Abstract
Free full text

Identification of the t Complex–encoded Cytoplasmic Dynein Light Chain Tctex1 in Inner Arm I1 Supports the Involvement of Flagellar Dyneins in Meiotic Drive
Abstract
The cytoplasmic dynein light chain Tctex1 is a candidate for one of the distorter products involved in the non-Mendelian transmission of mouse t haplotypes. It has been unclear, however, how the t-specific mutations in this protein, which is found associated with cytoplasmic dynein in many tissues, could result in a male germ cell–specific phenotype. Here, we demonstrate that Tctex1 is not only a cytoplasmic dynein component, but is also present both in mouse sperm and Chlamydomonas flagella. Genetic and biochemical dissection of the Chlamydomonas flagellum reveal that Tctex1 is a previously undescribed component of inner dynein arm I1. Combined with the recent identification of another putative t complex distorter, Tctex2, within the outer dynein arm, these results support the hypothesis that transmission ratio distortion (meiotic drive) of mouse t haplotypes involves dysfunction of both flagellar inner and outer dynein arms but does not require the cytoplasmic isozyme.
Dyneins are complex, microtubule-dependent molecular motors. These enzymes may be classified into four structurally and functionally distinct groups: cytoplasmic, outer arm, inner arm I1, and inner arms I2/3 (Witman et al., 1994). The flagellar dyneins generate motive force by causing interdoublet microtubule sliding that is ultimately converted to an axonemal bend (Warner et al., 1989). The outer arms provide most of the power for flagellar beating, whereas the heterogeneous inner arm system appears responsible for the initiation of a flagellar bend and for the shear amplitude of the propagated wave (Kamiya et al., 1989). Cytoplasmic dyneins exhibit a wide range of functions that include axonal vesicle transport, membrane trafficking, nuclear migration, and the positioning and anaphase movement of the mitotic spindle (Paschal and Vallee, 1987; Schroer et al., 1989; Corthésy-Theulaz et al., 1992; Li et al., 1993; Xiang et al., 1994; Cottingham and Hoyt, 1997). Despite these wide differences in function, flagellar and cytoplasmic dyneins exhibit some striking similarities in both their polypeptide composition and overall morphology (for reviews see Holzbaur et al., 1994; Mitchell, 1994).
The major isoform of cytoplasmic dynein and inner arm I1 both contain two heavy chains (HCs)1 (Vallee et al., 1988; Piperno et al., 1990). Flagellar outer arm contains either two or three HCs, depending on the source (Bell et al., 1979; Piperno and Luck, 1979; Pfister et al., 1982; Porter and Johnson, 1983), and each of the multiple versions of arms I2/3 contain a single HC (Piperno et al., 1990; Kagami and Kamiya, 1992; Porter et al., 1996). Each HC consists of a globular head domain that is responsible for ATP hydrolysis and force production and an NH2-terminal stem domain that, in the multimeric dyneins, connects the HCs to a common base. In addition to the ATP hydrolytic site, all HCs sequenced to date contain additional phosphate-binding loops, several of which also are involved in nucleotide binding and may function in allosteric regulation (Wilkerson, C.G., and G.B. Witman. 1995. Mol. Biol. Cell. 6:33a; Mocz and Gibbons, 1996).
Each dynein also contains a distinct complement of smaller components associated with the HCs. Cytoplasmic dynein has four light intermediate chains (LICs [53–59 kD]; Gill et al., 1994; Hughes et al., 1995), which have no known flagellar homologues, and two copies of a 74-kD intermediate chain (IC74; Paschal et al., 1992), which is a WD repeat protein related to the β subunit of heterotrimeric G proteins (Wilkerson et al., 1995; Sondek et al., 1996). Both outer arm dynein and inner arm I1 also contain members of the WD repeat family (Mitchell and Kang, 1991; Ogawa et al., 1995; Wilkerson et al., 1995; Yang, P., and W.S. Sale. 1996. Mol. Biol. Cell. 7:568a). These proteins are located at the base of the soluble dynein particle (King and Witman, 1990; Steffen et al., 1996) and appear to be important for attachment of the motor to its cargo (King et al., 1991, 1995; Karki and Holzbaur, 1995).
A variety of light chain (LC) components also are present in these enzymes. For example, inner arms I2/3 contain both a 28-kD protein, which is essential for arm assembly, and the Ca2+-binding protein centrin (LeDizet and Piperno, 1995). Likewise, multiple LCs have been found within outer arm dynein, including a novel calmodulin homologue (King and Patel-King, 1995a ) and two thioredoxins (Patel-King et al., 1996). The outer arm also contains a highly conserved 8,000-M r LC (King and Patel-King, 1995b ) that has been subsequently identified in several other enzyme systems, including cytoplasmic dynein (King et al., 1996a ), myosin V (Espindola, F.S., R.E. Cheney, S.M. King, D.M. Suter, and M.S. Mooseker. 1996. Mol. Biol. Cell. 7:372a), and neuronal nitric oxide synthase (Jaffrey and Snyder, 1996), where it apparently acts to regulate synthase activity by converting the active dimer to an inactive monomeric form. To date, the 8,000-M r LC is the only component that is reportedly shared by both flagellar and cytoplasmic dynein isozymes, and in Drosophila, it is essential for viability (Dick et al., 1996a ). Null mutants in both yeast and Chlamydomonas, however, are viable. The yeast mutant has no overt phenotype (Dick et al., 1996b ), whereas in Chlamydomonas, a wide variety of flagellar defects in the assembly of axonemal components and in retrograde intraflagellar transport are evident (Pazour, G.J., C.G. Wilkerson, and G.B. Witman. 1997. Mol. Biol. Cell. 8:162a).
Also associated with cytoplasmic dynein is a family of 14,000-M r LCs that includes the protein Tctex1 (King et al., 1996b ), which in mice is encoded within a 30–40-Mb region of chromosome 17 known as the t complex. Variant forms of this region termed t haplotypes exist, which exhibit several fascinating properties (for reviews see Silver, 1993; Olds-Clarke, 1997), including the non-Mendelian transmission of the t haplotype to almost all the progeny of heterozygous males. This phenomenon of transmission ratio distortion, a form of meiotic drive, is thought to derive from the action of mutant “distorter” and “responder” proteins (all encoded within the t haplotype) during spermiogenesis that lead to the inability of those sperm carrying the wildtype t complex to fertilize an oocyte. The Tctex1 protein has been of interest because it is a candidate for one of the distorter protein products responsible for the meiotic drive effect (Lader et al., 1989; O'Neill and Artzt, 1995). Intriguingly, we recently found that a Chlamydomonas outer arm dynein LC (Patel-King et al., 1997) was homologous to a second putative distorter termed Tctex2 (Huw et al., 1995).2 This observation raised the possibility that transmission ratio distortion might derive from the dysfunction of both cytoplasmic and flagellar dyneins in the testis (Patel-King et al., 1997). Although there is some evidence for the presence of Tctex1 in sperm (O'Neill and Artzt, 1995), it has remained unclear how a defect in a cytoplasmic dynein component found in many tissues could result in a testis-specific phenotype.
In this report, we describe the further characterization of Tctex1 and conclusively demonstrate that this cytoplasmic dynein component is present in mouse sperm and in the flagella of Chlamydomonas. Genetic dissection of the Chlamydomonas axoneme revealed that flagellar Tctex1 is specifically located in inner dynein arm I1. This same dynein was also found to contain the 8,000-M r LC and, thus, to closely resemble the cytoplasmic isozyme in terms of LC content. The data presented here suggest that the t-specific mutations in Tctex1 might contribute to meiotic drive through an effect on a flagellar inner dynein arm rather than (or perhaps in addition to) having consequences for cytoplasmic dynein function. Combined with our previous identification of the Tctex2 protein as an outer arm dynein LC (Patel-King et al., 1997), these results support a model whereby the differential incorporation of dysfunctional flagellar dyneins during spermiogenesis contributes to the meiotic drive of mouse t haplotypes.
Materials and Methods
Preparation of Mouse Sperm
Sperm were prepared from congenic +/+, tw5/+, and tw5/tw32 mice using the conditions described in Olds-Clarke et al. (1996). Whole sperm proteins were separated by electrophoresis in 15% acrylamide gels before immunoblot analysis.
Chlamydomonas Axoneme Isolation and Dynein Purification
Flagellar axonemes were prepared from Chlamydomonas reinhardtii using standard protocols (Witman, 1986; King, 1995). Dynein was extracted with 0.6 M NaCl and purified by centrifugation in a 5–20% sucrose density gradient (King et al., 1986). Gradient fractions were concentrated in a Centricon 30 unit (Amicon Corp., Danvers, MA). Nonspecific protein binding was minimized by preincubating the unit for 48 h with 5% Tween-20.
For the initial identification of the 14,000-M r protein, the wild-type strain cc124- was used. A mutant lacking the outer arm, oda9, was used for all subsequent purifications. Axonemes from mutants lacking inner arm components (ida1, ida2, ida3, and ida4), radial spokes (pf14), and the central pair microtubule complex (pf18) were used to localize the 14,000-M r protein.
Peptide Sequencing
Sucrose gradient fractions containing the 14,000-M r protein were concentrated, separated by electrophoresis in a 5–15% acrylamide gradient gel, and blotted to polyvinylidene difluoride (PVDF) membrane (Immobilin Psq; Millipore Corp., Bedford, MA) in 10 mM NaHCO3, 3 mM Na2CO3, 0.01% SDS, and 20% methanol. The blot was stained with 0.2% Ponceau S, and a thin strip was probed for the 14,000-M r protein. The immunoreactive band was then identified on the unprobed strip, excised, and treated with trypsin. Peptides eluting from the membrane were purified by reverse-phase chromatography on a C8 column. Two peptides were sequenced (model 492A sequencer; Applied Biosystems Inc., Foster City, CA) at the Protein Chemistry Facility at the Worcester Foundation for Biomedical Research (Shrewsbury, MA). For one peptide, a 12-h cycle of treatment with TFA vapor at 60°C was required to remove a blocked NH2 terminus via an Asp-Pro cleavage. Peptide masses were determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using a Linear Voyager Biospectrometry workstation (PerSeptive Biosystems, Framingham, MA).
