Abstract
Free full text

Synaptotagmin VII Regulates Ca2+-Dependent Exocytosis of Lysosomes in Fibroblasts
Abstract
Synaptotagmins (Syts) are transmembrane proteins with two Ca2+-binding C2 domains in their cytosolic region. Syt I, the most widely studied isoform, has been proposed to function as a Ca2+ sensor in synaptic vesicle exocytosis. Several of the twelve known Syts are expressed primarily in brain, while a few are ubiquitous (Sudhof, T.C., and J. Rizo. 1996. Neuron. 17: 379–388; Butz, S., R. Fernandez-Chacon, F. Schmitz, R. Jahn, and T.C. Sudhof. 1999. J. Biol. Chem. 274:18290–18296). The ubiquitously expressed Syt VII binds syntaxin at free Ca2+ concentrations ([Ca2+]) below 10 μM, whereas other isoforms require 200–500 μM [Ca2+] or show no Ca2+-dependent syntaxin binding (Li, C., B. Ullrich, Z. Zhang, R.G.W. Anderson, N. Brose, and T.C. Sudhof. 1995. Nature. 375:594–599). We investigated the involvement of Syt VII in the exocytosis of lysosomes, which is triggered in several cell types at 1–5 μM [Ca2+] (Rodríguez, A., P. Webster, J. Ortego, and N.W. Andrews. 1997. J. Cell Biol. 137:93–104). Here, we show that Syt VII is localized on dense lysosomes in normal rat kidney (NRK) fibroblasts, and that GFP-tagged Syt VII is targeted to lysosomes after transfection. Recombinant fragments containing the C2A domain of Syt VII inhibit Ca2+-triggered secretion of β-hexosaminidase and surface translocation of Lgp120, whereas the C2A domain of the neuronal- specific isoform, Syt I, has no effect. Antibodies against the Syt VII C2A domain are also inhibitory in both assays, indicating that Syt VII plays a key role in the regulation of Ca2+-dependent lysosome exocytosis.
Introduction
Recent studies revealed the existence of a large component of Ca2+-regulated exocytosis in cells previously thought to exhibit only constitutive secretion, such as CHO and other fibroblast lines (Chavez et al. 1996; Coorsen et al. 1996; Ninomiya et al. 1996). Capacitance measurements indicated that exocytic vesicles mobilized by low micromolar free Ca2+ concentrations ([Ca2+]) in CHO cells are large, with diameters between 0.4 and 1.5 μm (Ninomiya et al. 1996). This size range is compatible with the dimensions of mammalian lysosomes, which were shown, in a number of different assays, to undergo exocytosis in several cell lines when the intracellular [Ca2+] is elevated to 1–5 μM (Rodríguez et al. 1997). A similar population of large vesicles capable of Ca2+-regulated exocytosis was also detected by patch clamp studies in PC12 cells (Kasai et al. 1999), and by a combination of patch clamp and amperometry in chromaffin cells (Xu et al. 1998). Thus, it is becoming apparent that Ca2+-regulated secretion may be a property of all lysosomes, and not only of the lysosome-like granules of hemopoietic cells, as previously believed (Stinchcombe and Griffiths 1999).
The low micromolar [Ca2+] range in which lysosome exocytosis takes place suggests that a high affinity Ca2+ sensor might be present on lysosomal membranes. Although a number of Ca2+-binding proteins have been suggested to act as Ca2+ sensors in regulated exocytosis (Burgoyne and Morgan 1998), extensive genetic and functional evidence is available only for synaptotagmin (Syt) I, a synaptic vesicle transmembrane protein implicated in the regulation of neurotransmitter release (Sudhof and Rizo 1996). Interactions with members of the exocytic machinery including the SNAREs (soluble N-ethylmaleimide-sensitive factor [NSF] attachment protein receptors), SNAP-25 and syntaxin, the soluble factors β-SNAP and NSF, in addition to Ca2+ channels and phospholipids, have contributed to the notion that Syt I may function by modulating assembly of the membrane fusion complex (Schiavo et al. 1998).
Recently, however, a more complex picture regarding the role of Syts as Ca2+ sensors has emerged. This family has at least twelve identified isoforms, all of which have unique NH2-terminal ecto domains, and a highly conserved cytosolic domain containing the two Ca2+-binding C2 domains (C2A and C2B; Schiavo et al. 1998). The C2A domain of Syt IV, which does not appear to bind Ca2+ (von Poser et al. 1997), is nonetheless an effective inhibitor of Ca2+-mediated synaptic vesicle exocytosis (Thomas et al. 1999). Recent observations indicate that Syt IV forms hetero-oligomers with Syt I on the membrane of synaptic vesicles, an event that modulates the efficiency of Ca2+-secretion coupling (Littleton et al. 1999).
Most Syt isoforms identified so far are expressed predominantly in neuronal tissue, although the messages for several of them can be detected at low levels elsewhere (Butz et al. 1999). Syt I and II, the two major Syt isoforms in brain, are abundant transmembrane proteins of synaptic vesicles (Sudhof and Rizo 1996). Very little is known, however, about the subcellular localization of the additional isoforms. Syt III, the third most abundant Syt in brain, is enriched in synaptic plasma membranes (Butz et al. 1999), and it was also reported to be present on secretory granules of pancreatic β-cells (Mizuta et al. 1997). The latter localization study, however, used antibodies raised against recombinant Syt III including the highly conserved cytosolic domain, and such antibodies can cross-react with other Syt isoforms (Butz et al. 1999). Three Syt isoforms, VI, VII, and VIII, are expressed at significant levels in several tissues, and thus have been designated the ubiquitously expressed members of the Syt family (Li et al. 1995; Craxton and Goedert 1999). No subcellular localization information is available for Syt VI and Syt VIII, except that in brain, Syt VI appears not to be present in synaptic vesicles (Butz et al. 1999). In vitro binding studies performed with recombinant proteins revealed that Syt VI and Syt VIII do not exhibit Ca2+-dependent binding to either phospholipids or to syntaxin. On the other hand, Ca2+-dependent binding to syntaxin was detected with Syt VII, and, interestingly, the [Ca2+] range required for this interaction was found to be in the low micromolar range (Li et al. 1995). Thus, we decided to further investigate Syt VII as a candidate for involvement in the exocytosis of lysosomes, an event that occurs in many cell types when the intracellular [Ca2+] is elevated above 1 μM (Rodríguez et al. 1997). The data presented here are consistent with a role for the ubiquitously expressed Syt VII in the Ca2+-regulated exocytosis of conventional lysosomes.
Materials and Methods
Antibodies
Polyclonal antibodies against the NH2-terminal domain of Syt VII were generated by immunizing a rabbit with the synthetic peptide MYRDPEAASPGAC cross-linked to keyhole limpet hemocyanin using Sulfo-SMCC (Pierce Chemical Co.). Purified rabbit IgG (20 μg/ml) was preincubated with 1.5 mg/ml of the peptides MYRDPEAASPGAC or MAYAPREAGPDSC (scrambled control). A rabbit was immunized with Escherichia coli-expressed Syt VII C2A and the antibodies were affinity-purified on affigel 10 coupled to Syt VII C2A (BioRad). E41120 mAbs to EEA1 were obtained from Transduction Laboratories. The following additional antibodies were provided as gifts: rabbit anti-rab 7 by Susanne Pffefer (Stanford University, Stanford, CA) and C. Roy (Yale University, New Haven, CT); rabbit anticalnexin by A. Helenius (ETHZ, Switzerland); rabbit anticathepsin L by D. Portnoy (University of California, Berkeley, CA); rabbit anti-Syt I NH2-terminal peptide (MC17) by P. De Camilli, (Yale University); mouse anti-Lgp120 LYIC6 mAb by I. Mellman (Yale University).
