Abstract
Introduction
Aberrant DNA methylation has been found frequently in human breast cancers, associated with the loss of expression of a number of regulatory genes for growth and correlated to clinical outcomes. The present study was undertaken to determine whether methylation of a set of growth-suppressor genes would correlate to the expression of estrogen receptors (ERs) and progesterone receptors (PRs).Methods
We used a pyrosequencing methylation analysis to study the methylation of 12 known growth-suppressor genes in 90 pairs of malignant/normal breast tissues. We also examined the expression of ERs and PRs in those specimens by immunohistochemistry. Mutations of p53 in tumor cells were detected by direct sequencing.Results
Twelve tumor-suppressor genes: ARHI, RASSF1A, HIN-1, RARbeta2, hMLH1, 14-3-3 sigma, RIZ1, p16, E-cadherin, RIL, CDH13, and NKD2 were selected for this methylation study. Five of them (RIL, HIN-1, RASSF1A, CDH13, and RARbeta2) were frequently methylated in breast cancers (57%, 49%, 58%, 44%, and 17%, respectively) but not the normal breast (0-4%). Two panels of methylation profiles were defined. The methylation of the HIN-1/RASSFIA panel strongly correlated to the expression of ERs, PRs, and hormone receptors (HRs; which were defined as 'positive' if ERs and/or PRs were positive; p < 0.001). Conversely, the methylation of the RIL/CDH13 panel strongly correlated to negative ER, PR, and HR expression (p = 0.001, 0.025, and 0.001, respectively). The subset of triple-negative breast cancers (in other words, those with negative ER, PR, and HER-2/neu status) was positively associated with the methylation of the RIL/CDH13 panel and negatively associated with the HIN-1/RASSF1A panel. Mutations of p53 were found in nine breast tumors (11%), seven of which lacked methylation in both panels.Conclusion
We have defined two panels (HIN-1/RASSFIA, and RIL/CDH13) of methylation profiles, which correlated, either positively or negatively, to HR status.Free full text

Correlation between CpG methylation profiles and hormone receptor status in breast cancers
Abstract
Introduction
Aberrant DNA methylation has been found frequently in human breast cancers, associated with the loss of expression of a number of regulatory genes for growth and correlated to clinical outcomes. The present study was undertaken to determine whether methylation of a set of growth-suppressor genes would correlate to the expression of estrogen receptors (ERs) and progesterone receptors (PRs).
Methods
We used a pyrosequencing methylation analysis to study the methylation of 12 known growth-suppressor genes in 90 pairs of malignant/normal breast tissues. We also examined the expression of ERs and PRs in those specimens by immunohistochemistry. Mutations of p53 in tumor cells were detected by direct sequencing.
Results
Twelve tumor-suppressor genes: ARHI, RASSF1A, HIN-1, RARβ2, hMLH1, 14-3-3 σ, RIZ1, p16, E-cadherin, RIL, CDH13, and NKD2 were selected for this methylation study. Five of them (RIL, HIN-1, RASSF1A, CDH13, and RARβ2) were frequently methylated in breast cancers (57%, 49%, 58%, 44%, and 17%, respectively) but not the normal breast (0–4%). Two panels of methylation profiles were defined. The methylation of the HIN-1/RASSFIA panel strongly correlated to the expression of ERs, PRs, and hormone receptors (HRs; which were defined as 'positive' if ERs and/or PRs were positive; p < 0.001). Conversely, the methylation of the RIL/CDH13 panel strongly correlated to negative ER, PR, and HR expression (p = 0.001, 0.025, and 0.001, respectively). The subset of triple-negative breast cancers (in other words, those with negative ER, PR, and HER-2/neu status) was positively associated with the methylation of the RIL/CDH13 panel and negatively associated with the HIN-1/RASSF1A panel. Mutations of p53 were found in nine breast tumors (11%), seven of which lacked methylation in both panels.
Conclusion
We have defined two panels (HIN-1/RASSFIA, and RIL/CDH13) of methylation profiles, which correlated, either positively or negatively, to HR status.
Introduction
Over the past ten years, aberrant DNA methylation has been recognized as one of the most common molecular abnormalities in breast cancer [1,2]. A large body of evidence implicates potential hypermethylation of CpG islands in the loss of expression of a variety of crucial genes. Tumor-suppressor genes with aberrant methylation in breast cancers include ARHI [3,4], RASSF1A [5], HIN-1 [6], the retinoic acid receptor II gene (RARβ2) [7], hMLH1 [8], 14-3-3 σ [9], RIZ1 [10], p16 [11], the E-cadherin gene [12], PTEN [13], and BRCA1 [14]. Methylation in breast cancer has been related to clinical and pathologic characteristics evident at presentation and clinical outcomes. A higher prevalence of HIN-1 and RAR β2 methylation was found in the lymph nodes, bone, brain, and lung metastases than the primary tumor [15]. Widschwendter and colleagues [16] reported that the methylation of certain genes was associated with hormone receptor (HR) status, in addition to the response to treatment with tamoxifen. A high prevalence of PGR, HSD17B4, and CDH13 methylation has been associated with HER-2/neu-positive breast cancer [17].