Electrophoresis and Immunoblotting
All Chlamydomonas samples were electrophoresed in 5–15% polyacrylamide gradient gels and either stained with Coomassie brilliant blue or blotted to nitrocellulose (0.2 μm pore size; Schleicher & Schuell, Keene, NH) in 10 mM NaHCO3, 3 mM Na2CO3, 0.01% SDS, and 20% methanol. For immunoblotting, the nitrocellulose was blocked in 5% dried milk, and 0.1% Tween-20 in TBS before being probed with blot-purified primary antibody followed by a peroxidase-conjugated secondary antibody (King et al., 1996a ). After washing, the antibody signal was visualized using an enhanced chemiluminescent system (ECL; Amersham Corp., Arlington Heights, IL) and Rx film (Fuji Photo Film Co. Ltd., Tokyo, Japan). Total protein was subsequently detected using 0.2% Ponceau S. Antibodies used were R4058 vs. the Chlamydomonas 8,000-M r LC (King and Patel-King, 1995b ), R5205 vs. human Tctex1 (King et al., 1996b ), and R5391 vs. the Chlamydomonas Tctex2 homologue LC2 (Patel-King et al., 1997).
Quantitation of Coomassie blue–stained gels was performed using an IS1000 digital imaging system (Alpha Innotech, San Leandro, CA).
Molecular Cloning
A gene-specific primer (5′-GCGCGAATTCCTSCAGAACCAGCAGTAC- 3′) was designed based on the peptide sequence LQNQQY and incorporated the Chlamydomonas codon bias (Harris, 1989). The primer also incorporated an EcoRI site and a GC clamp at the 5′ end. The reverse primer was the standard oligo (dT) adapter primer (5′-GCGCGTCGACTCGAGT20V-3′) that contains SalI and XhoI sites at the 5′ end. 100-μl PCRs were performed in 10 mM Tris-Cl, pH 8.85, 25 mM KCl, 5 mM (NH4)2SO4, 2 mM Mg SO4, and 0.2 mM dNTP, and they contained 1 μg of each primer. The template used was first-strand cDNA made from mRNA isolated from cells actively regenerating their flagella. Samples were heated at 95°C for 5 min, cooled on ice, and 2.5 U of Pfu DNA polymerase (Stratagene, La Jolla, CA) were added. Samples were subjected to 35 rounds of the following program: 96°C for 1 min, 35°C for 1 min, and 75°C for 2 min. Products were subcloned into pBluescript II SK− (Stratagene) and were sequenced using Sequenase v2.0 and a 7-deaza-dGTP sequencing kit (U.S. Biochemical Co., Cleveland, OH).
The PCR product corresponding to the 14,000-M r protein was used to screen a λZapII cDNA library made from mRNA derived from cells actively regenerating flagella (Wilkerson et al., 1995). Phagemids were rescued using helper phage, and the longest clone was sequenced on both strands using a double-stranded DNA template. Northern and Southern blots were prepared by standard methods (Sambrook et al., 1989) and probed using the conditions described in King and Patel-King (1995b). The probes used were the 14,000-M r protein full-length cDNA, a full-length cDNA for LC4 (King and Patel-King, 1995a ), and a full-length calmodulin cDNA that had been isolated during a screen of the λZapII library for the calmodulin-like LC4 protein.
Computational Methods
Sequence assembly and analysis was performed using the GCG suite of software (Devereux et al., 1984). Searches of the GenBank database were made using BLAST (Altshul et al., 1990). Pairwise sequence comparisons were generated using GAP (Devereux et al., 1984), and multiple alignments were constructed with CLUSTALW (Thompson et al., 1994). The secondary structure prediction was made using the PHD program (Rost and Sander, 1993), and the helical segments were analyzed with HELICALWHEEL (Devereux et al., 1984). The phylogenetic tree was calculated with DISTANCES and plotted with GROWTREE.
Results
Tctex1 Is Present in the Flagellar Axoneme
We recently identified the mouse t complex–encoded protein Tctex1 as one of the 14,000-M r LCs associated with cytoplasmic dynein isolated from mammalian brain, kidney, liver, spleen, and testis (King et al., 1996b ). Intriguingly, a previous report had suggested that Tctex1 may also be present in the flagellum of mammalian sperm (O'Neill and Artzt, 1995). To confirm this observation, we examined sperm derived from congenic +/+, tw5/+, and tw5/tw32 mice for the presence of Tctex1 using the specific R5205 antibody described previously (King et al., 1996b ). In all samples, an immunoreactive band of ~14,000-M r was observed (Fig. (Fig.11 a, upper panel). To ensure that the reaction was specific, the blot-purified R5205 antibody was preadsorbed against recombinant Tctex1 before probing a companion blot; this yielded no signal (Fig. (Fig.11 a, lower panel). Thus, Tctex1 is indeed a component of mammalian sperm.
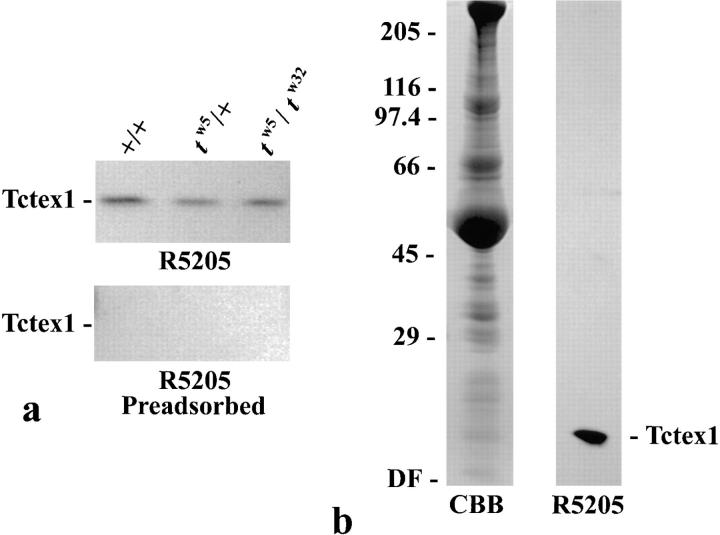
Tctex1 is present in both mouse sperm and Chlamydomonas axonemes. (a) Whole-cell extracts of equal numbers of sperm from +/+, tw 5/+, and tw 5/tw 32 mice were electrophoresed in a 15% acrylamide gel, blotted to Immobilon-P membranes, and probed with R5205 antibody (upper panel) or R5205 antibody that had been preadsorbed against recombinant Tctex1 (lower panel). Tctex1 is present in sperm from all genotypes. (b) 150 μg of Chlamydomonas axonemes were electrophoresed in a 5–15% acrylamide gradient gel and either stained with Coomassie blue (CBB) or blotted to nitrocellulose and probed with R5205 antibody. The locations of the relative molecular mass markers and the dye front (DF) are indicated on the left. A single 14,000-M r band was observed.
To further investigate the role of Tctex1 in the flagellum, axonemes were prepared from Chlamydomonas and probed with the R5205 antibody raised against human Tctex1. A single immunoreactive band migrating at ~14,000 M r was observed (Fig. (Fig.11 b), indicating that a Tctex1-like protein exists in Chlamydomonas and that it is a component of the flagellar axoneme. After treatment of these axonemes with 0.6 M NaCl, ~90% of the immunoreactive band was found in the high salt supernatant (Fig. (Fig.2),2), as would be expected for a component of axonemal dynein. Analysis of membrane/matrix fractions also revealed a very small pool of this protein (not shown). This pool does not appear to be distinct from the major axonemal fraction (see below).
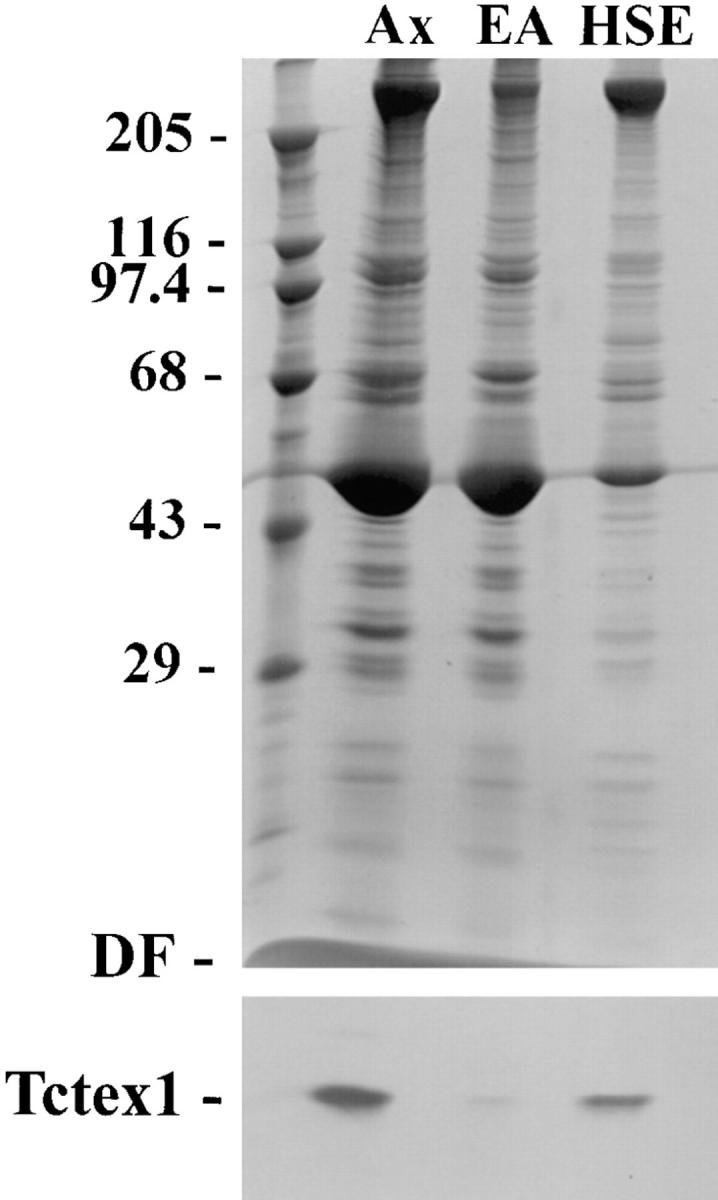
Distribution of Tctex1 in Chlamydomonas axonemes. 125 μg of axonemes were treated with 0.6 M NaCl. Equivalent amounts of axonemes (Ax), extracted axonemes (EA), and the high salt extract (HSE) were electrophoresed in 5–15% acrylamide gradient gels. One portion of the gel was stained with Coomassie blue (upper panel) while the other was blotted to nitrocellulose and probed with R5205 antibody (lower panel). The locations of the relative molecular mass markers and the dye front (DF) are indicated on the left. Approximately 90% of the Tctex1 band was extracted with 0.6 M NaCl, suggesting that it may be a component of axonemal dynein.