Subcellular Fractionation, Immunoprecipitation, and Western Blot
Normal rat kidney (NRK) cells plated 48 h before were scraped from the dish and homogenized in 1 ml buffer H (10 mM triethanolamine, 10 mM acetic acid, 1 mM EDTA, and 250 mM sucrose) by four passages in a ball bearing homogenizer. A postnuclear supernatant was prepared and top loaded on a 25% Percoll/20 mM sucrose density gradient solution prepared in buffer H. Centrifugation was carried out at 4°C in a 70.1 TI fixed-angle rotor and a Beckman L-80 centrifuge for 90 min at 18,000 g. Fractions were collected from the bottom of the gradient, centrifuged for Percoll removal, and concentrated with a centricon filter (Amicon) after solubilizing membranes in 1% NP-40. Total cell extracts of NRK, L6E9 rat myoblasts, and L mouse fibroblasts were obtained by solubilizing cells in 1 ml 150 mM NaCl, 50 mM Tris, pH 8.6, and 1% NP-40 containing protease inhibitors. For immunoprecipitation, 0.8 ml of NRK cell or rat brain lysates (containing 0.2 mg of protein) were mixed with 0.8 ml Tris 100 mM, pH 8.0, NaCl 10 mM, and EDTA 10 mM, and incubated overnight at 4°C with 80 μg of preimmune or affinity-purified anti-Syt VII C2A rabbit IgG. Protein A–Sepharose beads were then added and the mixture was rotated for 2 h at room temperature. After four washes with Tris 100 mM, pH 8.0, NaCl 10 mM, EDTA 10 mM, 1 mg/ml BSA, and 0.05% NP-40, immunoprecipitated proteins were eluted in SDS-PAGE sample buffer. After SDS-PAGE, proteins were transferred to immobilon filters and blots were probed with antibodies followed by ECL (New England Nuclear) detection.
Immunofluorescence and Endocytic Tracer Internalization
NRK cells were incubated with Texas red-conjugated 10,000 Mw dextran (Molecular Probes) at 0.5 mg/ml for 3 h at 37°C, followed by extensive washing and a 3-h chase in DME 10% FBS, followed by fixation in 2% paraformaldehyde (PFA). For immunofluorescent detection of the ectodomain of Syt VII, cells were fixed and permeabilized by 10 min incubation in 100% methanol at −20°C, rehydrated in PBS for 30 min, incubated with 10 μg/ml anti-Syt VII rabbit IgG containing 1 mM Syt VII NH2-terminal peptide, the scrambled control peptide, or with anti-EEA1 mouse mAbs, followed by fluorescein-conjugated secondary antibodies. For staining with anti-Syt VII C2A antibodies, cells were fixed in 2% PFA and permeabilized for 2 min in 0.1% Triton X-100. Rat Lgp120 was detected with the LYIC6 mAb in PFA-fixed cells permeabilized with 0.01% saponin, and rab 7 was detected with affinity-purified rabbit anti-rab 7 IgG in PFA-fixed cells permeabilized with 0.5% saponin. For transferrin detection, cells were incubated for 1 h at 37°C with 50 μg/ml Texas red or 25 μg/ml HRP-conjugated transferrin, followed by fixation. Peroxidase activity on the plasma membrane was abolished by incubation of cultures with MESNA for 10 min at 4°C before cell homogenization.
Fluorescence images were acquired in a Zeiss axiovert 135 microscope with an Orca II digital camera (Hamamatsu) controlled by MetaMorph software (Universal Imaging).
BSA-Gold Labeling of Lysosomes and Electron Microscopy
Lysosomes were loaded with BSA absorbed to 5-nm gold particles for 3 h, followed by a 3-h chase in DME 10% FBS at 37°C. Cells were fixed in 4% PFA/0.5% glutaraldehyde in 200 mM Hepes buffer, pH 7.2, scraped from the dish, pelleted, and embedded in 10% gelatin. Embedded pellets were infiltrated with 2.1 M sucrose in PBS, frozen by immersion in liquid nitrogen and cryosectioned. Thawed sections were labeled with anti-Syt VII rabbit IgG followed by a 10-nm protein A gold probe (from the Department of Cell Biology, University of Utrecht, The Netherlands).
Expression and Purification of Recombinant Syt Constructs
To obtain the Syt VII gene, the oligonucleotide primers: 5′-CTCGAAT TCATGTACCGGGACCCGAGGCG-3′ (forward) and 5′-CTCGAAT TCGGCTTTCAGCTGGTGCCACTG-3′ (reverse) containing EcoRI sites (underlined) were used to amplify cDNA synthesized from 10 μg of total NRK cell RNA. The amplification product (1.2 Kb) was cloned into the TA vector pCR2.1 (Invitrogen), and checked by sequencing. The Syt I (obtained from P. De Camilli, Yale University, New Haven, CT) and Syt VII genes were subcloned into the pET19b vector (Novagen) at the Xho restriction site and expressed as a NH2-terminal polyhistidine-tagged fusion proteins in Escherichia coli BL21 and purified on nickel-agarose, according to the manufacturer's instructions (Novagen).
To clone the C2A and C2B domains of Syt VII, the following primers were used for PCR using Syt VII DNA as template: 5′-CTCCTCGAGAGCCGAGAGAACCTG-3′ (forward) and 5′-CTCCTCGAGATCGCTGCATGGCTTC-3′ (reverse) for the C2A domain and 5′-CTCCTC GAGATGGGAGTGGGAGCCGAG-3′ (forward) and 5′-CTCCTCG-AGGGCTTTCAGCTGGTGCCACTG (reverse) for the C2B domain (underlined sequence represents the XhoI site). Equivalent primers designed based on the Syt I sequence were used for PCR using Syt I cDNA as template. PCR products were purified, digested with XhoI, cloned into pET19b, and used for expression and purification of the protein domains. The correct size of the fragments was checked by SDS-PAGE (see Fig. 5 a).