Methylation-specific PCR (MSP) is a sensitive assay used to detect methylation and analyze the methylation status of genes of interest. However, problems inherent to this assay (such as those caused by the use of different primers for the methylated or unmethylated alleles, the gel-based data analysis system used, and difficulties in quantitation) have caused frequent false-positive results in tissue-sample analyses. A new technique, pyrosequencing, has been adapted for use in highly sensitive and quantitative methylation analyses [18,19]. Pyrosequencing methylation analysis is a modification of the combined bisulfite restriction analysis (COBRA) that compares favorably with COBRA in sensitivity, specificity, and robustness [18]. Tost and colleagues also confirmed that the pyrosequencing technique is quantitative, amenable to the analysis of bisulfite-treated DNA derived from paraffin-embedded tissue samples, highly reproducible, and accurate [19]. Bisulfite pyrosequencing has been used in clinical trials of hypomethylating drug treatment and provides accurate and reliable results [20].
To investigate methylation profiles in breast cancer cells, we used bisulfite pyrosequencing to screen 12 known tumor-suppressor genes in 90 pairs of breast cancers and normal tissues. Although all 12 genes had been reported to exhibit hypermethylation in a fraction of breast cancer cases, our assays provided a comprehensive survey of their methylation status and confirmed that five genes could be useful in defining a methylation profile in breast cancer cells. Our findings also suggest that two panels of methylation profiles correlated, either positively or negatively, to HR status.
Materials and methods
Cell lines
Human breast cancer cell lines SKBr3, MDA-MB-435, MDA-MB-468, BT-20, MDA-MB-231, and MCF-7 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. Normal breast epithelial cells, HMEC 231 and HMEC234, were cultured in a 1:1 solution of MCDB 105 and medium 199 with 15% fetal bovine serum and 10 ng/ml epithelial growth factor (Sigma, St Louis, MO, USA), as described elsewhere [21].
Tissue samples
We used 90 samples, consisting of paired tissues and associated clinicopathologic data from the Breast Tumor Bank at The University of Texas MD Anderson Cancer Center (Houston, TX, USA). The samples of breast tumors and corresponding adjacent normal-appearing tissues (from tissues located at least 3 cm away from the site at which the tumor was sampled) came from 80 patients who had undergone surgery at The University of Texas MD Anderson Cancer Center in 2004 or 2005 and 10 patients who had been diagnosed with breast cancer between 1995 and 2003. All tissue samples had been fresh-frozen and stored at -80°C. No patient was recruited specifically for this study. All patients gave written informed consent permitting the use of their breast tissue for research at the time specimens were collected. This study was approved by The University of Texas MD Anderson Cancer Center's Institutional Review Board.
Histopathologic analysis
All breast specimens were reviewed by experienced pathologists. Slides were subject to immunoperoxidase staining for estrogen receptors (ERs; clone 6F11, Novocastra Laboratories Ltd, Benton Lane, UK) and progesterone receptors (PRs; clone1A6, Novocastra Laboratories Ltd), according to the manufacturer's recommendations. Cancers were considered receptor-positive if > 10% of malignant cells showed nuclear staining. Cancers were classified as HR-positive if the ER and/or PR status was positive. HER-2/neu gene amplification was assessed by interphase fluorescence in situ hybridization (FISH) using the PathVysion HER-2/neu probe kit (Vysis Inc., Downers Grove, IL, USA), according to the manufacturer's recommendations. Levels of the HER-2/neu:CEP17 signal ratio were considered normal if FISH detected ≤ 2.0 copies per cell.
Microdissection, DNA extraction, and sodium bisulfite treatment
To avoid contamination with normal tissues in the methylation analysis, we isolated breast cancer cells and paired normal breast epithelial cells from tissues by manual microdissection, following the National Cancer Institutes (Bethesda, MA, USA) protocols [22]. In brief, 5–10 μm sections were cut from each archival fresh-frozen tissue block. For each pair of tissues, the presence of tumor cells in malignant tissues and absence of cancer cells in normal tissues were confirmed by histopathologic examination. Frozen tissue sections were fixed with ethanol, stained with H & E, and microdissected using a needle. Each tumor sample contained > 70% tumor cells after microdissection. Genomic DNA was extracted from patient samples, breast cancer cell lines, and normal breast epithelial cells using the Dneasy tissue kit (Qiagen, Valencia, CA, USA). Bisulfite treatment of 1–2 μg of genomic DNA was performed, as previously described [3].