Tctex1 Is a Component of Inner Arm I1
To further define the intraflagellar associations of the Chlamydomonas Tctex1-like protein, axoneme samples were prepared from mutants lacking various axonemal structures, including the outer arm (oda9), inner arm I1 (ida1, ida2, and ida3), inner arms I2/I3 (ida4), the radial spokes (pf14), and the central pair complex (pf18). When these samples were probed with an antibody (R5391) against LC2 of the outer arm, which is a homologue of the putative t complex distorter Tctex2, this protein was found to be missing only in axonemes prepared from the oda9 mutant, as described previously (Patel-King et al., 1997). Interestingly, the minor 15,000-M r protein recognized by this antibody was present in all axoneme samples, indicating that it is not a component of the dynein arms, radial spokes, or central pair complex. In contrast, analysis of the same samples with the R5205 antibody revealed that the Tctex1-like protein was missing specifically in those strains (ida1–ida3) unable to assemble inner arm I1 (Fig. (Fig.3).3). This result strongly suggests that in Chlamydomonas, the Tctex1 protein is an integral component of the inner arm I1 complex.
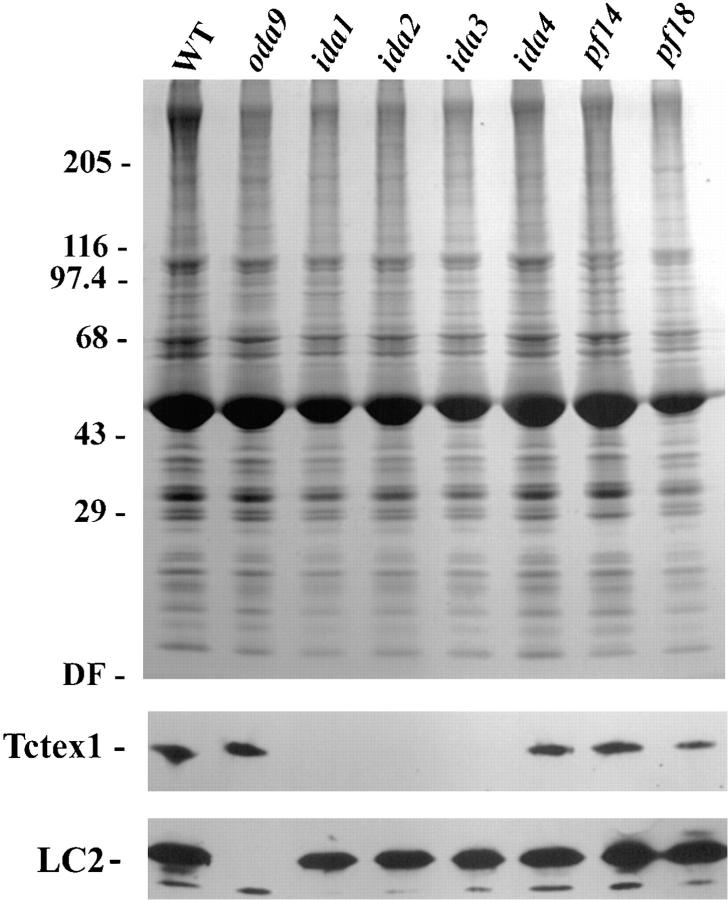
Intra-axonemal localization of Tctex1. Axonemes were prepared from wild-type Chlamydomonas (WT) and from strains lacking the outer arm (oda9), inner arm I1 (ida1, ida2, and ida3), inner arms I2/I3 (ida4), radial spokes (pf14), and central pair complex (pf18). 100 μg of each preparation was electrophoresed in a 5–15% acrylamide gradient gel and either stained with Coomassie blue (upper panel) or blotted to nitrocellulose (lower panels) and probed with R5205 and R5391 antisera to detect Tctex1 and LC2 (Tctex2) of outer arm dynein, respectively. LC2 is missing only in the oda9 mutant, which lacks the outer arm (the smaller immunoreactive band is present in all strains tested as described previously [Patel-King et al., 1997]). Tctex1 is specifically absent in those strains that fail to assemble inner arm I1, suggesting that it may be a component of that complex.
To further confirm the association deduced from genetic dissection, axonemes were prepared from the oda9 mutant and subjected to high salt extraction. The extract (which contained no outer arm components) was then sedimented through a 5–20% sucrose gradient. Electrophoretic and immunological analysis of the resulting fractions revealed that the Tctex1-like protein sedimented at ~18 S and indeed precisely comigrated with bona fide inner arm I1 components (Fig. (Fig.4).4). This result confirmed that Tctex1 is an inner arm I1 polypeptide. The small membrane pool of Tctex1 was missing from ida1 flagella, suggesting that it represents inner arm I1 that either dissociated during detergent extraction or had not yet been assembled into the axonemal superstructure (not shown).
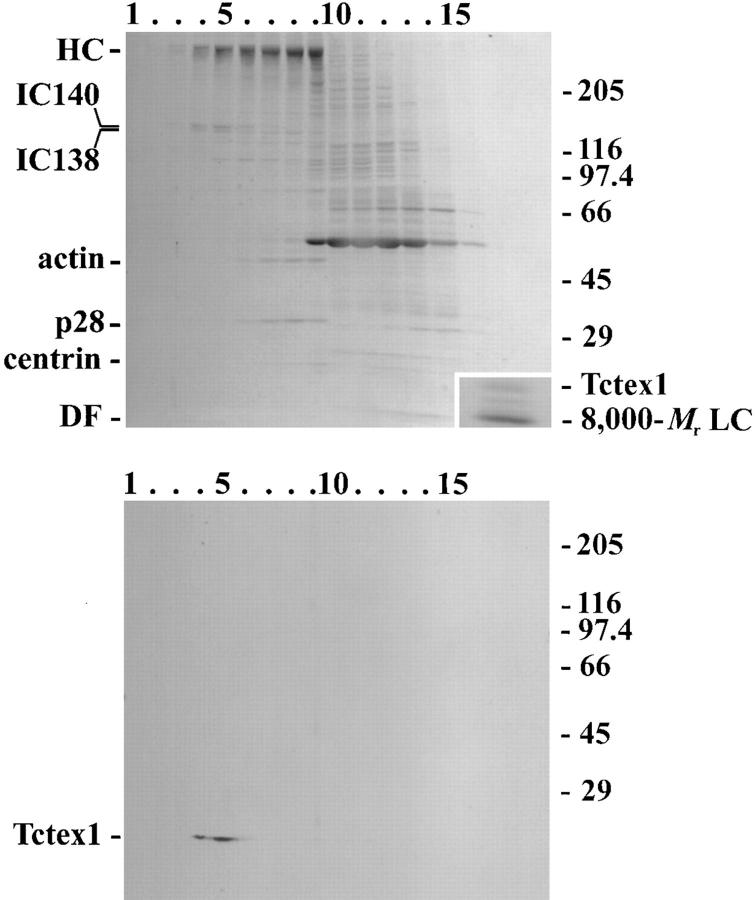
Tctex1 is a component of inner arm dynein I1. Proteins extracted from oda9 axonemes (which completely lack the outer arm) by high salt were separated by sucrose density gradient centrifugation. Equal volumes of each fraction were electrophoresed in 5–15% gradient gels. One gel was stained with Coomassie blue (upper panel), while the other was blotted to nitrocellulose and probed with R5205 antibody (lower panel). The bottom of the gradient is at the left. After immunoblotting, the nitrocellulose was restained with Ponceau S to reveal the location of individual lanes and the relative molecular mass markers. Tctex1 comigrates with inner arm I1 components (IC140 and IC138) at ~18 S, but it is clearly distinct from arms I2/3, which contain actin, p28, and centrin. (inset, upper panel) The I1 dynein–containing fractions from two gradients were pooled and concentrated in a Centricon-30 ultrafiltration unit and electrophoresed in a 5–15% acrylamide gel. The LC region of the Coomassie blue–stained gel is shown. Three bands are evident. The upper band was recognized by the R5205 antibody and is therefore Tctex1. The lower band was detected by the R4058 antibody, indicating that it represents an additional pool of the 8,000-M r LC (King and Patel-King, 1995b ). A third LC band of unknown origin is also present.
To further analyze the LC complement of this dynein, the I1-containing fractions from several gradients were pooled and concentrated in a Centricon 30 ultrafiltration unit. After electrophoresis and staining with Coomassie blue, three distinct bands were observed in the low molecular weight region (Fig. (Fig.4,4, inset). Immunological analysis revealed that the slowest migrating band was Tctex1. The fastest migrating band was recognized by antibody R4058 (King and Patel-King, 1995b ) and, thus, is likely to be identical to the 8,000-M r LC previously found in both outer arm and cytoplasmic dyneins, as well as in several other enzyme systems (Benashski et al., 1997). Intriguingly, a third band (~12,000-M r) was also observed in arm I1; at the present time, no further information is available concerning the identity of this protein.