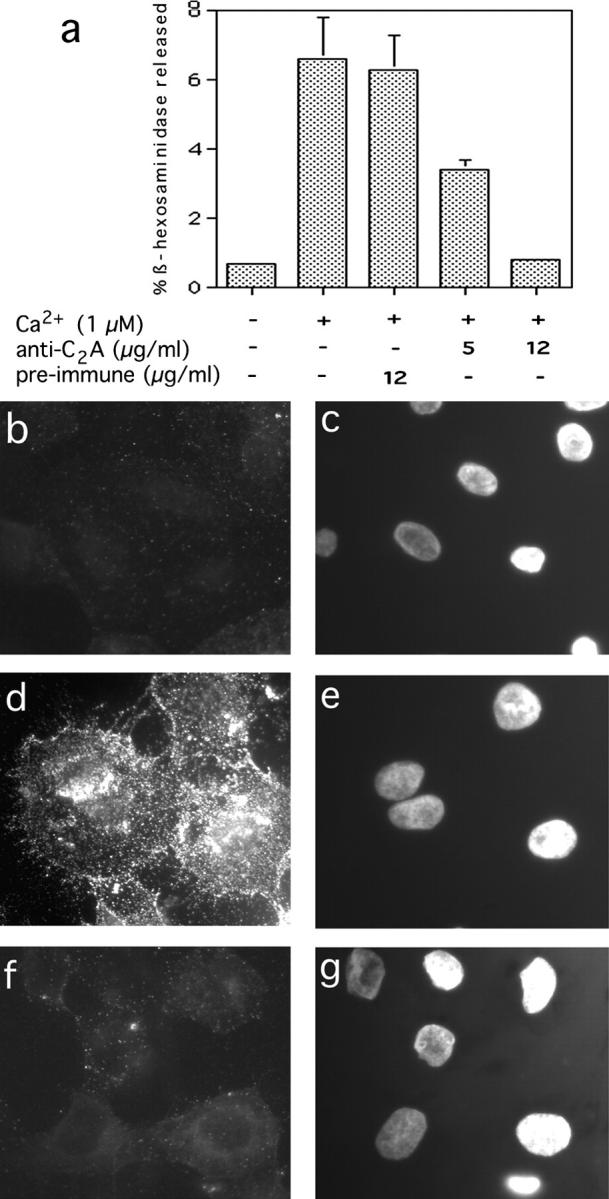
Antibodies against the Syt VII C2A domain inhibit lysosome exocytosis in NRK cells. a, Cells were permeabilized with SLO, stimulated or not with 1 μM Ca2+ in the presence of the indicated concentrations of preimmune rabbit IgG or anti-Syt VII C2A, and the supernatant was assayed after 10 min for released β-hexosaminidase. b, Surface immunofluorescence of Lgp120 in SLO-permeabilized cells in the absence of Ca2+. c, Same field as b, DAPI stain of cell nuclei. d, Surface immunofluorescence of Lgp120 in cells permeabilized in the presence of 1 μM Ca2+ and 12 μg/ml rabbit preimmune antibodies. e, Same as d, DAPI stain. f, Surface immunofluorescence of Lgp120 in cells permeabilized in the presence of 1 μM Ca2+ and 10 μg/ml anti-Syt VII C2A antibodies. g, Same as f, DAPI stain.
For construction of a Syt VII–GFP chimera, the Syt VII gene was subcloned into the EcoRI–KpnI site of the GFP expression vector, pEGFP-N2 (Clontech), and transfections were done using FuGENE 6 (Boehringer Mannheim Corp.) according to manufacturer's instructions.
Streptolysin O (SLO) Permeabilization, β-Hexosaminidase Secretion, and Lgp120 Surface Translocation Assays
Confluent monolayers of NRK cells were washed twice with 1 ml ice-cold buffer A (20 mM Hepes, 110 mM NaCl, 5.4 mM KCl, 0.9 mM Na2HPO4, 10 mM MgCl2, 2 mM CaCl2, and 11 mM glucose, pH 7.4) and incubated for 5 min at 4°C with SLO (Murex) at 0.5 U/ml in buffer A. After one wash with 1 ml ice-cold buffer B (20 mM Hepes, 100 mM K-glutamate, 40 mM KCl, and 5 mM EGTA, pH 7.2), and 0.5 ml of buffer B containing 2 mM ATP and 5 mM free Mg2+ (added as MgCl2) with or without Ca2+ (final [Ca2+] 1 μM), and containing antibodies or recombinant Syt C2 fragments, was added to the cells at 37°C for the indicated time points. The desired concentrations of free Mg2+ and Ca2+ were obtained with a Ca2+ or Mg2+/EGTA buffering system calculated using the software by Foehr and Warchol. Cell extracts were obtained by solubilization in buffer B 1% NP-40, followed by centrifugation at 11,000 g. For detection of β-hexosaminidase activity, 350 μl was incubated for 15 min at 37°C with 50 μl of 6 mM 4-methyl-umbellyferyl-N-acetyl-β-d-glucosaminide in sodium citrate-phosphate buffer, pH 4.5. The reaction was stopped by addition of 100 μl of 2 M Na2CO3, 1.1 M glycine, and the fluorescence was measured in a F-2000 spectrofluorimeter (Hitachi Instruments, Inc.) at excitation 365 nm, emmision 450 nm.
For detection of surface-translocated Lgp120, NRK cells plated on glass coverslips were permeabilized with SLO as described above and exposed to 100 μl of 1 μM Ca2+ buffer containing antibodies or recombinant Syt C2A fragments for 10 min at 37°C. Cells were then incubated for 30 min at 4°C with culture supernatant from a mouse hybridoma line (Ly1C6) producing antibodies to rat Lgp120, washed with PBS at 4°C, and fixed with 2% PFA for 15 min. After fluorescent staining with DAPI (for detection of cell nuclei) and a goat anti–mouse IgG conjugate (for detection of the anti-Lgp120 mAb), coverslips were examined under a Zeiss axiovert 135 fluorescence microscope.
Results and Discussion
Syt VII Is Localized on Mature, Dense Lysosomes of NRK Cells
The cytosolic COOH-terminal domains of Syts show a high degree of similarity, but their short intralumenal NH2-terminal domains are isoform-specific. This allowed us to generate antibodies specific for Syt VII by immunizing rabbits with a synthetic peptide corresponding to the twelve NH2-terminal amino acids of rat Syt VII (MYRDPEAASPGA; Li et al. 1995) coupled to keyhole limpet hemocyanin. Immunofluorescence of permeabilized NRK cells revealed that these antibodies recognize a population of intracellular vesicles in the presence of a scrambled control peptide (Fig. 1 a), but not of the correctly oriented Syt VII NH2-terminal peptide (Fig. 1 b). Specific label with anti-Syt VII antibodies was also observed by immunoelectron microscopy on electron-dense vesicles containing internalized BSA-gold complexes chased for several hours (Fig. 1 c). Anti-Syt VII antibodies also colocalized with fluorescent dextran chased into late endocytic compartments (Fig. 1, e–f). Syt VII-containing compartments did not colocalize with the early endosome markers EEA1 (Simonsen et al. 1998; Fig. 1, g–i) or transferrin (not shown).
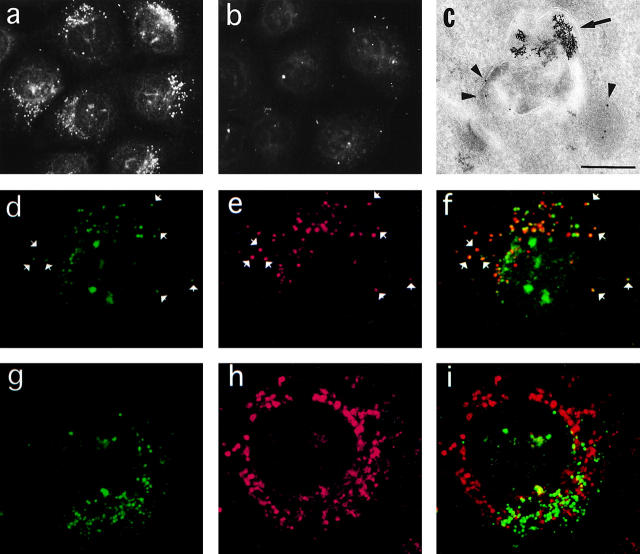
Syt VII is localized on late endocytic compartments of NRK cells. a, Immunofluorescence with anti-Syt VII antibodies in the presence of scrambled control peptide. b, Same as a, in the presence of Syt VII NH2-terminal peptide. c, Transmission EM of cryosections labeled with anti-Syt VII antibodies. Arrowheads point to 10-nm gold particles at the antibody binding sites; the arrow points to internalized BSA-gold complexes. Bar, 0.5 μm. d, Anti-Syt VII immunofluorescence. e, Dextran-Texas red chased into late endocytic compartments. f, Overlay of d and e. g, Anti-Syt VII immunofluorescence. h, Anti-EEA1 immunofluorescence. i, Overlay of g and h. Arrowheads in d–f point to examples of colocalization between Syt VII and dextran-Texas red. The large spots over the nucleus reactive with anti-Syt VII antibodies in d are artifacts of the methanol fixation.
