL-4 at the protein level and it remains uncertain if the IL-4 mRNA is translated and if IL-4 is secreted. Furthermore, because our data do not directly demonstrate a functional role for the NBNT, c-kit+, Fc
R1− cell population in enhancing Th2 polarization, but we have been unable to detect IL-4 at the protein level and it remains uncertain if the IL-4 mRNA is translated and if IL-4 is secreted. Furthermore, because our data do not directly demonstrate a functional role for the NBNT, c-kit+, Fc R1− cell population in mediating IL-25–dependent host protection, it remains possible that IL-25 could work through other cells/mechanisms to influence the type 2 response. Indeed, we have observed that further NBNT, c-kit− cell subsets can respond to IL-25 by secreting IL-13. Thus, although we only observed the expansion of the NBNT, c-kit+, Fc
R1− cell population in mediating IL-25–dependent host protection, it remains possible that IL-25 could work through other cells/mechanisms to influence the type 2 response. Indeed, we have observed that further NBNT, c-kit− cell subsets can respond to IL-25 by secreting IL-13. Thus, although we only observed the expansion of the NBNT, c-kit+, Fc R1− cell population, it is possible that IL-25 may also affect the function, rather than the expansion, of several other cell populations that may also contribute to resistance. It is also clear that, even in the absence of IL-25, a definitive Th2-cytokine response is eventually induced (though with slower kinetics), indicating that alternative pathways exist that can compensate for a deficiency in IL-25. Such functional redundancy is a common feature of cytokine-regulated systems, and several cellular sources of early IL-4 production have been reported, including T cells (33, 34), NK cells (35), mast cells and basophils (19, 24, 36, 37), and eosinophils (18, 19). A further possibility is that in vivo IL-25 may be able to act directly on T cells, thereby enhancing type 2 cytokine production, and that we and previous investigators have been unable to recapitulate the conditions required for this activity in vitro (13).
R1− cell population, it is possible that IL-25 may also affect the function, rather than the expansion, of several other cell populations that may also contribute to resistance. It is also clear that, even in the absence of IL-25, a definitive Th2-cytokine response is eventually induced (though with slower kinetics), indicating that alternative pathways exist that can compensate for a deficiency in IL-25. Such functional redundancy is a common feature of cytokine-regulated systems, and several cellular sources of early IL-4 production have been reported, including T cells (33, 34), NK cells (35), mast cells and basophils (19, 24, 36, 37), and eosinophils (18, 19). A further possibility is that in vivo IL-25 may be able to act directly on T cells, thereby enhancing type 2 cytokine production, and that we and previous investigators have been unable to recapitulate the conditions required for this activity in vitro (13).
It is possible that these MLN NBNT, c-kit+, Fc R1−, type 2 cytokine–producing cells correspond to similar c-kit+ cells identified in the lungs of N. brasiliensis–infected IL-4/GFP reporter mice (19), although in this study we have not detected these cells in the lung. It was speculated (19) that this c-kit+ lung cell population is a possible common basophil/mast cell precursor as described previously in the human system (38). Indeed, the IL-25–dependent NBNT, c-kit+, Fc
R1−, type 2 cytokine–producing cells correspond to similar c-kit+ cells identified in the lungs of N. brasiliensis–infected IL-4/GFP reporter mice (19), although in this study we have not detected these cells in the lung. It was speculated (19) that this c-kit+ lung cell population is a possible common basophil/mast cell precursor as described previously in the human system (38). Indeed, the IL-25–dependent NBNT, c-kit+, Fc R1−, type 2 cytokine–producing cells that we show in the MLN of N. brasiliensis–infected mice may be previously described, but functionally uncharacterized c-kit+ mast cell–committed progenitor cell that has also been reported after N. brasiliensis infection (39). Despite characterizing the expression of a panel of eosinophil, mast cell, and basophil genes, we have been unable to define the NBNT, c-kit+, Fc
R1−, type 2 cytokine–producing cells that we show in the MLN of N. brasiliensis–infected mice may be previously described, but functionally uncharacterized c-kit+ mast cell–committed progenitor cell that has also been reported after N. brasiliensis infection (39). Despite characterizing the expression of a panel of eosinophil, mast cell, and basophil genes, we have been unable to define the NBNT, c-kit+, Fc R1−, type 2 cytokine–producing cells as belonging to a specific lineage, suggesting that they may represent a novel lineage or a precursor cell population.