Quantitative densitometry of Coomassie blue–stained gels was used to determine the stoichiometry of the Tctex1 and 8,000-M r LCs within the I1 dynein (Table (TableI).I). This analysis revealed that there are two copies of Tctex1 and suggested the presence of 8–12 copies of the 8,000-M r LC per I1 arm (this latter value is likely an overestimate; see Discussion). Furthermore, based on the presence of two HCs (Iα and Iβ), the data indicate that the I1 particle contains one to two copies of IC140, a single IC138, and one to two copies of IC97. A 34,000-M r protein has also been suggested to be part of the I1 complex (King and Dutcher, 1997). Although a band of that relative molecular mass is present in the appropriate region of the sucrose gradients, the 34,000-M r polypeptide peak is offset from that of I1 dynein by one fraction and, instead, it appears to comigrate with 100,000- and 106,000-M r proteins.
Table I
Stoichiometry of Components within Inner Dynein Arm I1*
| Component‡ | Relative stoichiometry§ | Copies per dynein arm | ||
|---|---|---|---|---|
| HC | 1.00 | 2‖ | ||
| IC140 | 0.71 0.53 0.63 (0.5–1.0) | 1–2 | ||
| IC138 | 0.53 (0.5) | 1 | ||
| IC97 | 0.95 0.67 0.56 (0.5–1.0) | 1–2 | ||
| Tctex 1 | 1.28 1.12 0.71 (1.0) | 2 | ||
| 8,000-M r LC | 5.50 4.00 (4.0–5.5) | 8–12 ¶ |
 Determined by quantitative densitometry of Coomassie blue–stained gels.
Determined by quantitative densitometry of Coomassie blue–stained gels.  The ~12,000-M
r protein observed in I1 samples is present at a stoichiometry of one per particle. This polypeptide, however, has not yet been formally demonstrated to be a dynein LC.
The ~12,000-M
r protein observed in I1 samples is present at a stoichiometry of one per particle. This polypeptide, however, has not yet been formally demonstrated to be a dynein LC.  The values are quoted relative to the HCs. The most likely stoichiometry is in parentheses.
The values are quoted relative to the HCs. The most likely stoichiometry is in parentheses.  Previously published results indicate that there are two HCs (Iα and Iβ) per particle (Piperno et al., 1990).
Previously published results indicate that there are two HCs (Iα and Iβ) per particle (Piperno et al., 1990).  35S-Labeling of Chlamydomonas proteins in vivo suggests this value may be an overestimate by approximately twofold (Wilkerson, C.G., and G.B. Witman, personal communication).
35S-Labeling of Chlamydomonas proteins in vivo suggests this value may be an overestimate by approximately twofold (Wilkerson, C.G., and G.B. Witman, personal communication). Molecular Analysis of Chlamydomonas Tctex1
To conclusively identify the 14,000-M r protein as the Chlamydomonas homologue of Tctex1, it was essential to compare the sequences of the algal and mammalian proteins. Therefore, the sucrose gradient–purified inner arm I1 from the oda9 mutant was concentrated in a Centricon 30, and the components were separated by electrophoresis and blotted to a PVDF membrane. The 14,000-M r band was identified using blot-purified R5205 antibody, excised from the PVDF blot, and digested in situ with trypsin. Eluted peptides were purified by reverse-phase chromatography (Fig. (Fig.5,5, a). Mass spectrometry revealed that peak (i) contained a single peptide with a mass (M+H+) of 1,825 D (Fig. (Fig.5,5, b). This peptide subsequently yielded the sequence ESIDAVLQNQQYSEAK, which has a calculated molecular weight of 1,823 D. Analysis of peak (Fig. (Fig.55 a, ii) revealed a major peptide of 2,463 D and a minor one of 2,479 D (Fig. (Fig.5,5, c). As the mass difference between these two molecules is 16 D, the latter very likely represents a methionine oxidation product of the former. Initially, Edman degradation of the peak (Fig. (Fig.55 a, ii) peptide yielded no products, indicating that the NH2 terminus was blocked. However, after prolonged treatment with TFA, which cleaves Asp-Pro bonds, the sequence PAVEEAAFVADDVSNII was obtained.
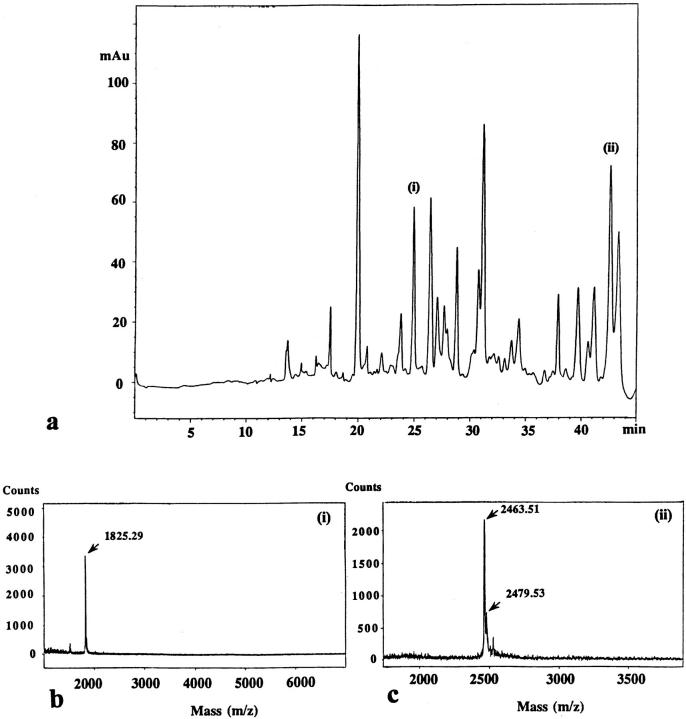
Peptide purification from Tctex1. The I1-containing fractions from seven gradients were pooled and concentrated in a Centricon 30 ultrafiltration unit. The concentrate was electrophoresed in a 5–15% acrylamide gradient gel and blotted to polyvinylidene difluoride. A strip was excised and probed with R5205 to localize the Tctex1 band. The remaining Tctex1 band was then excised from the unprobed PVDF and digested in situ with trypsin. Peptides eluting from the membrane were purified by reverse-phase chromatography on a C8 column (a). Peaks (i) and (ii) were sequenced. Mass spectrometry (b and c) revealed a single peptide of 1,825 D present in peak (i). Peak (ii) contained a single major peptide of 2,463 D and a minor amount of its methionine oxidation product at 2,479 D.
A gene-specific primer based on the sequence LQNQQY and an oligo (dT) adaptor primer were then used in the PCR using first-strand cDNA derived from mRNA enriched for flagella sequences and generated a product of ~1 kb. Sequencing of the 5′ end of this product revealed that the predicted residues were encoded immediately after the primer. Therefore, this product was used to screen a λZapII cDNA library made from RNA derived from Chlamydomonas actively regenerating their flagella (Wilkerson et al., 1995). Multiple clones were obtained, and the longest was sequenced on both strands.
The largest clone isolated was 1,190 bp in length and contained a single open reading frame of 342 bp encoding a protein of 114 residues with a calculated molecular weight of 12,668 D and a predicted isoelectric point of 5.17 (Fig. (Fig.6).6). The reading frame is preceded by a 115-bp 5′ untranslated region that contains three in-frame stop codons before the first Met residue. The reading frame terminates with a single stop codon followed by a 707-bp 3′ untranslated region before the poly A tail. Both peptide sequences obtained from the authentic molecule were found within the predicted coding sequence (33/33 residues correct), confirming that the clone indeed encodes Chlamydomonas Tctex1.
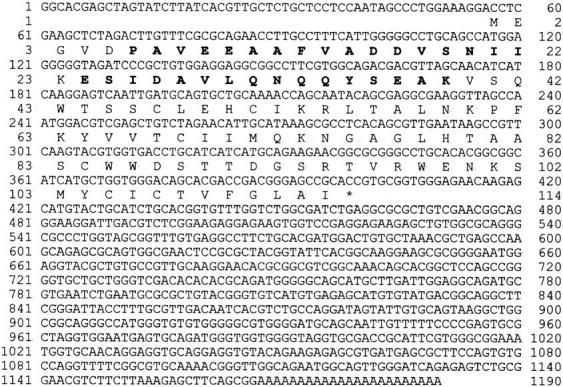
Sequence of Chlamydomonas Tctex1. Nucleotide and predicted amino acid sequence of the cDNA clone encoding Tctex1 are shown. The residues shown in bold type were obtained from peptide sequencing (33/33 residues correct) and confirm the identity of the clone. The 5′ untranslated region contains three in-frame stop codons. These sequence data are available from GenBank/EMBL/DDBJ under accession number AF039437.
The peak (i) peptide was found to be preceded by a Lys residue, as required for a tryptic fragment. The peptide from peak (ii) represents the NH2 terminus of the protein. It contains the predicted acid-sensitive Asp-Pro bond immediately before the peptide sequence obtained. Intriguingly, the actual mass of the blocked NH2-terminal peptide was 42-D greater than calculated from the encoded sequence. This mass difference strongly suggests that the modification resulting in NH2-terminal blockage was caused by acetylation of the initial methionine residue.
Southern blot analysis of Chlamydomonas genomic DNA revealed a single band after digestion with BamHI, PstI, PvuII, and SmaI (Fig. (Fig.77 a), suggesting that a single gene for this protein exists within Chlamydomonas. Two messages for this protein, however, were detected on Northern blots (Fig. (Fig.77 b). The larger message of ~1.35 kb was observed in total RNA samples from nondeflagellated cells, but it was not detected in samples from cells that were undergoing flagellar regeneration. In contrast, the smaller ~1.30-kb mRNA was greatly upregulated in cells actively regrowing flagella. This differential regulation of both message levels was very apparent when compared with the levels of the single messages for two other proteins (Fig. (Fig.77 c). The mRNA for LC4 of the outer arm was essentially undetectable in nondeflagellated cells, but it was highly upregulated during regeneration (King and Patel-King, 1995a ). Calmodulin mRNA exhibited a different pattern and was readily detectable in both samples because the same protein is used constitutively in the cytoplasm but is also upregulated and required for flagellar assembly and/or function (Gitelman and Witman, 1980; Zimmer et al., 1988).