On Western blots, the rabbit anti-Syt VII NH2-terminal antibodies specifically recognized a band of ~66 kD on extracts of NRK fibroblasts and L6E9 rat myoblasts, and also reacted with recombinant Syt VII expressed in E. coli (Fig. 2 a, left and middle). Recombinant, his-tagged Syt VII migrated as an ~48-kD band, as predicted from the 403 amino acid Syt VII sequence (Li et al. 1995) without any posttranslational modifications. The MC17 antibody specific for the Syt I NH2-terminal domain did not react with NRK or L6E9 cells, but clearly recognized an ~65-kD band in rat brain extracts, and also the recombinant Syt I expressed in E. coli, which, similarly to Syt VII, migrates faster than the native protein on SDS-PAGE (Fig. 2 a, right). These results are thus consistent with the previously reported synaptic localization of Syt I and the ubiquitous expression of Syt VII, and demonstrate that the antibodies used in this study are isoform-specific. The lack of detection of Syt VII in brain in this experiment reflects the small amount of extract analyzed (see Fig. 4 d). As further discussed below, this observation most probably reflects the fact that brain preparations are enriched in nerve terminals, and thus in Syt I-containing synaptic vesicles.
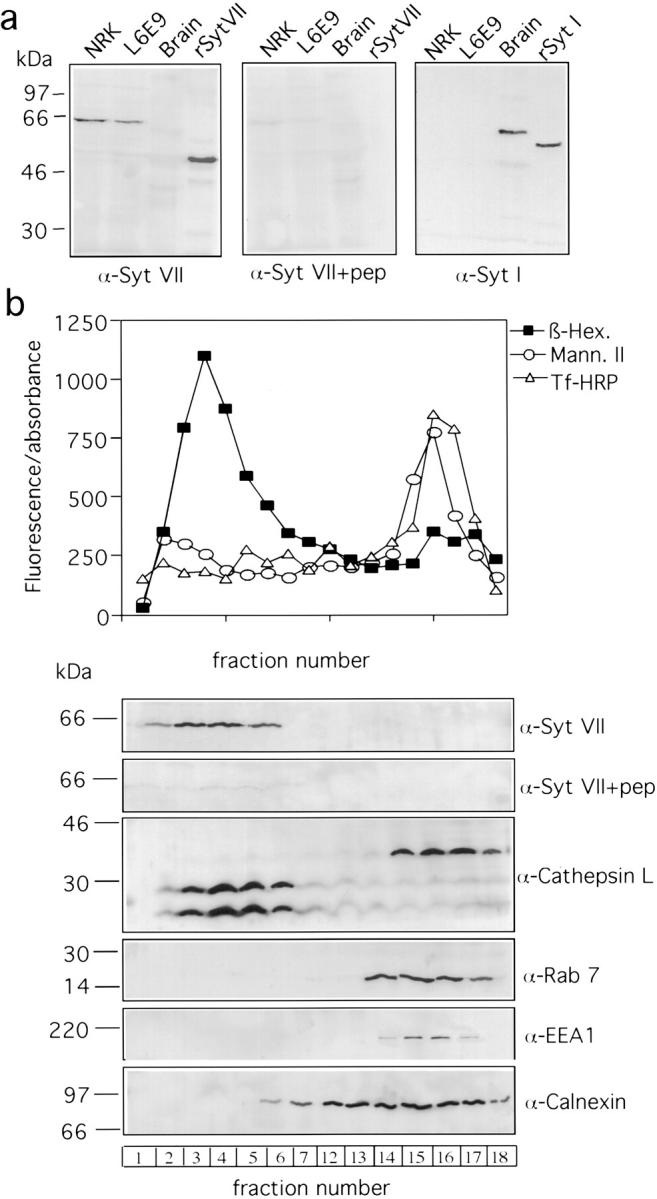
Syt VII colocalizes with dense lysosomes on subcellular fractionation. a, Western blot of NRK fibroblasts, L6E9 myoblasts, and rat brain extracts (5 μg/lane), and recombinant Syt VII (rSyt VII) with anti-Syt VII antibodies in the presence of the scrambled control peptide (left), or in the presence of the NH2-terminal Syt VII peptide (center). Right, Western blot with antibodies to Syt I (MC17) of the same extracts, and recombinant Syt I (rSyt I). b, Percoll density gradient fractionation of NRK cells. Top, detection of β-hexosaminidase, mannosidase II, and transferrin–HRP on gradient fractions collected from the bottom (activities assayed using 4-methyl-umbellyferyl-N-acetyl-β-d-glucosaminide, 4-methylumbelliferyl a-D-mannopyranoside, and 3,3′, 5,5′-tetramethylbenzidine as substrates, respectively). Bottom, Western blot detection of Syt VII, cathepsin L, rab 7, EEA1, and calnexin on gradient fractions.
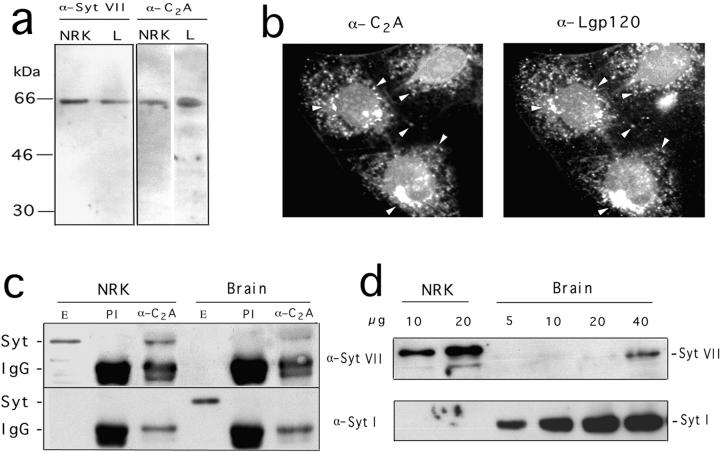
Specificity of the antibodies generated against the NH2-terminal and C2A domains of Syt VII. a, Western blot of NRK or L mouse fibroblast extracts (5 μg/lane) with antibodies to Syt VII NH2 terminus peptide (α-Syt VII) or to Syt VII recombinant C2A domain (α-C2A). b, Immunofluorescence of NRK cells with anti-Syt VII C2A antibodies (left) or anti-Lgp120 (right). c, Immunoprecipitation of NRK or rat brain extracts with anti-Syt VII C2A antibodies, Western blotted with antibodies to the NH2 terminus of Syt VII (top) or Syt I (bottom). Syt indicates the position of Syt VII or Syt I, and IgG the position of the heavy chain of rabbit IgG. E, Total extract; PI, immunoprecipitate obtained with preimmune rabbit IgG; α-C2A, immunoprecipitate with anti-Syt VII C2A affinity-purified IgG. d, Western blot of increasing amounts of NRK (10 or 20 μg) or rat brain (5–40 μg) extracts with antibodies against the NH2 terminus of Syt VII (top) or Syt I (bottom).