R1−, type 2 cytokine–producing cells as belonging to a specific lineage, suggesting that they may represent a novel lineage or a precursor cell population.
The reduced frequency of NBNT, c-kit+, Fc R1−, type 2 cytokine–producing cells in the MLN of the il25
−/− mice after infection may result from a failure of these cells to migrate to the draining lymph node or it may be the result of inefficient expansion or differentiation of this population in situ. Although IL-25 has not been demonstrated to have direct growth factor activity on mouse T and B cells (11), spleens from IL-25–treated mice have been reported to contain significantly elevated numbers of hematopoietic progenitor cells, though the mechanism for this remains undefined (13). A role for IL-25 in regulating cell migration has also been suggested by its ability to stimulate expression of the chemokines IL-8 (10) and GROα (12) and the adhesion molecules ICAM-1 and VCAM-1 (12). However, in vitro assays have failed to demonstrate eosinophil chemoattractant activity for IL-25 (30). Further studies will be necessary to determine the origin of these cells in the lymph node.
R1−, type 2 cytokine–producing cells in the MLN of the il25
−/− mice after infection may result from a failure of these cells to migrate to the draining lymph node or it may be the result of inefficient expansion or differentiation of this population in situ. Although IL-25 has not been demonstrated to have direct growth factor activity on mouse T and B cells (11), spleens from IL-25–treated mice have been reported to contain significantly elevated numbers of hematopoietic progenitor cells, though the mechanism for this remains undefined (13). A role for IL-25 in regulating cell migration has also been suggested by its ability to stimulate expression of the chemokines IL-8 (10) and GROα (12) and the adhesion molecules ICAM-1 and VCAM-1 (12). However, in vitro assays have failed to demonstrate eosinophil chemoattractant activity for IL-25 (30). Further studies will be necessary to determine the origin of these cells in the lymph node.
This is the first report of the functional importance of IL-25 in the immune response to the intestinal parasite N. brasiliensis. Our results clearly demonstrate that IL-25 is an important early regulatory molecule in the genesis of type 2 immunity. IL-25 regulates innate type 2 immune cells whose cytokine expression precedes the subsequent induction of known adaptive Th2 cell–mediated immunity.
MATERIALS AND METHODS
Mice.
BALB/c mice were obtained from Harlan UK and maintained in the SABU/CBS facilities or in Trinity College, Dublin, in specific pathogen-free environments. 4get (26) and rag1 −/− (40) mice, on a BALB/c background, were purchased from Jackson ImmunoResearch Laboratories and bred in Trinity College, Dublin or in SABU/CBS facilities. il4 −/− il5 −/− il9 −/− il13 −/− mice on a BALB/c background were as described previously (15). il25 −/− mice were generated and maintained as described in supplemental Materials and methods (available at http://www.jem.org/cgi/content/full/jem.20051615/DC1). All animal experiments outlined in this report were undertaken with the approval of the UK Home Office or the Department of Health and Children, Ireland.
Helminth infection.
Individual mice were inoculated subcutaneously with 500 viable third-stage N. brasiliensis larvae. Animals were killed 5, 10, 15, and 20 d after infection, the intestinal worm burdens were determined, and serum was taken. N. brasiliensis–infected mice treated with PBS or IL-25 (see IL-25 administration and assay) were killed on days 2–5 after infection and lung and intestinal worm counts were determined. For lung counts, the lungs were removed, chopped finely with scissors, and incubated for 3 h at 37°C in saline. Emergent larvae were counted using a dissecting microscope. Feces were collected for fecal egg counts. Jejunal tissue was removed and processed for histology as described previously (41). In addition, MLN cells were harvested, counted, and analyzed by flow cytometry or stimulated in vitro at either 2.5 × 106 cells/ ml with plate-bound anti-CD3 or at 5 × 106 cells/ml with 50 μg/ml of parasite antigens (NbES) (42) for 72 h. Supernatants were harvested and analyzed for cytokines. Lungs were removed and chopped before digestion in 1 mg/ml collagenase D (Roche Diagnostics GmbH) in RPMI 1640 with FCS for 30 min at 37°C with gentle shaking. Lung digests were filtered through 100-μm Falcon cell strainers (Becton Dickinson) followed by three washes and further filtering through 40-μm cell strainers before analysis by flow cytometry. For isolation of blood granulocytes, mice were exsanguinated by cardiac puncture. Blood was collected on ice over citrate-phosphate-dextrose solution (1.4:10; Sigma-Aldrich) and centrifuged immediately. Plasma was removed and red blood cells were lysed by two rounds of exposure to ammonium chloride.