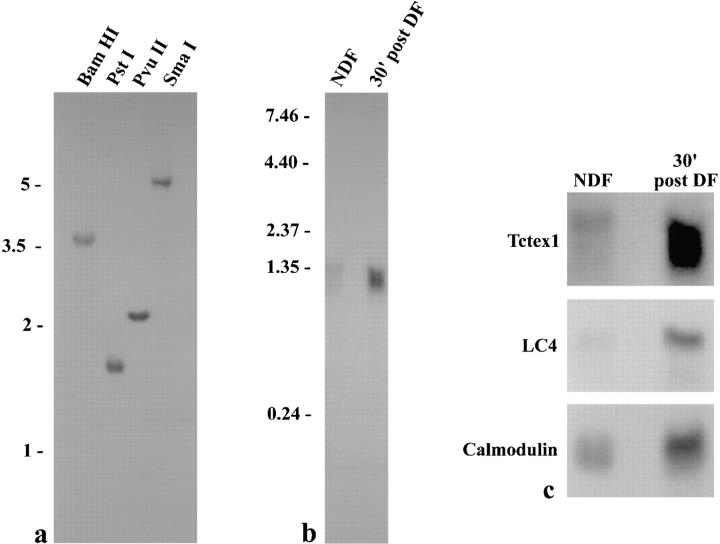
Southern and Northern blot analyses. (a) Southern blot of 10 μg genomic DNA from Chlamydomonas strain S1D2 digested with BamHI, PstI, PvuII, and SmaI. Single bands were observed in all lanes, suggesting that there is only a single gene for this LC within Chlamydomonas. Standards are indicated on the left in kilobases. (b) Northern blot of 20 μg total RNA obtained from nondeflagellated cells (NDF) and from cells that had been deflagellated and allowed to regenerate flagella for 30 min (30′ post DF). Standards are shown on the left in kilobases. Two messages were observed. The message at ~1.35 kb was detected only in the NDF sample, while a smaller message (~1.30 kb) was greatly upregulated after deflagellation. (c) Enlargement of Fig. Fig.77 b (upper panel) and comparison with both a deflagellation-regulated message (LC4 of outer arm dynein) and one that is present constitutively but is also upregulated (calmodulin).
The secondary structure for Chlamydomonas Tctex1 was predicted using PHD (Fig. (Fig.88 a; Rost and Sander, 1993). The NH2-terminal half of the molecule has two segments that have a high probability of being helical. Significant portions of both segments (Fig. (Fig.88 a, i and ii) are predicted to be highly amphiphilic and may therefore be involved in protein–protein interactions. The COOH-terminal region consists of a series of extended sheet structures.
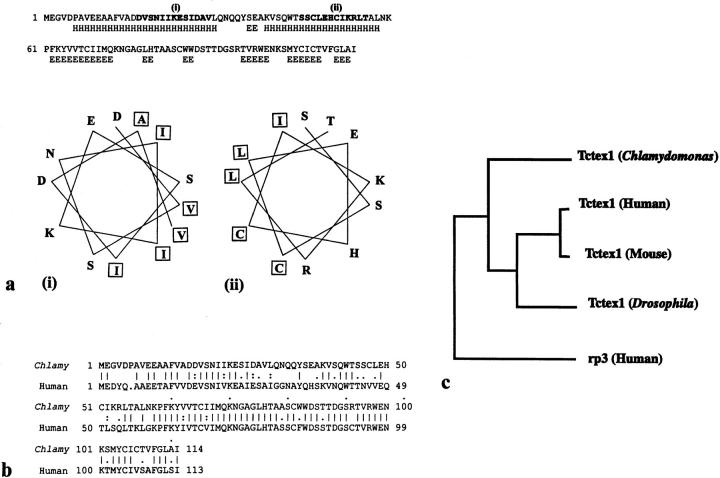
Sequence analysis of Chlamydomonas flagellar Tctex1. (a) The secondary structure of Tctex1 was predicted using PHD (Rost and Sander, 1993). E, extended sheet; H, helix. Helical stretches (i) and (ii) are amphiphilic and displayed using HELICALWHEEL. Hydrophobic and hydrophilic residues cluster to opposite sides of the helix. (b) Sequence comparison between Chlamydomonas flagellar Tctex1 and human Tctex1 (D50663). The alignment was generated by GAP using the default parameters. These proteins share 62% identity (68% similarity) with the smallest Poisson probability P (n) = 6.3 × 10−45 (calculated by BLAST). (c) Phylogenetic analysis of the members of the Tctex1 protein family. The relationship was calculated with DISTANCES and plotted with GROWTREE (UPGMA option). Chlamydomonas flagellar Tctex1 is more closely related to mammalian and insect Tctex1 than it is to human rp3, which is an additional cytoplasmic dynein LC sharing 55% identity with Tctex1 (King, S.M., E. Barbarese, J.F. Dillman III, S.E. Benashski, K.T. Do, R.S. Patel-King, and K.K. Pfister. 1997. Mol. Biol. Cell. 8:163a).
Homology of the Chlamydomonas and Murine Tctex1 Proteins
Examination of the GenBank and Expressed Sequence Tag databases using BLAST revealed that the Tctex1 protein from Chlamydomonas flagella is closely related to mammalian Tctex1. A comparison between the Chlamydomonas and human proteins generated by GAP using the default parameters is shown in Fig. Fig.88 b. These proteins share 62% identity (68% similarity) with a smallest Poisson probability P (n) = 6.3 × 10−45 (calculated by BLAST), indicating that the match is highly significant. In addition to human Tctex1, the Chlamydomonas protein is closely related to murine Tctex1 (60% identity; P (n) = 1.2 × 10−43) and Drosophila Tctex1 (56% identity; P (n) = 1.9 × 10−39). Chlamydomonas Tctex1 is more distantly related to the human protein rp3 (46% identity, P (n) = 2.1 × 10−35; Roux et al., 1994), which we have recently shown to be an additional LC of cytoplasmic dynein (King, S.M., E. Barbarese, J.F. Dillman III, S.E. Benashski, K.T. Do, R.S. Patel-King, and K.K. Pfister. 1997. Mol. Biol. Cell. 8:163a). These proteins are also distantly related to another t complex distorter candidate (Tctex2; Huw et al., 1995) and its Chlamydomonas homologue, which is an LC of outer arm dynein (Patel-King et al., 1997), as well as to several mammalian, nematode, and trypanosome homologues present in the Expressed Sequence Tag database (not shown).
Phylogenetic analysis (Fig. (Fig.88 c) revealed that the Chlamydomonas Tctex1 protein is more closely related to mammalian and insect Tctex1 proteins than is the mammalian Tctex1 homologue rp3. This strongly supports the hypothesis that the Chlamydomonas protein is indeed the algal equivalent of Tctex1 rather than another member of this divergent protein family.
Discussion
In this report, we demonstrate that the t complex–encoded cytoplasmic dynein LC Tctex1 is also an integral stoichiometric component of flagellar inner dynein arm I1. This conclusion is supported by several observations. First, a 14,000-M r protein in both mouse sperm and Chlamydomonas flagella is specifically recognized by an antibody raised against human Tctex1. Second, the Chlamydomonas protein copurifies with inner arm I1 and is missing only in those strains that fail to assemble this particular dynein complex. Third, there are two messages for this protein in Chlamydomonas; one is expressed constitutively, as expected for a cytoplasmic dynein component, while a second is subject to upregulation after deflagellation, as predicted for an axonemal protein. Finally, molecular analysis revealed that the Chlamydomonas protein shares considerable sequence identity with murine Tctex1.
The Chlamydomonas 14,000-Mr Protein Is Closely Related to Murine Tctex1
The Tctex1 protein family consists of two major branches exemplified by Tctex1 (shown in Fig. Fig.88 c) and by murine Tctex2 and its Chlamydomonas homologue LC2 (Patel-King et al., 1997). Molecular cloning of the Chlamydomonas 14,000-M r protein described here revealed that it shares 62% identity with mammalian Tctex1. The closest known mammalian homologue of Tctex1 is the rp3 polypeptide that was originally cloned by Roux et al. (1994) and is ~55% identical with Tctex1. The rp3 protein has recently been demonstrated to be an additional cytoplasmic dynein LC that is most prevalent in cells and tissues distinct from those expressing Tctex1 (King, S.M., E. Barbarese, J.F. Dillman III, S.E. Benashski, K.T. Do, R.S. Patel-King, and K.K. Pfister. 1997. Mol. Biol. Cell. 8:163a). Importantly then, the dendrogram clearly distinguishes rp3 from the Chlamydomonas, insect, and mammalian Tctex1 proteins. Thus, phylogenetic analysis strongly supports the identification of the 14,000-M r protein as the Chlamydomonas version of murine Tctex1. Furthermore, there are several partial and complete sequences of additional mammalian, nematode, and trypanosome Tctex1 homologues present in the Expressed Sequence Tag database (not shown). All of these proteins are significantly less related to Tctex1 than is the Chlamydomonas LC and, indeed, several of them group with the much more diverse Tctex2 branch of this family.
