Abstract
Free full text

Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli.
Abstract
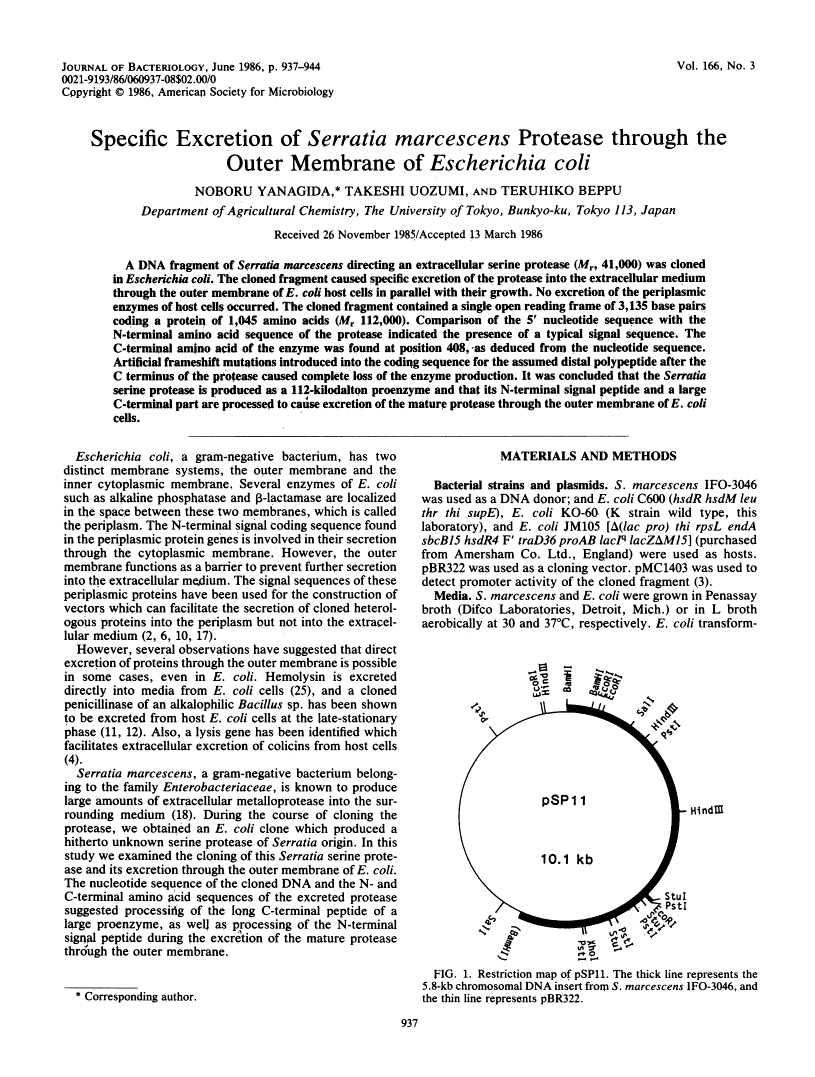
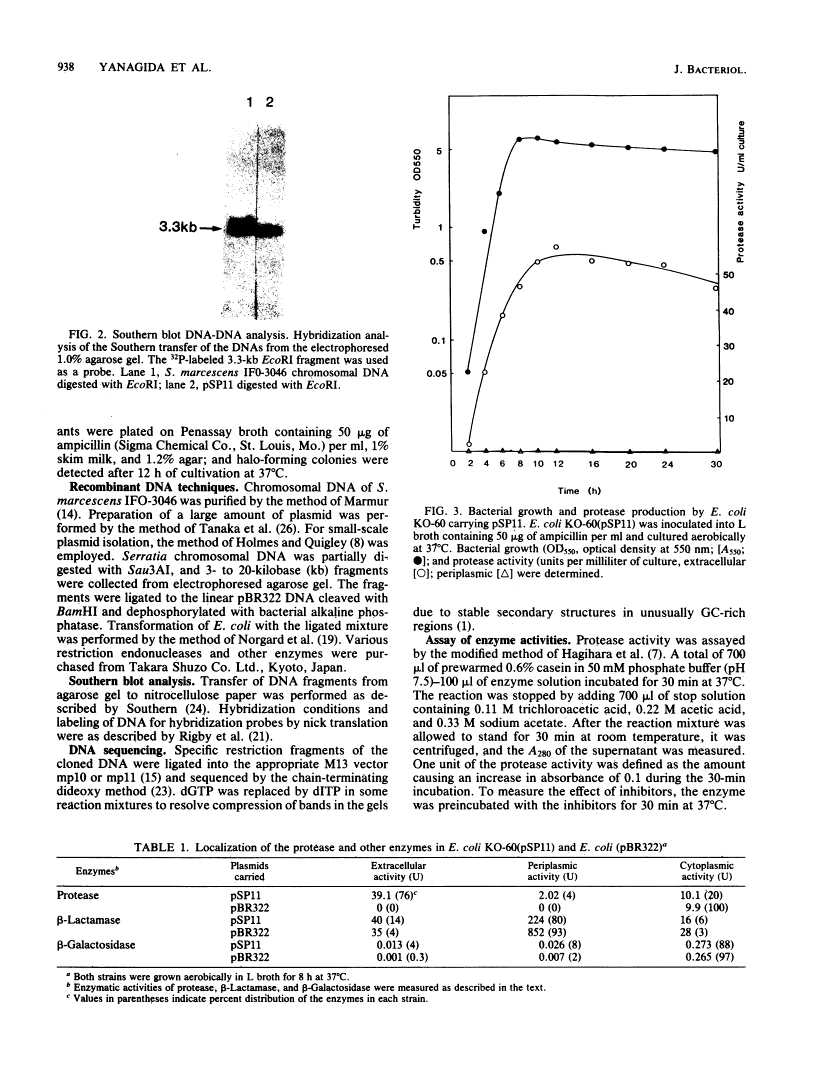
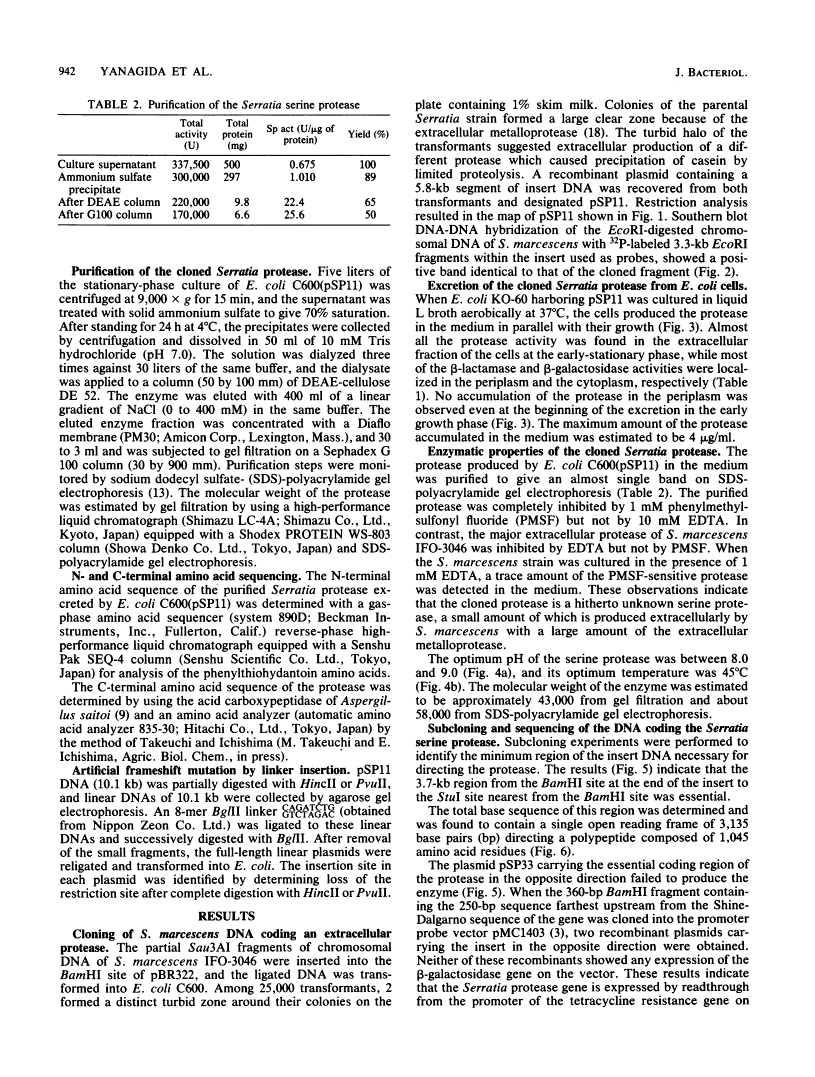
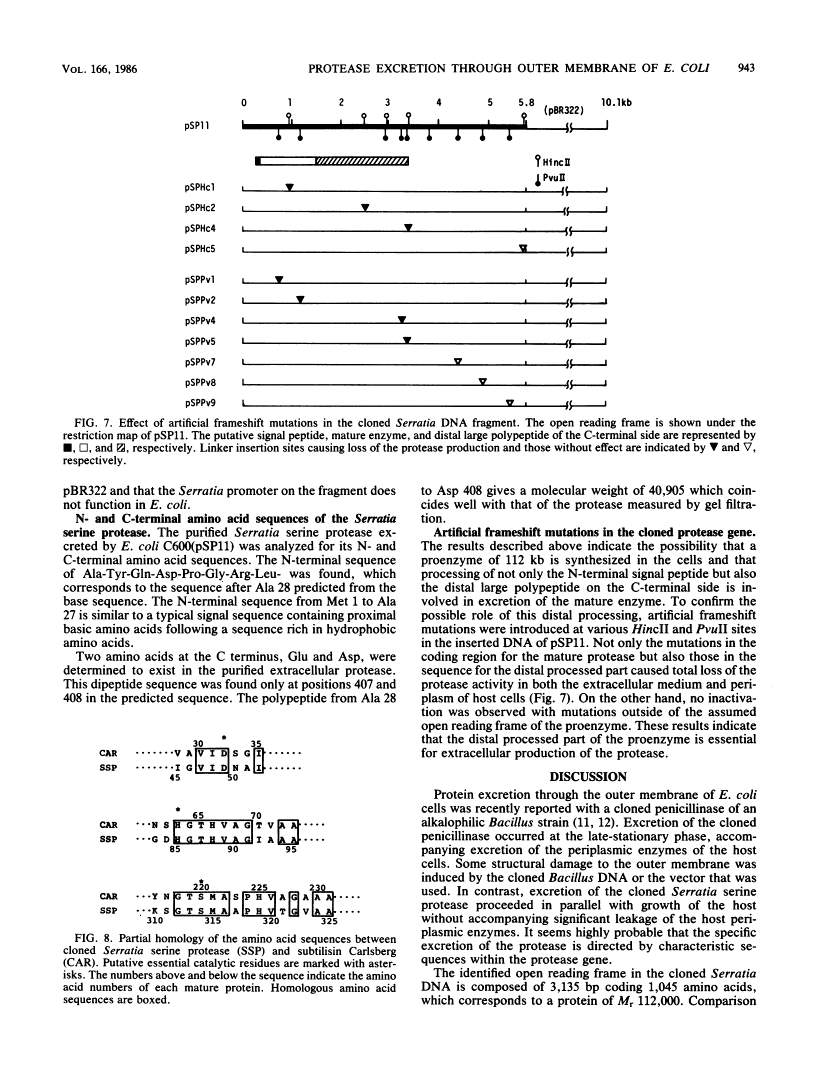
A DNA fragment of Serratia marcescens directing an extracellular serine protease (Mr, 41,000) was cloned in Escherichia coli. The cloned fragment caused specific excretion of the protease into the extracellular medium through the outer membrane of E. coli host cells in parallel with their growth. No excretion of the periplasmic enzymes of host cells occurred. The cloned fragment contained a single open reading frame of 3,135 base pairs coding a protein of 1,045 amino acids (Mr 112,000). Comparison of the 5' nucleotide sequence with the N-terminal amino acid sequence of the protease indicated the presence of a typical signal sequence. The C-terminal amino acid of the enzyme was found at position 408, as deduced from the nucleotide sequence. Artificial frameshift mutations introduced into the coding sequence for the assumed distal polypeptide after the C terminus of the protease caused complete loss of the enzyme production. It was concluded that the Serratia serine protease is produced as a 112-kilodalton proenzyme and that its N-terminal signal peptide and a large C-terminal part are processed to cause excretion of the mature protease through the outer membrane of E. coli cells.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes WM, Bevan M, Son PH. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. [Abstract] [Google Scholar]