The colocalization of Syt VII with endocytic tracers chased for several hours into NRK cells (Fig. 1, c–f) suggested that this isoform was present on either late endosomes or lysosomes. To clarify this issue, we performed subcellular fractionation of NRK cells on Percoll density gradients under conditions that separate dense lysosomes from late endosomes (Green et al. 1987; Czekay et al. 1997). As shown in Fig. 2 a, the lysosomal enzyme β-hexosaminidase was enriched in high density fractions, whereas Golgi and early endosome markers were detected in lower density fractions. Western blot detection of additional markers (Fig. 2 b) showed that the early endosome protein EEA1 and the late endosome marker rab 7 (Feng et al. 1995) were also present in low density fractions. The ER marker, calnexin (Hebert et al. 1995), showed a more spread distribution, but it was absent from the higher density fractions. Antibodies to cathepsin L detected the unprocessed 36 kD form of the enzyme in low density fractions of the gradient, whereas the lysosomally processed 28 and 21 kD forms (Portnoy et al. 1986) were, as expected, present only in the high density fractions containing mature lysosomes. Anti-Syt VII antibodies reacted with a ~66 kD band on the same high density gradient fractions containing the lysosomally processed forms of cathepsin L (but not rab 7), and the labeling was specifically inhibited by the Syt VII NH2-terminal peptide. Our results thus suggest that Syt VII is localized on dense, mature lysosomes of NRK fibroblasts.
GFP-tagged Syt VII Is Targeted to Lgp120-positive, rab 7-negative Compartments
The lysosomal localization of Syt VII was confirmed by expression of a GFP-tagged Syt VII construct in NRK cells. Cells expressing relatively low amounts of GFP were chosen for analysis, to avoid possible mislocalization due to overexpression. Syt VII–GFP (COOH-terminal fusion) was targeted to vesicles that colocalize with the lysosomal membrane glycoprotein, Lgp120 (Fig. 3, a–c), and with internalized dextran-rhodamine chased for several hours (not shown). The Syt VII–GFP-containing compartments did not colocalize with transferrin (Fig. 3, d–f) or EEA1 (not shown). The great majority of Syt VII–GFP-containing compartments also did not stain with antibodies against the late endosome marker, rab 7 (Fig. 3, g–i). These findings are consistent with the subcellular fractionation data discussed above, which showed that endogeneous Syt VII is exclusively localized on dense organelles, whereas the early and late endosome markers, EEA1 and rab 7, are detected only on lighter density fractions (Fig. 2). Our observations thus suggest that Syt VII contains specific targeting signals for dense, mature lysosomes of NRK cells, and that these signals are distinct from the targeting information present in Syt I, which is expressed on the plasma membrane when transfected into CHO fibroblasts (Feany and Buckley 1993; Feany et al. 1993).
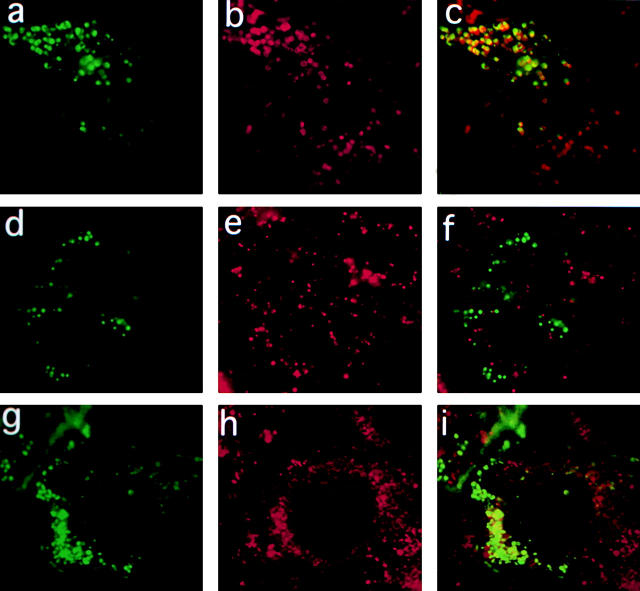
Transfected Syt VII-GFP is targeted to mature lysosomes of NRK cells. a, Syt VII-GFP. b, Anti-Lgp120 immunofluorescence. c, Overlay of a and b. d, Syt VII-GFP. e, Transferrin/Texas red-containing early endosomes. f, Overlay of d and e. g, Syt VII-GFP. h, Anti-rab 7 immunofluorescence. i, Overlay of g and h.
The C2A Domain of Syt VII Regulates Ca2+-triggered Exocytosis of Lysosomes
Extensive evidence implicating Syt C2A and C2B domains in Ca2+-triggered exocytosis was obtained through inhibition of exocytosis after introduction of specific peptides, soluble recombinant fragments, or antibodies into neurons (Bommert et al. 1993; Elferink et al. 1993; Mikoshiba et al. 1995; Mochida et al. 1997) or certain endocrine cells (Lang et al. 1997; Mizuta et al. 1997; Ohara-Imaizumi et al. 1997). We took a similar approach to investigate the role of the C2 domains of Syt VII on Ca2+-regulated exocytosis of lysosomes in NRK fibroblasts. Initially, a polyclonal rabbit antiserum was produced by imunization with the purified recombinant C2A domain of Syt VII. The antibodies were affinity-purified, and shown by Western blot to recognize an ~66-kD band in rat NRK and mouse L fibroblast cell extracts, which comigrates with the band recognized in NRK fibroblasts and L6E9 myoblasts by the antibodies against the Syt VII NH2 terminus peptide (Fig. 4 a).
On immunofluorescence assays with NRK cells, the anti-C2A antibodies labeled a vesicular population that also contained the lysosomal glycoprotein, Lgp120 (Fig. 4 b). To confirm that these anti-Syt VII C2A antibodies reacted with the same molecule recognized by the anti-Syt VII NH2 terminus antibodies, immunoprecipitation was performed, followed by Western blot. NRK cell (Fig. 4 c, NRK) or rat brain extracts (Fig. 4 c, Brain) were immunoprecipitated with purified preimmune (Fig. 4 c, PI) or anti-C2A (Fig. 4 c, α-C2A) IgG. The immunoprecipitates were then run on SDS-PAGE, together with samples of NRK or brain extracts, and processed for Western blot detection with anti-Syt VII or anti-Syt I NH2-terminal antibodies. Since all antibodies used in this experiment were generated in rabbits, the Western blot also revealed the heavy chain of rabbit IgG, migrating at ~55 kD. It is evident from Fig. 4 c that the preimmune antibodies, although loaded in higher amounts than the immune IgG, did not immunoprecipitate Syt VII or Syt I. On the other hand, Syt VII was clearly detected migrating above the IgG heavy-chain band, after immunoprecipitation of NRK extracts with anti-C2A antibodies. Smaller amounts of Syt VII were also detected after immunoprecipitation of brain extracts with anti-C2A antibodies (Fig. 4 c, top). Although Syt I was clearly detected on whole brain extracts, it was not immunoprecipitated by the anti-C2A antibodies (Fig. 4 c, bottom). These results demonstrate that Syt VII is effectively recognized in NRK cells by the antibodies generated against the Syt VII C2A domain, and suggest that these antibodies do not cross-react with the C2A domain of Syt I. Again, similarly to what is shown in Fig. 2 a, Syt VII was not detected in 5 μg of rat brain extract, whereas it was clearly detected on the same amount of NRK cell extract (Fig. 4 c, top).