Implications of a Flagellar Form of Tctex1 for the Mechanism of Meiotic Drive
Meiotic drive or transmission ratio distortion is thought to derive from defects in spermiogenesis, where sperm bearing the t haplotype–containing chromosome are normal, but those with the wild-type t complex are dysfunctional and unable to fertilize an oocyte (Olds-Clarke and Peitz, 1985). This phenotype could be caused by aberrant flagellar motility because sperm from t/+ males exhibit subtle defects in motility that have been shown to affect their ability to reach the oocytes both in vivo and in vitro (see Olds-Clarke [1997] for recent review). Genetic analysis suggests that meiotic drive derives from the interaction of three to four distorter proteins with a responder that is expressed after meiosis (Lyon, 1984, 1986). To achieve distortion, the responder must be kept in close association with the nucleus that encoded it. We have recently suggested that distortion might be achieved if the responder acts as the gatekeeper or sorting mechanism for flagella assembly located at or near the basal body (Patel-King et al., 1997). The wild-type responder is thought to interact with both wild-type and t mutant distorter proteins, whereas the mutant responder may offer a protective effect by interacting poorly with mutant distorters and thereby incorporating mainly the wild-type versions (Lyon, 1984, 1986; Cebra-Thomas et al., 1991). The consequences of this model for incorporation of Tctex1, Tctex2, and the Tcd2 distorter into + and t sperm are illustrated in Fig. Fig.9.9. Simultaneous analysis of the speed and path shape of sperm populations from t/+ males shows two distinct peaks, one of which resembles that of wild-type sperm and one that is similar to sperm from mice carrying two t haplotypes (Olds-Clarke and Johnson, 1993). These data support the idea that t/+ males produce two subpopulations of sperm that differ in their motility characteristics.
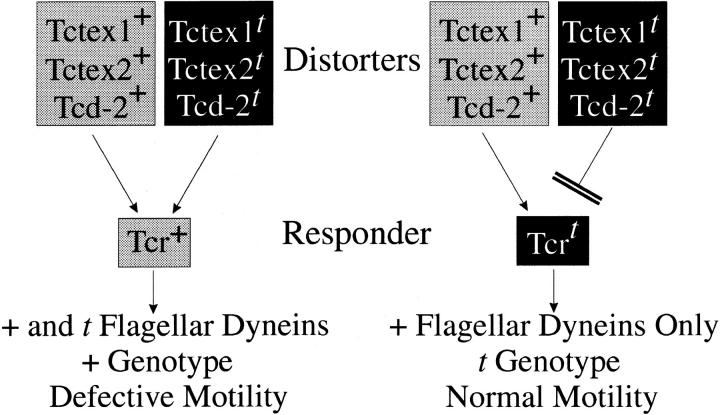
A mechanism for flagellar dynein–mediated meiotic drive. In this model, the responder is hypothesized to be a “gatekeeper” or sorting mechanism that determines what may enter the growing flagellum during spermiogenesis. Because t mutations in both putative distorters Tctex1 and Tctex2 could lead to flagellar dynein dysfunction, their incorporation into + sperm by the wild-type responder (Tcr +) might result in defective motility. In contrast, the t mutant responder (Tcrt) is thought to protect the t sperm with which it associates by being unable to interact with (or having a lower affinity for) the mutant distorters. In the heterozygous case, this would lead to incorporation of only wild-type dyneins into t haplotype–bearing sperm and thus to normal motility. The molecular identities of all the distorters are unknown at the present time. In this model, it is hypothesized that Tctex1 and Tctex2 are the Tcd1 and Tcd3 distorters, respectively.
Tctex1 and Tctex2 are located in the appropriate regions of the t haplotype to be candidates for distorter factors Tcd1 and Tcd3, respectively (Lader et al., 1989; Rappold et al., 1987; Huw et al., 1995). The t haplotype forms of both proteins contain mutations that are likely to affect dynein function. In Tctex1, the mutation Q41H disrupts a tripeptide sequence that has been completely conserved in all Tctex1 proteins (except for Drosophila, which contains Asn at the equivalent position) and in the closely related rp3. Similarly, the t form of Tctex2 contains a three-residue deletion and a proline insertion within a predicted helical segment. As Tctex2 is an essential outer arm component, it is relatively easy to see how its dysfunction might affect sperm motility. However, identification of Tctex1 as a cytoplasmic dynein LC was more problematic, because this protein is present in many tissues but heterozygous t haplotype–bearing mice show an abnormal phenotype only in male germ cells. If Tctex1 is a component of an inner arm in mouse sperm flagella, this would provide a mechanism by which the t haplotype allele could lead to alterations in sperm activity.
The action of the distorter proteins is cumulative such that the degree of ratio distortion is directly related to the particular alleles present in a given haplotype (Lyon, 1984, 1986). These allele-specific effects can be readily understood through increasingly severe consequences for flagellar dynein function and, thus, for the motile properties of sperm. In our model, distortion arises because the t responder interacts only weakly with the t distorters and, therefore, preferentially incorporates the wild-type forms into the growing sperm tail. Sperm from tx/ty mice3 are known to contain both Tctex1 and Tctex2 (Huw et al., 1995; O'Neill and Artzt, 1995; this study). All sperm from these mice, however, exhibit defective motility and are completely nonprogressive (Olds-Clarke and Johnson, 1993). This observation is also predicted by our model (Table (TableII).II). The tx/ty homozygous animals represent the sole occasion on which the t forms of both the distorters and responder are the only versions present within the cell. We suggest that in the absence of the competing “high affinity” + distorters, the “low affinity” t forms would interact with the t responder and become incorporated by default. Thus, in the absence of competition with the high affinity distorters, low affinity interactions could suffice for the insertion of the t mutant distorters. The predicted variation in motile properties between + and t sperm from heterozygotes may then reflect a qualitative difference in the type of distorter protein (+ or t) incorporated into the two sperm classes.
Table II
Predicted Sperm Motility Phenotypes
| Sperm genotype | Spermatocyte genotype | Predicted incorporation of + and t dyneins | Predicted motility | |||
|---|---|---|---|---|---|---|
| + | +/+ | + | Normal | |||
| + | t/+ | + and t | Defective | |||
| t | t/+ | + | Normal | |||
| t | t/t | t | Very poor |
Tctex1 and Tctex2 are candidates for the proximal Tcd1 and Tcd3 distorters. Analysis of rare partial haplotypes has revealed, however, that the strongest distorter (Tcd2) is located in the distal portion of the t complex (see Fig. Fig.11 in Pilder et al., 1993). The molecular identity of this protein, which may be encoded at the Hybrid Sterility-6 locus (Pilder et al., 1993), is unknown at the present time. Sperm from the Hst6 mutant exhibit an abnormal flagellar curvature as well as poor motility. This phenotype is only seen in motile gametes, raising the possibility that it derives from the misregulation of flagellar beating, perhaps through direct effects on the dynein arm (and thus potentially on Tctex1 and/or Tctex2) or radial spoke systems. Intriguingly, a presumptive axonemal dynein HC has recently been mapped to the distal region of the t complex (Dnahc8; Vaughan et al., 1996). Although the precise location of the Dnahc8 gene relative to Hst6 is not yet clear, this does raise the possibility that the distal distorter could be a dynein component. Such a scenario might then explain how Tcd2 t greatly enhances the effects due to the other distorters.
A Generic Requirement for Tctex1 Family LCs in Dyneins Containing Multiple HCs
Tctex1 is part of a diverse protein family, members of which have now been identified in the major cytoplasmic dynein isoform (Tctex1 [King et al., 1996b ] and rp3 [King, S.M., E. Barbarese, J.F. Dillman III, S.E. Benashski, K.T. Do, R.S. Patel-King, and K.K. Pfister. 1997. Mol. Biol. Cell. 8:163a]), outer arm dynein (Tctex2; Patel-King et al., 1997), and inner arm I1 (Tctex1; this study). At least in the outer arm, the Tctex2 LC is an essential component and null mutants fail to assemble the entire structure (Pazour, G.J., A. Koutoulis, H. Sheng, R.S. Patel-King, S.M. King, and G.B. Witman, unpublished data). This LC family, however, is not apparently represented in the inner arm I2/3 complexes, all the components of which are now known (LeDizet and Piperno, 1995). Thus, Tctex1 family– containing dyneins are distinct in that they all include two or more HCs and at least one IC that is a member of the WD repeat protein family. In outer arm and cytoplasmic dynein, the Tctex1 family LCs are known to interact with the ICs and form part of the basal IC/LC complex (Mitchell and Rosenbaum, 1986; King and Witman, 1990; for review see Witman et al., 1991; King, S.M., E. Barbarese, J.F. Dillman III, S.E. Benashski, K.T. Do, R.S. Patel-King, and K.K. Pfister. 1997. Mol. Biol. Cell. 8:163a). By analogy, a similar IC-associated location is likely in inner arm I1 and suggests that the Tctex1 homologues play a generic and essential role in maintaining the integrity of these multi-HC complexes.
The Highly Conserved 8,000-Mr LC Is also Present in Inner Arm I1
Electrophoretic analysis of inner arm I1 revealed the presence of three distinct LC components. The smallest LC was recognized by antibody R4058 and is, therefore, the highly conserved 8,000-M r LC that was first identified as a component of Chlamydomonas outer arm dynein (King and Patel-King, 1995). Since then, this dimeric protein has been found as an integral component of several other enzyme systems, including mammalian cytoplasmic dynein (King et al., 1996a ), the unconventional actin-based motor myosin V (Espindola, F.S., R.E. Cheney, S.M. King, D.M. Suter, and M.S. Mooseker. 1996. Mol. Biol. Cell. 7:372a), and neuronal nitric oxide synthase (Jaffrey and Snyder, 1996), where it apparently acts to control synthase activity. These studies have led to the suggestion that this 8,000-M r protein acts as a generalized regulatory element, perhaps in a manner analogous to calmodulin (discussed in Benashski et al., 1997). Unsurprisingly then, this LC is essential in multicellular organisms. In Drosophila, partial loss of function leads to morphogenetic defects, female sterility, and alterations in axonal guidance; total loss of function results in apoptosis and embryonic lethality (Dick et al., 1996a ; Phillis et al., 1996). In both Chlamydomonas and Saccharomyces cerevisiae, null mutants have essentially no effect on viability (Dick et al., 1996b ; Pazour, G., C.G. Wilkerson, and G.B. Witman. 1997. Mol. Biol. Cell. 8:162a). The Chlamydomonas mutant, however, does exhibit defects in both outer and inner arm dyneins, intradoublet microtubule projections, radial spokes, and in retrograde intraflagellar transport that is likely caused by the dysfunction of a cytoplasmic dynein (Pazour, G., C.G. Wilkerson, and G.B. Witman. 1997. Mol. Biol. Cell. 8:162a).