- Bassford PJ, Jr, Silhavy TJ, Beckwith JR. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. [Europe PMC free article] [Abstract] [Google Scholar]
- Casadaban MJ, Chou J, Cohen SN. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. [Europe PMC free article] [Abstract] [Google Scholar]
- Cole ST, Saint-Joanis B, Pugsley AP. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. [Abstract] [Google Scholar]
- Cornelis P, Digneffe C, Willemot K. Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet. 1982;186(4):507–511. [Abstract] [Google Scholar]
- Emr SD, Hall MN, Silhavy TJ. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. [Europe PMC free article] [Abstract] [Google Scholar]
- Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. [Abstract] [Google Scholar]
- Ichishima E, Arai T. Specificity and mode of action of acid carboxypeptidase from Aspergillus saitoi. Biochim Biophys Acta. 1973 Feb 15;293(2):444–450. [Abstract] [Google Scholar]
- Ito K, Beckwith JR. Role of the mature protein sequence of maltose-binding protein in its secretion across the E. coli cytoplasmic membrane. Cell. 1981 Jul;25(1):143–150. [Abstract] [Google Scholar]
- Kudo T, Kato C, Horikoshi K. Excretion of the penicillinase of an alkalophilic Bacillus sp. through the Escherichia coli outer membrane. J Bacteriol. 1983 Nov;156(2):949–951. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. [Abstract] [Google Scholar]