IL-25 administration and assay.
0.4 μg of rIL-25 (R&D Systems) in PBS was administered per mouse intraperitoneally on days 0–4 for N. brasiliensis infections. Mice were culled on day 5 and tissues were harvested for analysis. For rIL-25 treatment alone, mice were administered 0.4 μg of rIL-25 (R&D Systems or produced in-house [see supplemental Materials and methods]) on days 0–3; mice were culled on day 4 and tissues were harvested for analysis. Control animals received PBS only.
NBNT, c-kit−, Fc R1− and NBNT, c-kit+, Fc
R1− and NBNT, c-kit+, Fc R1− cells were sorted from naive MLN or spleen and plated at 105 cells/well. rIL-25 was added at 10 ng/ml and the cells were incubated for 4 d. Cytokine production was assessed by ELISA.
R1− cells were sorted from naive MLN or spleen and plated at 105 cells/well. rIL-25 was added at 10 ng/ml and the cells were incubated for 4 d. Cytokine production was assessed by ELISA.
Harvest of NBNT from bone marrow.
Bone marrow was flushed aseptically from femora and tibias of 8-wk-old mice. The cell suspension was incubated with biotin-conjugated anti-CD4, -CD8, and -CD19 (eBioscience) then M280 streptavidin Dynabeads (Dynal) were used to remove labeled cells according to the manufacturer's instructions.
Flow cytometry.
Cell surface marker expression was assessed by flow cytometry using a FACScan flow cytometer (Becton Dickinson). The following mAbs were used: Tri-color conjugated anti-CD19 (6D5; Caltag), Tri-color conjugated anti-CD4 (CT-CD4; Caltag); Tri-color conjugated anti-CD8a (5H10; Caltag); PE-conjugated anti-CCR-3 (83101.111; BD Biosciences); FITC-conjugated anti-Fc R1 (MAR-1; eBioscience); PE-conjugated CD117 (c-kit; ACK45; BD Biosciences); Gr-1 (RB6-8C5; BD Biosciences); CD11b (M1/70; BD Biosciences); CD11c (HL3; BD Biosciences); B220 (RA36B2; BD Biosciences), MHCII (14–4-45; BD Biosciences); CD80 (16-10A1; BD Biosciences); CD86 (GL1; BD Biosciences); Thy1.2 (30-H-12; BD Biosciences); CD34 (RAM34; BD Biosciences); and DX5 (eBioscience). Gates were set using isotype control antibodies. Cells were counted and resuspended in ice-cold FACS buffer (2% FCS, 0.05% sodium azide in PBS) at 2 × 106 cells/ml on a 96-well plate. Cells were stained with surface antibodies for 30 min on ice and washed three times in FACS buffer.
R1 (MAR-1; eBioscience); PE-conjugated CD117 (c-kit; ACK45; BD Biosciences); Gr-1 (RB6-8C5; BD Biosciences); CD11b (M1/70; BD Biosciences); CD11c (HL3; BD Biosciences); B220 (RA36B2; BD Biosciences), MHCII (14–4-45; BD Biosciences); CD80 (16-10A1; BD Biosciences); CD86 (GL1; BD Biosciences); Thy1.2 (30-H-12; BD Biosciences); CD34 (RAM34; BD Biosciences); and DX5 (eBioscience). Gates were set using isotype control antibodies. Cells were counted and resuspended in ice-cold FACS buffer (2% FCS, 0.05% sodium azide in PBS) at 2 × 106 cells/ml on a 96-well plate. Cells were stained with surface antibodies for 30 min on ice and washed three times in FACS buffer.
NBNT cells were gated as cells negative for CD19, CD4, and CD8 (reference 18). Blood eosinophils were identified as NBNT, CCR-3+, SSChigh, whereas blood basophils were NBNT, c-kit−, Fc R1+, SSClow. IL-4–expressing cells in 4get mice were detected in the FL1 channel on cells surface stained as described in the previous paragraph.
R1+, SSClow. IL-4–expressing cells in 4get mice were detected in the FL1 channel on cells surface stained as described in the previous paragraph.