Previous studies established the predominantly neuronal expression of Syt I, and the ubiquitous distribution of Syt VII (Li et al. 1995; Sudhof and Rizo 1996). Therefore, it was initially puzzling to us the lack of detection of Syt VII in rat brain, particularly in view of reverse transcriptase (RT)-PCR and Western blot data that suggested a higher expression of Syt VII in brain than in other tissues (Li et al. 1995). To clarify this issue, we performed Western blots on increasing amounts of NRK or rat brain extracts, with the antibodies specific for the NH2 terminus of Syt VII or Syt I. As shown in Fig. 4 d, top, Syt VII was only detected in brain when about eight times more material was loaded in the gels. Syt I, as expected, was detected in all concentrations of brain extract, but not in NRK cells (Fig. 4 d, bottom). These results reinforce our interpretation that this effect is due to the lower abundance of lysosomes in relation to synaptic vesicles in brain extracts. It is important to note that our experiments involved highly specific antibodies against the unique ectodomain of Syt VII, which exclusively detect full-length, transmembrane Syt VII. Li et al. 1995, on the other hand, generated antibodies against the cytosolic region of Syt VII containing the conserved C2 domains, which, in addition to a possible cross-reactivity with other isoforms (Butz et al. 1999), do not distinguish between full-length and truncated forms generated by alternative splicing. A recent study reported that the vast majority of RT-PCR products of Syt VII obtained from rat brain lacked the transmembrane region, apparently due to exon skipping. Importantly, that study also showed that all the exon-skipped forms of Syt obtained by RT-PCR resulted in frame-shifted messages (Craxton and Goedert 1999). This implies that the original NH2-terminal sequence of Syt VII would not be present in the truncated forms, explaining the lack of reactivity with our antibodies generated against the NH2-terminal sequence of full-length Syt VII. It was suggested that unknown upstream exons may import novel NH2-terminal sequences to these exon-skipped forms, or, alternatively, that frame-shifted forms may be initiated downstream, to produce double C2 domain-containing proteins (Craxton and Goeder, 1999). Although the physiological function of these truncated forms of Syt VII remains to be determined, it is noteworthy that alternative splicing appears to be a mechanism commonly used in the brain of higher organisms for increasing functional diversity (Black 1998).
Having established that the anti-Syt VII C2A antibodies recognize lysosome-associated Syt VII in NRK cells, β-hexosaminidase secretion assays (Rodríguez et al. 1997) were performed to verify if the antibodies interfered with Ca2+-triggered lysosome exocytosis. When SLO-permeabilized NRK cells were exposed to 1 μM [Ca2+] in the presence of 5 or 12 μg/ml affinity-purified anti-Syt VII C2A antibodies, inhibition levels of 46 and 87%, respectively, were observed in relation to the level of β-hexosaminidase released in the presence of preimmune rabbit antibodies (Fig. 5 a). Next, we followed the translocation of lysosomal membrane proteins to the cell surface, as an additional assay to examine the inhibitory effect of the anti-Syt VII C2A antibodies on Ca2+-triggered lysosome exocytosis. The antibodies generated against the Syt VII NH2 terminus ectodomain peptide were not used for these surface-labeling assays because they only recognize Syt VII in cells fixed and permeabilized with methanol (possibly reflecting a need to extract other lysosomal proteins to expose the epitopes). Thus, we used the previously characterized assay of Ca2+-dependent surface translocation of the lumenal domain of Lgp120 (Rodríguez et al. 1997), which colocalizes with Syt VII in NRK cells (Fig. 3, a–c). The cells were permeabilized with SLO, exposed to buffer containing or not 1 μM Ca2+ and 12 μg/ml preimmune IgG or anti-Syt VII C2A affinity-purified antibodies, and surface-labeled at 4°C with an mAb against a lumenal domain epitope of Lgp120. As shown in Fig. 5b–e, Fig. 1 μM Ca2+ triggers the translocation of Lgp120 to the plasma membrane of SLO-permeabilized NRK cells, resulting in a punctate surface fluorescence pattern identical to that previously reported for ionomycin-treated intact NRK cells (Rodríguez et al. 1997). When the cells were exposed to Ca2+ in the presence of anti-Syt VII C2A antibodies, a significant reduction in surface-exposed Lgp120 was observed (Fig. 5, f–g).
These findings strongly suggested a functional role for the C2A domain of Syt VII in the regulation of lysosome exocytosis. To confirm these results, we proceeded to verify, using the two assays described above, if introduction of soluble Syt C2 domains had a specific and direct effect on lysosome exocytosis. Histidine-tagged C2A, C2B, or C2A–C2B domains of Syt VII and of the exclusively neuronal isoform Syt I were expressed in E. coli and purified (Fig. 6 a). NRK cells were permeabilized and exposed to Ca2+ for 10 min in the presence of increasing concentrations of the Syt VII C2A domain, and the supernatant was assayed for released β-hexosaminidase. A clear dose-dependent inhibition in exocytosis was observed, reaching 90% at 200 μg/ml. The same concentrations of the Syt I C2A domain had no effect (Fig. 6 b).
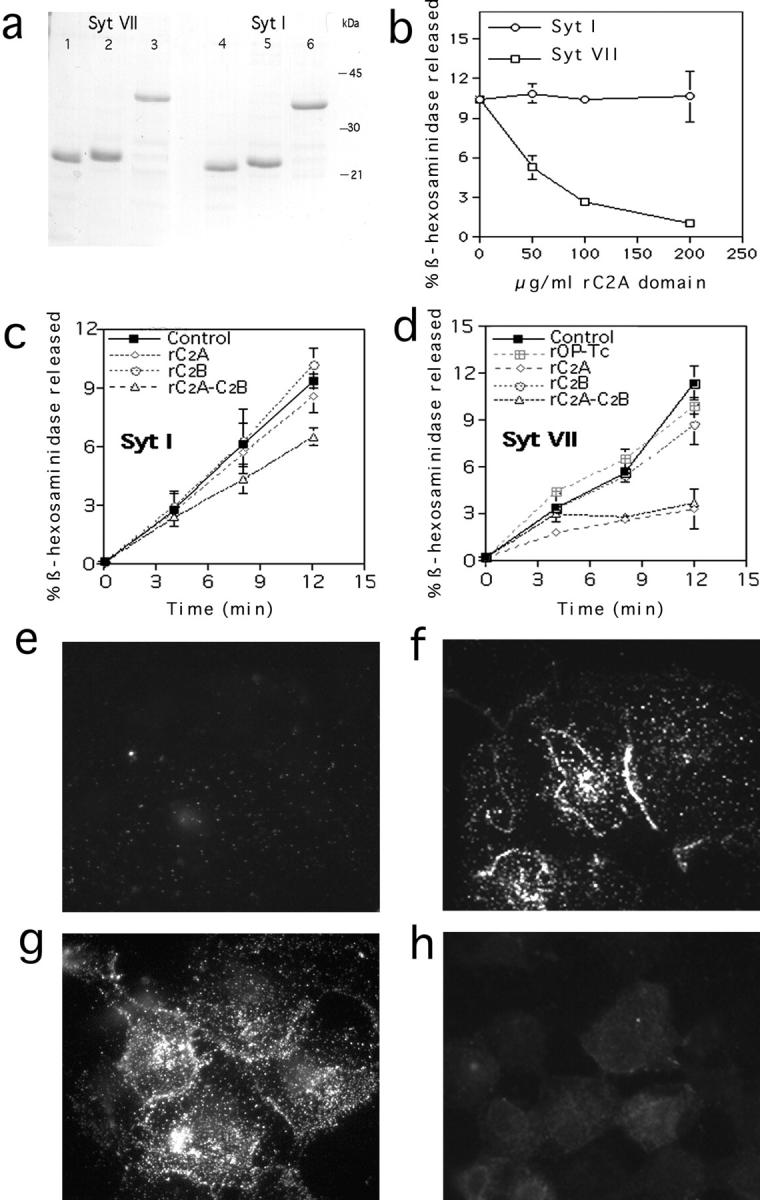
Syt VII C2A domain inhibits lysosome exocytosis in NRK cells. a, SDS-PAGE (10 μg/lane) of recombinant Syt VII C2A (1); Syt VII C2B (2); Syt VII C2A-B (3); Syt I C2A (4); Syt I C2B (5); and Syt I C2A-B (6). b, Permeabilized cells were stimulated with 1 μM Ca2+ in the absence or presence of the indicated concentrations of Syt VII or Syt I C2A domains, and the supernatant was assayed after 10 min for released β-hexosaminidase. c, Permeabilized cells were stimulated with Ca2+ in the absence or presence of 200 μg/ml Syt I fragments, and the supernatant was assayed for released β-hexosaminidase at the indicated time points. d, Same as c, except that 200 μg/ml Syt VII fragments were used. Op-Tc (d) corresponds to an unrelated protein expressed in E. coli and purified under the same conditions. The data is expressed as percentage of the total cellular content of β-hexosaminidase (mean of triplicates ± SD). e–h, Permeabilized cells were stained for surface Lgp120 after incubation in buffer containing: e, no Ca2+; f, 1 μM Ca2+; g, 1 μM Ca2+ plus 200 μg/ml recombinant Syt I C2A fragments; and h, 1 μM Ca2+ plus 200 μg/ml recombinant Syt VII C2A fragments.