Genes studied
Twelve tumor-suppressor genes were selected for this study. ARHI (CpG I and II), RASSF1A, HIN-1 (SCGB3A1), RARβ2, hMLH1, the 14-3-3 σ gene, RIZ1, p16 (CDKN2A), and the E-cadherin gene (CDH1) were selected on the basis of previous reports of elevated methylation rates in breast cancers [3-12]. RIL (PDLIM4) [23], CDH13, and NKD2 were identified as hypermethylated genes using genome-wide methylated CpG island amplification (MCA) from multiple tumors (24). Global methylation was estimated by testing methylation levels of LINE1 repetitive elements [25]. We also assessed the methylation of ERα and PGRB genes.
Pyrosequencing methylation analysis
Bisulfite pyrosequencing was used to detect methylation of all 15 genes. Pyrosequencing primers were designed using Assay Design software 1.0 (Biotage, Westborough, MA, USA). For each gene, we selected the CpG island region flanking the transcription start site at the 5'UTR. Two to six CpG sites were studied for each particular CpG island. The primers for pyrosequencing and PCR conditions are listed in Additional file 1. Bisulfite-treated DNA (1 μl) was amplified in 50 μl of reaction mixture, containing primers and 0.2 U of Taq polymerase (New England Biolabs, Ipswich, MA, USA). For the amplification of HIN-1, RARβ2, hMLH1, the 14-3-3 σ gene, RIZ1, the E-cadherin gene, NKD2, and PGRB, we used a universal primer approach [18]. The PCR product was purified and methylation was quantitated using the PSQ HS 96A pyrosequencing system and Pyro gold reagents (Biotage). Methylation data are presented as the percentage of average methylation in all observed CpG sites. To set the controls for pyrosequencing, we used cancer cell lines and normal cells that were consistently positive or negative with stable levels of methylation. In this study, each PCR assay included a positive control (the MDA-MB-231 breast cancer cell line, which is highly methylated in most genes) and a negative control (normal breast epithelial cells, HMEC231, which are unmethylated in all genes, except LINE1, ARHI, and the 14-3-3 σ gene). RKO, a colon cancer cell line that is highly methylated in hMLH1 and p16, was also used as a positive control.
p53 mutation detection
Genomic DNA was extracted from microdissected tumor tissue using the QIAGEN DNA purification kit (Qiagen). PCR was performed using primers that amplify p53 exons 5–6 and 7–9. The PCR products were then purified by the gel purification system (Qiagen) or Exonuclease I and shrimp alkaline phosphatase (USB, Cleveland, OH, USA). Mutations were determined by direct sequencing.
Statistical methods and analysis
In the studied cohort, adjacent normal breast tissue was taken from each of 90 patients during surgery. Taking advantage of paired normal/tumor samples in this study, we chose the value of normal samples as the reference. If using the sample mean plus two times the standard deviation of the pooled normal samples (and a minimum of 10% methylation) as a cut-off point, there is > 97% probability that the methylation level for a normal tissue will be lower than the cut-off point. It is reasonable to assume that a value larger than the cut-off point is likely to be abnormal (or positive). Panels of genes, that is to say, HIN-1/RASSF1A and RIL/CDH13 were considered positive if both markers in each panel were positive. Descriptive analyses were performed first for exploratory purposes. Pair-wise scatter plots are presented to show the correlations among the genes methylated. Heat maps were plotted to show levels of gene expression or methylation using hierarchical clustering to visually represent the association of different genes or samples by histopathologic tumor characteristics (ERs, PRs, and HER-2/neu). Chi-square or Fisher's exact test were used to assess the dependence between two categorical variables. Pearson's correlation coefficient was used to assess the relationship between two continuous variables. The Wilcoxon rank-sum test was used to compare either continuous or categorical variables between two groups. Analysis of variance (ANOVA) was applied to compare the values of gene methylation with tumor characteristics. Multivariate logistic regression was used to assess the ability of various levels of gene methylation to predict the ER, PR, HR, or HER-2/neu status. All reported p values are two-sided and considered statistically significant if p < 0.05. Analyses were performed using S-PLUS 2000 software (Insightful Corp., Seattle, WA, USA).