Cell sorting was used to isolate the NBNT, c-kit+, SSClow cells from single cell suspensions of the MLN or spleen. Cells were stained with Tri-color–conjugated anti-CD4, CD8, and CD19; PE or allophycocyanin-conjugated anti-CD117 (c-kit); and sorted on a FACSAria (Conway Institute) or MoFlo cell sorter (MRC-LMB, Cambridge). In some experiments, biotin-conjugated anti-Fc R1 was also included to exclude mature mast cells. The sorted cells were >97% pure.
R1 was also included to exclude mature mast cells. The sorted cells were >97% pure.
In vitro T helper cell differentiation assays.
MLN cells or splenocytes were cultured on anti-CD3 antibody-coated plates (1 μg/ml of clone 2C11; Becton Dickinson) in the presence of exogenous cytokines or anti-cytokine antibody as indicated. 10 ng/ml IL-2 (R&D Systems) was added to all cultures. Th2 cell differentiation was promoted in the presence of 100 ng/ml IL-4 (R&D Systems) and anti-IFNγ antibody (10 μg/ml of clone XMG1.2; Becton Dickinson), whereas Th1 differentiation was promoted by anti–IL-4 antibody at 10 μg/ml (clone 11B11; DNAX Research Institute) and 1 ng/ml IL-12 (Genzyme). Cells were cultured for 5 d, washed, and resuspended at 106 cells/ml for 24 h in the presence of plate-bound anti-CD3. Supernatants were analyzed by cytokine ELISA. In some cases, cells were harvested after 48 h for mRNA preparation.
Mast cells and RNA preparation.
Bone marrow was aseptically flushed from femora and tibias of 8-wk-old mice. The cell suspension was cultured at 4 × 105 cells/ml in the presence of 5 ng/ml of IL-3 and 10% WEHI-3B conditioned supernatant for 3–4 wk, with media changes every 7 d. The resultant cell populations were 95% c-kit+ and Gr-1+ when analyzed by flow cytometry (BD Biosciences). After washing, cells were resuspended at 106 cells/ml and stimulated for 1 h with the polyclonal activators PMA at 50 ng/ml and/or calcium ionophore A23187 at 500 ng/ml.
NBNT cell/T cell coculture.
NBNT, c-kit−, Fc R1− and NBNT, c-kit+, Fc
R1− and NBNT, c-kit+, Fc R1− cells were sorted from MLN of IL-13–deficient mice after treatment with IL-25 (see IL-25 administration and assay). NBNT, c-kit−, Fc
R1− cells were sorted from MLN of IL-13–deficient mice after treatment with IL-25 (see IL-25 administration and assay). NBNT, c-kit−, Fc R1−, and NBNT, c-kit+, Fc
R1−, and NBNT, c-kit+, Fc R1− cells were cocultured on anti-CD3–coated (145-2C11; BD Biosciences) plates (0.3 μg/ml), with CD3+ cells purified from untreated wild-type spleens by cell sorting using PE-conjugated anti-CD3 (145-2C11; BD Biosciences) (CD3+ cells were >97% pure). Where indicated, IL-25 was added to the cultures at a concentration of 10 ng/ml. The cells were cocultured for 72 h before the supernatant was harvested and assessed for the presence of IL-13 using the Quantikine Murine IL-13 Kit (R&D Systems).
R1− cells were cocultured on anti-CD3–coated (145-2C11; BD Biosciences) plates (0.3 μg/ml), with CD3+ cells purified from untreated wild-type spleens by cell sorting using PE-conjugated anti-CD3 (145-2C11; BD Biosciences) (CD3+ cells were >97% pure). Where indicated, IL-25 was added to the cultures at a concentration of 10 ng/ml. The cells were cocultured for 72 h before the supernatant was harvested and assessed for the presence of IL-13 using the Quantikine Murine IL-13 Kit (R&D Systems).
Real-time PCR.
Real-time PCR was performed for IL-13 using TaqMan-labeled probe chemistry and using a FAM-labeled probe to enable the detection of amplified product as it accumulates during PCR (Table S1, available at http://www.jem.org/cgi/content/full/jem.20051615/DC1). PCR for IL-4, IL-5, was performed using Sybr green I dye chemistry. Primers and probe sequences were as listed in Table S1. Real-Time PCR analysis was performed on an ABI7900 Real-Time PCR cycler (Applied Biosystems). Hypoxanthine phosphoribosyltransferase (HPRT) PCR was performed in parallel for each gene. Expression levels for each gene were quantified relative to the internal HPRT control before comparison of mRNA levels of each gene between the different cell populations. Expression of all other genes was determined using standard PCR using primers sequences as listed in Table S1.