Kinetic β-hexosaminidase secretion assays were then performed in the presence of 200 μg/ml recombinant Syt I and Syt VII C2A, C2B, and C2A–C2B fragments. No inhibition was observed with the C2A or C2B domains of Syt I, and only a partial reduction (of ~30%) in β-hexosaminidase release was detected in the presence of the larger Syt I fragment containing both the C2A and C2B domains (Fig. 5 c). In contrast, a strong inhibition, detectable after the initial five minutes, was observed in the presence of both constructs containing the Syt VII C2A domain (Fig. 6 d). Only a small reduction in exocytosis (of <20%) was detected at the later time points in the presence of the Syt VII C2B domain alone. An unrelated protein (OP-Tc), expressed and purified under the same conditions, had no effect (Fig. 6 d). Lgp120 surface-translocation assays confirmed the specific inhibitory effect of the Syt VII C2A domain on lysosome exocytosis. Whereas the typical pattern of Ca2+-triggered Lgp120 surface immunofluorescence was observed in cells exposed or not to Syt I C2A (Fig. 6, f–g), the presence of Syt VII C2A fragments was strongly inhibitory (Fig. 6 h).
The results of these functional exocytosis assays suggest a specific role for Syt VII in the regulation of Ca2+-induced lysosome exocytosis. In particular, our data points to the involvement of C2A domain-specific functions of Syt VII, which include the binding of syntaxin at low micromolar [Ca2+] (Li et al. 1995). Although our findings are consistent with a function for Syt VII as a high affinity Ca2+ sensor, additional studies are needed to clarify the basis for this activity, and to further characterize the structural and functional differences between the C2 domains of different Syt isoforms.
The presence of Syt VII on the membrane of dense lysosomes may explain earlier observations of assembly of AP-2–containing clathrin coats on mature lysosomes of permeabilized NRK cells (Traub et al. 1996). AP-2 has been shown to bind to the C2B domain of Syt I (Zhang et al. 1994) and several other Syt isoforms, including Syt VII (Li et al. 1995; Haucke and De Camilli 1999). It is thus conceivable that Syt VII is also involved in the retrieval of lysosomal components delivered to the plasma membrane by exocytosis, in an analogous manner to the proposed role of Syt I in synaptic vesicle recycling (Fukuda et al. 1995; Jorgensen et al. 1995).
Acknowledgments
We are grateful to Gian-Carlo Ochoa and Pietro De Camilli for rat brain extracts and anti-Syt I antibodies; Volker Hauke and Pietro De Camilli for Syt I expression plasmids; Ari Helenius, Ira Mellman, Susanne Pfeffer, Dan Portnoy, and Craig Roy for antibodies; Ana Rodríguez and Swathi Rao for useful discussions; and Henry Tan for photography.
Jennifer Morehead is the recipient of a KO8 MCSD award, and this work was supported by a grant from the National Intitutes of Health and a Burroughs Wellcome Scholar Award to Norma W. Andrews.
Footnotes
Abbreviations used in this paper: [Ca2+], free calcium concentration; GFP, green fluorescent protein; NRK, normal rat kidney; PFA, paraformaldehyde; SLO, streptolysin O; Syt, synaptotagmin.
References
- Black D.L. Splicing in the inner eara common tune, but what are the instruments? Neuron. 1998;20:165–168. [Abstract] [Google Scholar]
- Bommert K., Charlton M.P., DeBello W.M., Chin G.J., Betz H., Augustine G.J. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993;363:163–165. [Abstract] [Google Scholar]
- Burgoyne R.D., Morgan A. Calcium sensors in regulated exocytosis. Cell Calcium. 1998;24:367–376. [Abstract] [Google Scholar]
- Butz S., Fernandez-Chacon R., Schmitz F., Jahn R., Sudhof T.C. The subcellular localizations of atypical synaptotagmins III and VI. Synaptotagmin III is enriched in synapses and synaptic plasma membranes but not in synaptic vesicles. J. Biol. Chem. 1999;274:18290–18296. [Abstract] [Google Scholar]
- Chavez R.A., Miller S.G., Moore H.-P.H.. A biosynthetic regulated secretory pathway in constitutive secretory cells. J. Cell Biol. 1996;133:1177–1191. [Europe PMC free article] [Abstract] [Google Scholar]
- Coorsen J.R., Schmitt H., Almers W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:3787–3791. [Europe PMC free article] [Abstract] [Google Scholar]
- Craxton M., Goedert M. Alternative splicing of synaptotagmins involving transmembrane exon skipping. FEBS Lett. 1999;460:417–422. [Abstract] [Google Scholar]
- Czekay R.-P., Orlando R.A., Woodward L., Lunstrom M., Farquhar M.G. Endocytic trafficking of megalin/RAP complexesdissociation of the complexes in late endosomes. Mol. Biol. Cell. 1997;8:517–532. [Europe PMC free article] [Abstract] [Google Scholar]
- Elferink L.A., Peterson M.R., Scheller R.H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. [Abstract] [Google Scholar]
- Feany M.B., Buckley K.M. The synaptic vesicle protein synaptotagmin promotes formation of filopodia in fibroblasts. Nature. 1993;364:537–540. [Abstract] [Google Scholar]
- Feany M.B., Yee A.G., Delvy M.L., Buckley K.M. The synaptic vesicle proteins SV2, synaptotagmin, and synaptophysin are sorted to separate cellular compartments in CHO fibroblasts. J. Cell Biol. 1993;123:575–584. [Europe PMC free article] [Abstract] [Google Scholar]
- Feng Y., Press B., Wandinger-Ness A. Rab 7an important regulator of late endocytic membrane traffic. J. Cell Biol. 1995;131:1435–1452. [Europe PMC free article] [Abstract] [Google Scholar]
- Fukuda M., Moreira J.E., Lewis F.M., Sugimori M., Niinobe M., Mikoshiba K., Llinas R. Role of the C2B domain of synaptotagmin in vesicular release and recycling as determined by specific antibody injection into the squid giant synapse preterminal. Proc. Natl. Acad. Sci. USA. 1995;92:10708–10712. [Europe PMC free article] [Abstract] [Google Scholar]
- Green S.A., Zimmer K.P., Griffiths G., Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J. Cell Biol. 1987;105:1227–1240. [Europe PMC free article] [Abstract] [Google Scholar]
- Haucke V., De Camilli P. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science. 1999;285:1268–1271. [Abstract] [Google Scholar]
- Hebert D.N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. [Abstract] [Google Scholar]
- Jorgensen E.M., Hartwig E., Schuske K., Nonet M.L., Jin Y., Horvitz H.R. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans . Nature. 1995;378:196–199. [Abstract] [Google Scholar]