- Miyake T, Oka T, Nishizawa T, Misoka F, Fuwa T, Yoda K, Yamasaki M, Tamura G. Secretion of human interferon-alpha induced by using secretion vectors containing a promoter and signal sequence of alkaline phosphatase gene of Escherichia coli. J Biochem. 1985 May;97(5):1429–1436. [Abstract] [Google Scholar]
- Norgard MV, Keem K, Monahan JJ. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. [Abstract] [Google Scholar]
- Rigby PW, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. [Abstract] [Google Scholar]
- Ross GW, O'Callaghan CH. Beta-lactamase assays. Methods Enzymol. 1975;43:69–85. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Springer W, Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. [Europe PMC free article] [Abstract] [Google Scholar]
- Tanaka T, Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.166.3.937-944.1986
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc215216?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/166/3/937
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/166/3/937
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jb.166.3.937-944.1986
Article citations
Crystal structure of a subtilisin-like autotransporter passenger domain reveals insights into its cytotoxic function.
Nat Commun, 14(1):1163, 01 Mar 2023
Cited by: 0 articles | PMID: 36859523 | PMCID: PMC9977779
Phylogenetic survey of the subtilase family and a data-mining-based search for new subtilisins from Bacillaceae.
Front Microbiol, 13:1017978, 26 Sep 2022
Cited by: 3 articles | PMID: 36225363 | PMCID: PMC9549277