Results
Patients' clinical data
Patient characteristics are presented in Table Table1.1. Normal paired tissues were unavailable in two cases. Information regarding the grade, HER-2/neu status, and ER and PR status were unavailable for one, ten, and three patients, respectively.
Table 1
Patient characteristics
| Clinicopathologic factors | Number of sample |
| Age | 90 |
| Median, range | 57 years, 29–81 years |
| Tumor grade (BNG) | 89 |
| 1 | 3 |
| 2 | 47 |
| 3 | 39 |
| Tumor size | 90 |
| T1 | 19 |
| T2 | 36 |
| T3 | 18 |
| T4 | 17 |
| Stage | 90 |
| I | 15 |
| II | 37 |
| III | 38 |
| Lymph-node metastasis | 90 |
| pN0 | 39 |
| pN1 | 28 |
| pN2 | 9 |
| pN3 | 14 |
| Lymphatic invasion | 78 |
| Positive | 33 |
| Negative | 45 |
| Vascular invasion | 78 |
| Positive | 32 |
| Negative | 46 |
| ER status | 87 |
| Positive | 52 |
| Negative | 35 |
| PR status | 87 |
| Positive | 40 |
| Negative | 47 |
| Hormone receptor status | 87 |
| Positive (ER- and/or PR-positive) | 55 |
| Negative (both ER- and PR-negative) | 32 |
| HER-2/neu status | 80 |
| Positive | 7 |
| Negative | 73 |
| Histology | 90 |
| Ductal | 62 |
| Lobular | 8 |
| Mixed ductal and lobular | 9 |
| Other | 11 |
BNG, Black's nuclear grade; ER, estrogen receptor; PR, progesterone receptor.
Accuracy, reproducibility, and quantitation of results with bisulfite pyrosequencing
To confirm the reliability of bisulfite pyrosequencing for the quantitation of methylation levels, we measured the methylation of ARHI CpG islands I and II using pyrosequencing and COBRA and compared the resulting data. COBRA detected only one CpG site, whereas pyrosequencing detected four to eight CpG sites in one CpG island (Figure (Figure1).1). From six breast cancer cell lines and two cultures of normal breast epithelial cells, methylation data were matched in CpG island II but not completely matched in CpG island I. COBRA showed two breast cancer cell lines were partially methylated in CpG island I (50% and 54% in one CpG site), but they were shown to be hypermethylated in CpG island I by pyrosequencing (the mean of four CpG sites per island was 92% and 91%, respectively). On the basis of these results, pyrosequencing was more sensitive for methylation detection than COBRA.
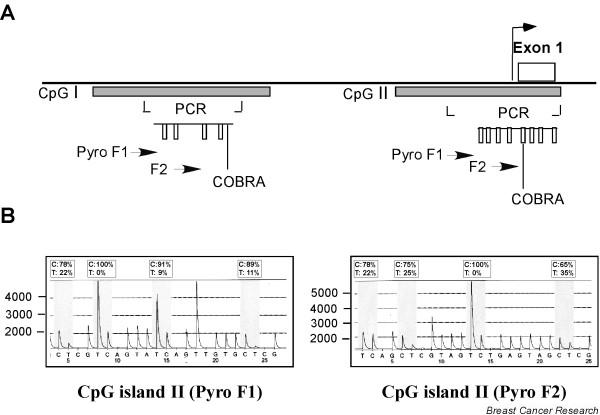
Pyrosequencing analysis for ARHI methylation. (a) Design of pyrosequencing primers for ARHI CpG islands I and II. The vertical bars indicate CpG sites in PCR products. COBRA indicated the restriction enzyme cutting site. (b) Pyrosequencing results for ARHI CpG island II methylation in MB-MDA-231 cells using two detection primers (Pyro F1 and F2). Methylation levels ranged from 65% to 100%, with a mean of 84%. Gray highlighting marks the CpG sites tested and the percentage reading of C and T. COBRA, combined bisulfite restriction analysis.
To confirm the reproducibility of pyrosequencing, the methylation of HIN-1 and RIL was measured repeatedly in 22 samples using bisulfite treatment and pyrosequencing assays. The correlation between repeated and initial values was high (for HIN-1, Pearson's correlation coefficient R = 0.99 and p < 0.0001; for RIL, R = 0.99 and p < 0.0001). In addition, the variation between individual assays was < 6%: the mean differences and 95% confidence intervals (CIs) for HIN-1 and RIL were 1.9% with 95% CIs of -0.1% to 3.9% and 3.0% with 95% CIs of 0.1% to 6.0%, respectively.