- Kasai H., Kishimoto T., Liu T.T., Miyashita Y., Podini P., Grohovaz F., Meldolesi J. Multiple and diverse forms of regulated exocytosis in wild type and defective PC12 cells. Proc. Natl. Acad. Sci. USA. 1999;96:945–949. [Europe PMC free article] [Abstract] [Google Scholar]
- Lang J., Fukuda M., Zhang H., Mikoshiba K., Wollheim C.B. The first C2 domain of synaptotagmin is required for exocytosis of insulin from pancreatic β-cellsaction of synaptotagmin at low micromolar calcium. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5837–5846. [Europe PMC free article] [Abstract] [Google Scholar]
- Li C., Ullrich B., Zhang J.Z., Anderson R.G.W., Brose N., Sudhof T.C. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. [Abstract] [Google Scholar]
- Littleton J.T., Serano T.L., Rubin G.M., Ganetzky B., Chapman E.R. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature. 1999;400:757–760. [Abstract] [Google Scholar]
- Mikoshiba K., Fukuda M., Moreira J.E., Lewis F.M., Sugimori M., Niinobe M., Llinas R. Role of the C2A domain of synaptotagmin in transmitter release as determined by specific antibody injection into the squid giant synapse preterminal. Proc. Natl. Acad. Sci. USA. 1995;92:10703–10707. [Europe PMC free article] [Abstract] [Google Scholar]
- Mizuta M., Kurose T., Miki T., Shoji-Kasai Y., Takahashi M., Seino S., Matsukura S. Localization and functional role of synaptotagmin III in insulin secretion in pancreatic beta-cells. Diabetes. 1997;46:2002–2006. [Abstract] [Google Scholar]
- Mochida S., Fukuda M., Niinobe M., Kobayashi H., Mikoshiba K. Roles of synaptotagmin C2 domains in neurotransmitter secretion and inositol high-polyphosphate binding at mammalian cholinergic synapses. Neuroscience. 1997;77:937–943. [Abstract] [Google Scholar]
- Ninomiya Y., Kishimoto T., Miyashita Y., Kasai H. Ca2+-dependent exocytic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J. Biol. Chem. 1996;271:17751–17754. [Abstract] [Google Scholar]
- Ohara-Imaizumi M., Fukuda M., Niinobe M., Misonou H., Ikeda K., Murakami T., Kawasaki M., Mikoshiba K., Kumakura K. Distinct roles of C2A and C2B domains of synaptotagmin in the regulation of exocytosis in adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 1997;94:287–291. [Europe PMC free article] [Abstract] [Google Scholar]
- Portnoy D.A., Erickson A.H., Kochan J., Ravetch J., Unkeless J.C. Cloning and characterization of a mouse cysteine proteinase. J. Biol. Chem. 1986;261:14697–14703. [Abstract] [Google Scholar]
- Rodríguez A., Webster P., Ortego J., Andrews N.W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997;137:93–104. [Europe PMC free article] [Abstract] [Google Scholar]
- Schiavo G., Osborne S.L., Sgouros J.G. Synaptotagminsmore isoforms than functions? Biochem. Biophys. Res. Comm. 1998;248:1–8. [Abstract] [Google Scholar]
- Simonsen A., Lippe R., Christoforidis S., Gaullier J.M., Brech A., Callaghan J., Toh B.H., Murphy C., Zerial M., Stenmark H. EEA1 links PI(3)K function to Rab 5 regulation of endosome fusion. Nature. 1998;394:494–498. [Abstract] [Google Scholar]
- Stinchcombe J.C., Griffiths G.M. Regulated secretion from hemopoietic cells. J. Cell Biol. 1999;147:1–5. [Europe PMC free article] [Abstract] [Google Scholar]
- Sudhof T.C., Rizo J. SynaptotagminsC2 domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. [Abstract] [Google Scholar]
- Thomas D.M., Ferguson G.D., Herschman H.R., Elferink L.A. Functional and biochemical analysis of the C2 domains of synaptotagmin IV. 1999. Mol. Biol. Cell. 1999;10:2285–2295. [Europe PMC free article] [Abstract] [Google Scholar]
- Traub L., Bannykh S.I., Rodel J.E., Aridor M., Balch W.E., Kornfeld S. AP-2–containing clathrin coats assemble on mature lysosomes. J. Cell Biol. 1996;135:1801–1814. [Europe PMC free article] [Abstract] [Google Scholar]
- von Poser C., Ichtchenko K., Shao X., Rizo J., Sudhof T.C. The evolutionary pressure to inactivate. J. Biol. Chem. 1997;272:14314–14319. [Abstract] [Google Scholar]
- Xu T., Binz T., Niemann H., Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat. Neurosci. 1998;1:192–200. [Abstract] [Google Scholar]
- Zhang J.Z., Davletov B.A., Sudhof T.C., Anderson R.G.W. Synaptotagmin I is a high affinity receptor for clathrin AP-2implications for membrane recycling. Cell. 1994;78:751–760. [Abstract] [Google Scholar]
Articles from The Journal of Cell Biology are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1083/jcb.148.6.1141
Read article for free, from open access legal sources, via Unpaywall:
http://jcb.rupress.org/content/148/6/1141.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1083/jcb.148.6.1141
Article citations
The 8-oxoguanine DNA glycosylase-synaptotagmin 7 pathway increases extracellular vesicle release and promotes tumour metastasis during oxidative stress.
J Extracell Vesicles, 13(9):e12505, 01 Sep 2024
Cited by: 1 article | PMID: 39235072 | PMCID: PMC11375530
The endolysosomal system in conventional and unconventional protein secretion.
J Cell Biol, 223(9):e202404152, 12 Aug 2024
Cited by: 0 articles | PMID: 39133205 | PMCID: PMC11318669
Review Free full text in Europe PMC
The lysosomal trafficking regulator "LYST": an 80-year traffic jam.
Front Immunol, 15:1404846, 07 May 2024
Cited by: 0 articles | PMID: 38774881 | PMCID: PMC11106369
Review Free full text in Europe PMC
Lysosomes in Cancer-At the Crossroad of Good and Evil.
Cells, 13(5):459, 05 Mar 2024
Cited by: 1 article | PMID: 38474423 | PMCID: PMC10930463
Review Free full text in Europe PMC
Wound Repair of the Cell Membrane: Lessons from Dictyostelium Cells.
Cells, 13(4):341, 14 Feb 2024
Cited by: 0 articles | PMID: 38391954 | PMCID: PMC10886852
Review Free full text in Europe PMC
Go to all (209) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins.
Nature, 375(6532):594-599, 01 Jun 1995
Cited by: 388 articles | PMID: 7791877
Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+ -dependent exocytosis in PC12 cells.
J Biol Chem, 279(50):52677-52684, 28 Sep 2004
Cited by: 61 articles | PMID: 15456748
Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells.
J Biol Chem, 277(27):24499-24505, 02 May 2002
Cited by: 49 articles | PMID: 12006594
Synaptotagmin regulates mast cell functions.
Immunol Rev, 179:25-34, 01 Feb 2001
Cited by: 21 articles | PMID: 11292024
Review