Cytokine ELISA.
IL-13 ELISA was performed using the Quantikine Murine IL-13 Kit (R&D Systems). All other cytokine ELISAs used the sandwich format with capture and detection antibodies purchased from Becton Dickinson. ELISAs were performed according to Becton Dickinson ELISA protocol.
Mouse mast cell protease-1 ELISA.
Mouse mast cell protease-1 levels in sera were assayed using a mouse MCP-1 ELISA kit purchased from Moredun Scientific. ELISA was performed according to the manufacturer's protocol.
Statistical analysis.
The significance of the differences between experimental groups was analyzed using a Student's unpaired Student's t test.
Online supplemental material.
Fig. S1 shows inactivation of the il25 gene by homologous recombination. Fig. S2 shows worm counts in lungs and intestines of N. brasiliensis–infected wild-type mice after treatment with rIL-25 or control saline. Fig. S3 shows that in vitro Th1 and Th2 cell development is normal in cells from il25 −/− mice. Table S1 shows PCR primers used in this study. The supplemental Materials and methods section describes generation of il25 −/− mice and production of IL-25. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20051615/DC1.
Acknowledgments
We thank P. Smith for technical assistance. We also thank S. Bell and the McKenzie lab for critical reading of this manuscript and the MRC SABU/CBS staff, especially R. Burke and T. Butcher. Hugh Miller kindly provided the initial culture of helminth larvae.
P.G. Fallon is supported by Science Foundation Ireland. A. Dasvarma, A. McIlgorm, and D.R. Hewett were supported by a grant from the Leukaemia Research Fund.
The authors have no conflicting financial interests.
Notes
Abbreviations used: MLN, mesenteric lymph node; NBNT, non–B, non–T.
P.G. Fallon, S.J. Ballantyne, and N.E. Mangan contributed equally to this work.
References
 RI-mediated activation. Blood.
101:3594–3596.
[Abstract] [Google Scholar]
RI-mediated activation. Blood.
101:3594–3596.
[Abstract] [Google Scholar]Articles from The Journal of Experimental Medicine are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1084/jem.20051615
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jem/article-pdf/203/4/1105/1156260/1105.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102378216
Article citations
Body Weight and Allergic Asthma: A Narrative Review.
J Clin Med, 13(16):4801, 15 Aug 2024
Cited by: 0 articles | PMID: 39200943 | PMCID: PMC11355285
Review Free full text in Europe PMC
Spatial adaptation of eosinophils and their emerging roles in homeostasis, infection and disease.
Nat Rev Immunol, 09 Jul 2024
Cited by: 0 articles | PMID: 38982311
Review
LSD1 drives intestinal epithelial maturation and controls small intestinal immune cell composition independent of microbiota in a murine model.
Nat Commun, 15(1):3412, 22 Apr 2024
Cited by: 0 articles | PMID: 38649356 | PMCID: PMC11035651
Epithelial regulation of microbiota-immune cell dynamics.
Mucosal Immunol, 17(2):303-313, 28 Feb 2024
Cited by: 0 articles | PMID: 38428738
Review
Regulatory role of T helper 9/interleukin-9: Transplantation view.
Heliyon, 10(4):e26359, 15 Feb 2024
Cited by: 2 articles | PMID: 38420400 | PMCID: PMC10900956
Review Free full text in Europe PMC
Go to all (483) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nippostrongylus brasiliensis: cytokine responses and nematode expulsion in normal and IL-4-deficient mice.
Exp Parasitol, 84(1):65-73, 01 Oct 1996
Cited by: 75 articles | PMID: 8888733
Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity.
Nature, 464(7293):1367-1370, 03 Mar 2010
Cited by: 1385 articles | PMID: 20200518 | PMCID: PMC2862165
Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion.
Proc Natl Acad Sci U S A, 111(48):E5169-77, 17 Nov 2014
Cited by: 52 articles | PMID: 25404305 | PMCID: PMC4260590
Critical role of IL-25 in nematode infection-induced alterations in intestinal function.
J Immunol, 185(11):6921-6929, 25 Oct 2010
Cited by: 73 articles | PMID: 20974983 | PMCID: PMC2988083
Funding
Funders who supported this work.
Medical Research Council (1)
Investigation of immune and haematopoietic disorders
Dr Andrew McKenzie, MRC Laboratory of Molecular Biology
Grant ID: MC_U105178805