Identification of the 8,000-M r LC in inner arm I1 suggests that this protein is also a ubiquitous component of all dynein classes that contain two or more HCs. Stoichiometry calculations based on dye binding suggest that inner arm I1 contains 8–12 copies of this LC per particle. A similarly high number was obtained for outer arm dynein (for review see King and Witman, 1989). However, based on recent studies measuring 35S incorporation into outer arm components in vivo (Wilkerson, C.G., and G.B. Witman, personal communication), it is likely that these numbers represent a significant overestimate (by approximately twofold), presumably because the Chlamydomonas version of this LC has an unusually high affinity for the dye. Thus, it seems most likely that inner arm I1, like the outer arm, contains four 8,000-M r LCs (i.e., two dimers) per particle.
In conclusion, we describe here a set of LCs from flagellar inner arm I1 that are apparently identical to recently identified components of cytoplasmic dynein. Because one of these LCs is the t complex–encoded protein Tctex1, these observations provide a mechanism by which the t haplotype mutations in murine Tctex1 might result in a testis-specific phenotype. Combined with our previous identification of another putative distorter, Tctex2 within the outer arm, this supports the hypothesis that transmission ratio distortion of t haplotypes involves the dysfunction of flagellar dyneins. Further structural and functional analyses of this intriguing class of dynein components will likely provide additional insight into this example of the fascinating phenomenon of meiotic drive.
Acknowledgments
We thank S.E. Benashski and R.S. Patel-King for their assistance, and Dr. J. Leszyk (Worcester Foundation for Biomedical Research, Shrewsbury, MA) for peptide sequencing.
This study was supported by a New Investigator award from the Patrick and Catherine Weldon Donaghue Medical Research Foundation (to S.M. King) and by grants GM 51293 (to S.M. King) and HD 15045 (to P. Olds-Clarke) awarded by the National Institutes of Health.
Abbreviations used in this paper
| HC | heavy chain |
| IC | intermediate chain |
| LC | light chain |
| LIC | light intermediate chain |
| PVDF | polyvinylidene difluoride |
Footnotes
Address all correspondence to Stephen M. King, Department of Biochemistry, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06032-3305. Tel.: (860) 679-3347. Fax: (860) 679-3408. E-mail: ude.chcu.adnap@gnik
3. tx/ty indicates mice that carry t haplotypes with different embryonic lethal factors that do not affect sperm function (Silver, 1993).
2. Because of a recent revision in the nomenclature of murine genes, the tctex2 gene has been formally reassigned as tcte3 (see the Mouse Genome Database on the World Wide Web at www.informatics.jax.org). We have retained the Tctex2 designation here, however, to allow for continuity with previous reports.
References
- Altshul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic alignment search tool. J Mol Biol. 1990;215:403–410. [Abstract] [Google Scholar]
- Bell CW, Fronk E, Gibbons IR. Polypeptide subunits of dynein 1 from sea urchin sperm flagella. J Supramol Struct. 1979;11:311–317. [Abstract] [Google Scholar]
- Benashski SE, Harrison A, Patel-King RS, King SM. Dimerization of the highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. [Abstract] [Google Scholar]
- Cebra-Thomas JA, Decker CL, Snyder LC, Pilder SH, Silver LM. Allele- and haploid-specific product generated by alternative splicing from a mouse t complex responderlocus candidate. Nature. 1991;349:239–241. [Abstract] [Google Scholar]
- Corthésy-Theulaz J, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. [Europe PMC free article] [Abstract] [Google Scholar]
- Cottingham FR, Hoyt MA. Mitotic spindle positioning in Saccharomyces cerevisiaeis accomplished by antagonistically acting microtubule motor proteins. J Cell Biol. 1997;138:1041–1053. [Europe PMC free article] [Abstract] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. [Europe PMC free article] [Abstract] [Google Scholar]
- Dick T, Ray K, Salz HK, Chia W. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. . Mol Cell Biol. 1996a;16:1966–1977. [Europe PMC free article] [Abstract] [Google Scholar]
- Dick T, Surana U, Chia W. Molecular and genetic characterization of SLC1, a putative Saccharomyces cerevisiaehomolog of the metazoan cytoplasmic dynein light chain 1. Mol Gen Genet. 1996b;251:38–43. [Abstract] [Google Scholar]
- Gill SR, Cleveland DW, Schroer TA. Characterization of DLC-A and DLC-B, two families of cytoplasmic dynein light chain subunits. Mol Biol Cell. 1994;5:645–654. [Europe PMC free article] [Abstract] [Google Scholar]
- Gitelman SE, Witman GB. Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J Cell Biol. 1980;87:764–770. [Europe PMC free article] [Abstract] [Google Scholar]
- Harris, E.H. 1989. The Chlamydomonas source book. Academic Press, San Diego, CA. 392–394.
- Holzbaur, E.L.F., A. Mikami, B.M. Paschal, and R.B. Vallee. 1994. Molecular characterization of cytoplasmic dynein. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, Inc., New York. 251–267.
- Hughes SM, Vaughn KT, Herskovits JS, Vallee RB. Molecular analysis of a cytoplasmic dynein light intermediate chain reveals homology to a family of ATPases. J Cell Sci. 1995;108:17–24. [Abstract] [Google Scholar]
- Huw L-I, Goldsborough AS, Willison K, Artzt K. Tctex2: a sperm tail surface protein mapping to the tcomplex. Dev Biol. 1995;170:183–194. [Abstract] [Google Scholar]
- Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. [Abstract] [Google Scholar]
- Kagami O, Kamiya R. Translocation and rotation of microtubules caused by multiple species of Chlamydomonasinner-arm dynein. J Cell Sci. 1992;103:653–664. [Google Scholar]
- Kamiya, R., E. Kurimoto, H. Sakakibara, and T. Okagaki. 1989. A genetic approach to the function of inner and outer arm dynein. In Cell Movement, Vol. 1. The Dynein ATPases. F.D. Warner, P. Satir, and I.R. Gibbons, editors. Alan R. Liss, Inc., New York. 209–218.
- Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. [Abstract] [Google Scholar]
- King SJ, Dutcher SK. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtiiis altered in phototactic mutant strains. J Cell Biol. 1997;136:177–191. [Europe PMC free article] [Abstract] [Google Scholar]
- King SM. Large scale isolation of Chlamydomonasflagella. Methods Cell Biol. 1995;47:9–12. [Abstract] [Google Scholar]
- King SM, Patel-King RS. Identification of a Ca2+-binding light chain within Chlamydomonasouter arm dynein. J Cell Sci. 1995a;108:3757–3764. [Abstract] [Google Scholar]
- King SM, Patel-King RS. The Mr=8,000 and 11,000 outer arm dynein light chains from Chlamydomonasflagella have cytoplasmic homologues. J Biol Chem. 1995b;270:11445–11452. [Abstract] [Google Scholar]
- King, S.M., and G.B. Witman. 1989. Molecular structure of outer arm dynein. In Cell Movement, Vol. 1: The Dynein ATPases. F.D. Warner, P. Satir, and I.R. Gibbons, editors. Alan R. Liss, Inc., New York. 61–75.