Of linkers and autochaperones: an unambiguous nomenclature to identify common and uncommon themes for autotransporter secretion.
Mol Microbiol, 95(1):1-16, 24 Nov 2014
Cited by: 25 articles | PMID: 25345653 | PMCID: PMC4275399
Review Free full text in Europe PMC
An alternative outer membrane secretion mechanism for an autotransporter protein lacking a C-terminal stable core.
Mol Microbiol, 90(5):1028-1045, 20 Oct 2013
Cited by: 17 articles | PMID: 24118465 | PMCID: PMC3857143
Autotransporter protein secretion.
Biomol Concepts, 2(6):525-536, 01 Dec 2011
Cited by: 2 articles | PMID: 25962052
Go to all (93) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Detection of large COOH-terminal domains processed from the precursor of Serratia marcescens serine protease in the outer membrane of Escherichia coli.
J Biochem, 111(5):627-632, 01 May 1992
Cited by: 8 articles | PMID: 1639760
Characterization of the precursor of Serratia marcescens serine protease and COOH-terminal processing of the precursor during its excretion through the outer membrane of Escherichia coli.
J Bacteriol, 171(12):6566-6572, 01 Dec 1989
Cited by: 41 articles | PMID: 2687244 | PMCID: PMC210548
Two genes encoding serine protease homologues in Serratia marcescens and characterization of their products in Escherichia coli.
J Biochem, 121(5):902-913, 01 May 1997
Cited by: 15 articles | PMID: 9192732
Extracellular production of a Serratia marcescens serine protease in Escherichia coli.
Biosci Biotechnol Biochem, 60(10):1551-1558, 01 Oct 1996
Cited by: 9 articles | PMID: 8987650
Review