- King SM, Witman GB. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J Biol Chem. 1990;265:19807–19811. [Abstract] [Google Scholar]
- King SM, Otter T, Witman GB. Purification and characterization of Chlamydomonasflagellar dyneins. Methods Enzymol. 1986;134:291–306. [Abstract] [Google Scholar]
- King SM, Wilkerson CG, Witman GB. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with α-tubulin in situ. . J Biol Chem. 1991;266:8401–8407. [Abstract] [Google Scholar]
- King SM, Patel-King RS, Wilkerson CG, Witman GB. The 78,000-M r intermediate chain of Chlamydomonasouter arm dynein is a microtubule-binding protein. J Cell Biol. 1995;131:399–409. [Europe PMC free article] [Abstract] [Google Scholar]
- King SM, Barbarese E, Dillman JF, III, Patel-King RS, Carson JH, Pfister KK. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved M r8,000 light chain. J Biol Chem. 1996a;271:19358–19366. [Abstract] [Google Scholar]
- King SM, Dillman JF, III, Benashski SE, Lye RJ, Patel-King RS, Pfister KK. The mouse tcomplex–encoded protein Tctex-1 is a light chain of brain cytoplasmic dynein. J Biol Chem. 1996b;271:32281–32287. [Abstract] [Google Scholar]
- Lader E, Ha H-S, O'Neill M, Artzt K, Bennett D. tctex-1: a candidate gene family for a mouse tcomplex sterility locus. Cell. 1989;58:969–979. [Abstract] [Google Scholar]
- LeDizet M, Piperno G. The light chain p28 associates with a subset of inner dynein arm heavy chains in Chlamydomonas axonemes. . Mol Biol Cell. 1995;6:697–711. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y-Y, Yeh E, Hays T, Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci USA. 1993;90:10096–10100. [Europe PMC free article] [Abstract] [Google Scholar]
- Lyon MF. Transmission ratio distortion in mouse thaplotypes is due to multiple distorter genes acting on a responder locus. Cell. 1984;37:621–628. [Abstract] [Google Scholar]
- Lyon MF. Male sterility of the mouse t-complex is due to homozygosity of the distorter genes. Cell. 1986;44:357–363. [Abstract] [Google Scholar]
- Mitchell DR. Cell and molecular biology of flagella dyneins. Int Rev Cytol. 1994;155:141–180. [Abstract] [Google Scholar]
- Mitchell DR, Kang Y. Identification of oda6 as a Chlamydomonasdynein mutant by rescue with the wild type gene. J Cell Biol. 1991;113:835–842. [Europe PMC free article] [Abstract] [Google Scholar]
- Mitchell DR, Rosenbaum JL. Protein-protein interactions in the 18 ATPase of Chlamydomonasouter dynein arms. Cell Motil Cytoskel. 1986;6:510–520. [Abstract] [Google Scholar]
- Mocz G, Gibbons IR. Phase partition analysis of nucleotide binding to axonemal dynein. Biochemistry. 1996;35:9204–9211. [Abstract] [Google Scholar]
- Ogawa K, Kamiya R, Wilkerson CG, Witman GB. Interspecies conservation of outer arm dynein intermediate chair sequences defines two intermediate chain subclasses. Mol Biol Cell. 1995;6:685–696. [Europe PMC free article] [Abstract] [Google Scholar]
- Olds-Clarke P. Models for male infertility: the thaplotypes. Rev Reprod. 1997;2:157–164. [Abstract] [Google Scholar]
- Olds-Clarke P, Johnson LR. thaplotypes in the mouse compromise sperm flagellar function. Dev Biol. 1993;155:14–25. [Abstract] [Google Scholar]
- Olds-Clarke P, Peitz B. Fertility of sperm from t/+ mice: evidence that + bearing sperm are dysfunctional. Genet Res. 1985;47:49–52. [Abstract] [Google Scholar]
- Olds-Clarke P, Pilder SH, Visconti PE, Moss SB, Orth JM, Kopf GS. Sperm from mice carrying two thaplotypes do not possess a tyrosine phosphorylated form of hexokinase. Mol Reprod Dev. 1996;43:94–104. [Abstract] [Google Scholar]
- O'Neill MJ, Artzt K. Identification of a germ-cell-specific transcriptional repressor in the promoter of Tctex-1. Development (Camb) 1995;121:561–568. [Abstract] [Google Scholar]
- Paschal BM, Vallee RB. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987;330:181–183. [Abstract] [Google Scholar]
- Paschal BM, Mikami A, Pfister KK, Vallee RB. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992;118:1133–1143. [Europe PMC free article] [Abstract] [Google Scholar]
- Patel-King RS, Benashski SE, Harrison A, King SM. Two functional thioredoxins containing redox-sensitive vicinal dithiols from the Chlamydomonasouter dynein arm. J Biol Chem. 1996;271:6283–6291. [Abstract] [Google Scholar]
- Patel-King RS, Benashski SE, Harrison A, King SM. A Chlamydomonas homologue of the putative murine tcomplex distorter Tctex-2 is an outer arm dynein light chain. J Cell Biol. 1997;137:1081–1090. [Europe PMC free article] [Abstract] [Google Scholar]
- Pfister KK, Fay RB, Witman GB. Purification and polypeptide composition of dynein ATPases from Chlamydomonasflagella. Cell Motil. 1982;2:525–547. [Abstract] [Google Scholar]
- Phillis R, Statton D, Caruccio P, Murphey RK. Mutations in the 8 kDa dynein light chain gene disrupt sensory axon projections in the Drosophilaimaginal CNS. Development (Camb) 1996;122:2955–2963. [Abstract] [Google Scholar]
- Pilder SH, Olds-Clarke P, Phillips DM, Silver LM. Hybrid Sterility-6: a mouse tcomplex locus controlling sperm flagellar assembly and movement. Dev Biol. 1993;159:631–642. [Abstract] [Google Scholar]
- Piperno G, Luck JL. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii: purification of two dyneins. J Biol Chem. 1979;254:3084–3090. [Abstract] [Google Scholar]
- Piperno G, Ramanis Z, Smith EF, Sale WS. Three distinct inner dynein arms in Chlamydomonasflagella: molecular composition and location in the axonemes. J Cell Biol. 1990;110:379–389. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter ME, Johnson KA. Characterization of the ATP-sensitive binding of Tetrahymena30S dynein to bovine brain microtubules. J Biol Chem. 1983;258:6575–6581. [Abstract] [Google Scholar]
- Porter ME, Knott JA, Myster SH, Farlow SJ. The dynein gene family in Chlamydomonas reinhardtii. . Genetics. 1996;144:569–585. [Europe PMC free article] [Abstract] [Google Scholar]
- Rappold GA, Stubbs L, Labeit S, Crkvenjakov RB, Lehrach H. Identification of a testis-specific gene from the mouse t-complex next to a CpG-rich island. EMBO (Eur Mol Biol Organ) J. 1987;6:1975–1980. [Europe PMC free article] [Abstract] [Google Scholar]
- Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. [Abstract] [Google Scholar]
- Roux A-F, Rommens J, McDowell C, Anson-Cartwright L, Bell S, Schappert K, Fishman GA, Musarella M. Identification of a gene from Xp21 with similarity to the tctex-1 gene of the murine tcomplex. Hum Mol Genet. 1994;3:257–263. [Abstract] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 7.46–7.48 and 9.34–9.38.
- Schroer TA, Steuer ER, Sheetz MP. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell. 1989;56:937–946. [Abstract] [Google Scholar]
- Silver LM. The peculiar journey of a selfish chromosome: mouse thaplotypes and meiotic drive. Trends Genet. 1993;9:250–254. [Abstract] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein βγ dimer at 2.1 Å resolution. Nature. 1996;379:369–374. [Abstract] [Google Scholar]
- Steffen W, Hodgkins JL, Wiche G. Immunogold localization of the intermediate chain within the protein complex of cytoplamsic dynein. J Struct Biol. 1996;117:227–235. [Abstract] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. [Europe PMC free article] [Abstract] [Google Scholar]
- Vallee RB, Wall JS, Paschal BM, Shpetner HS. Microtubule- associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988;332:561–563. [Abstract] [Google Scholar]
- Vaughan KT, Mikami A, Paschal BM, Holzbaur ELF, Hughes SM, Echeverri CJ, Moore KJ, Gilbert DJ, Copeland NG, Jenkins NA, Vallee RB. Multiple mouse chromosomal loci for dynein-based motility. Genomics. 1996;36:29–38. [Abstract] [Google Scholar]
- Warner, F.D., P. Satir, and I.R. Gibbons. 1989. Cell Movement, Vol. 1. The dynein ATPases. Alan R. Liss, Inc., New York.
- Wilkerson CG, King SM, Koutoulis A, Pazour GJ, Witman GB. The 78,000-M r intermediate chain of Chlamydomonasouter arm dynein is a WD-repeat protein required for arm assembly. J Cell Biol. 1995;129:169–178. [Europe PMC free article] [Abstract] [Google Scholar]
- Witman GB. Isolation of Chlamydomonasflagella and flagellar axonemes. Methods Enzymol. 1986;134:280–290. [Abstract] [Google Scholar]
- Witman, G.B., C.G. Wilkerson, and S.M. King. 1994. The biochemistry, genetics and molecular biology of flagellar dynein. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, Inc., New York. 229–249.
- Xiang X, Beckwith SM, Morris NR. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. . Proc Natl Acad Sci USA. 1994;91:2100–2104. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimmer WE, Schloss JA, Silflow CD, Youngblom J, Watterson DM. Structural organization, DNA sequence and expression of the calmodulin gene. J Biol Chem. 1988;263:19370–19383. [Abstract] [Google Scholar]
Articles from The Journal of Cell Biology are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1083/jcb.140.5.1137
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jcb/article-pdf/140/5/1137/1279977/33004.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1083/jcb.140.5.1137
Article citations
Structure and Function of Dynein's Non-Catalytic Subunits.
Cells, 13(4):330, 11 Feb 2024
Cited by: 1 article | PMID: 38391943 | PMCID: PMC10886578
Review Free full text in Europe PMC
Consensus nomenclature for dyneins and associated assembly factors.
J Cell Biol, 221(2):e202109014, 10 Jan 2022
Cited by: 20 articles | PMID: 35006274 | PMCID: PMC8754002
Review Free full text in Europe PMC
Loss of Axdnd1 causes sterility due to impaired spermatid differentiation in mice.
Reprod Med Biol, 21(1):e12452, 01 Jan 2022
Cited by: 3 articles | PMID: 35386379 | PMCID: PMC8968163
Composition and function of ciliary inner-dynein-arm subunits studied in Chlamydomonas reinhardtii.
Cytoskeleton (Hoboken), 78(3):77-96, 01 Mar 2021
Cited by: 12 articles | PMID: 33876572 | PMCID: PMC8217317
Review Free full text in Europe PMC
LAX28 is required for the stable assembly of the inner dynein arm f complex, and the tether and tether head complex in Leishmania flagella.
J Cell Sci, 133(2):jcs239855, 23 Jan 2020
Cited by: 2 articles | PMID: 31932510 | PMCID: PMC7747692
Go to all (85) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (2)
- (1 citation) ENA - AF039437
- (1 citation) ENA - D50663
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Tctex1/Tctex2 class of dynein light chains. Dimerization, differential expression, and interaction with the LC8 protein family.
J Biol Chem, 276(17):14366-14373, 22 Jan 2001
Cited by: 39 articles | PMID: 11278908
A Chlamydomonas homologue of the putative murine t complex distorter Tctex-2 is an outer arm dynein light chain.
J Cell Biol, 137(5):1081-1090, 01 Jun 1997
Cited by: 43 articles | PMID: 9166408 | PMCID: PMC2136226
A novel Tctex2-related light chain is required for stability of inner dynein arm I1 and motor function in the Chlamydomonas flagellum.
J Biol Chem, 279(20):21666-21676, 11 Mar 2004
Cited by: 26 articles | PMID: 15020587
Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation.
Cell Motil Cytoskeleton, 64(8):569-579, 01 Aug 2007
Cited by: 49 articles | PMID: 17549744
Review
Funding
Funders who supported this work.
NICHD NIH HHS (1)
Grant ID: HD 15045
NIGMS NIH HHS (2)
Grant ID: R01 GM051293
Grant ID: GM 51293