Frequent methylation of RIL, HIN-1, RASSF1A, CDH13, and RARβ2
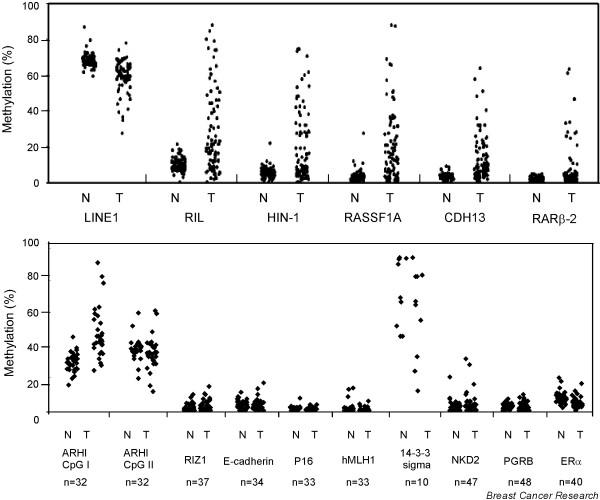
We evaluated the methylation status of 12 putative tumor-suppressor genes (ARHI, RASSF1A, HIN-1, RARβ2, hMLH1, the 14-3-3 σ gene, RIZ1, p16, the E-cadherin gene, RIL, CDH13, and NKD2), LINE1, ER, and PGRB in both culture cell lines and primary cancer tissues. The results from the analysis of six breast cancer cell lines and two normal breast epithelial cell samples (Additional file 2) indicated that 7 of 12 genes (RIL, HIN-1, RASSF1A, ARHI, CDH13, RARβ2, and NKD2) were densely methylated (marked bold) in cancer cell lines but not in normal breast epithelial cell lines. We also tested the methylation profiles of the 12 genes in breast cancer and the adjacent normal breast tissues from the same patients. In our pilot study, we screened 12 tumor-suppressor genes in 32–37 paired tissues. Five tumor-suppressor genes (RASSF1A, RIL, HIN-1, CDH13, and RARβ2) were frequently methylated in breast cancer tissues (the positive rate ranged from 17% to 58%) but not in normal breast tissues (the positive rate ranged from 0% to 4.5%). Another five genes (RIZ1, the E-cadherin gene, p16, hMLH1, and NKD2) were not frequently methylated in either malignant or normal breast tissue (the positive rate ranged from 0% to 9%, all < 10%). The 14-3-3 σ gene is highly methylated in both malignant and normal breast tissues. ARHI, as an imprinted gene, is at least partially methylated in all cases (Figure (Figure22 and Table Table2).2). Consequently, seven genes were unsuitable for development of methylation profiles. To correlate the methylation profile to clinical outcomes, we focused on the five highly methylated genes by assaying 53 more paired samples. PGRB was not frequently methylated in the 48 cases of breast tumor or normal breast tissue. ER was not highly methylated in the 40 cases of breast tumor or normal breast tissue, but higher methylation levels were observed in normal tissues. LINE1, a global methylation marker, was highly methylated in all normal and malignant tissues, but the methylation levels were significant lower in tumors (60.0 ± 8.8) than normal tissues (68.2 ± 3.5; p < 0.001).
Table 2
Methylation levels of 15 genes from pairs of normal and malignant breast tissues
| Gene | Number of cases studied | Methylation level (mean ± SD) (Positive rate*) | ||
| Normal | Cancer | pvalue** | ||
| LINE1 | 90 | 68 ± 4 | 60 ± 9 | < 0.001 |
| RIL | 90 | 10 ± 4 (4.5) | 27 ± 22 (57) | < 0.001 |
| HIN-1 | 90 | 5 ± 3 (2.3) | 20 ± 20 (49) | < 0.001 |
| RASFF1A | 90 | 3 ± 3 (3.4) | 19 ± 20 (58) | < 0.001 |
| CDH13 | 90 | 3 ± 2 (0) | 14 ± 13 (44) | < 0.001 |
| RARβ2 | 90 | 2 ± 1 (0) | 7 ± 12 (17) | < 0.001 |
| ARHI CpG I | 32 | 30 ± 6 (0) | 47 ± 16 (49) | < 0.001 |
| ARHI CpG II | 32 | 38 ± 7 (2) | 36 ± 10 (2) | 0.250 |
| RIZ1 | 37 | 2 ± 2 (0) | 3 ± 4 (5) | 0.111 |
| E-cadherin | 34 | 4 ± 3 (3) | 4 ± 4 (9) | 0.163 |
| p16 | 33 | 2 ± 1 (0) | 1 ± 1 (0) | 0.044 |
| hMLH1 | 33 | 2 ± 3 (6) | 1 ± 1 (0) | 0.864 |
| 14-3-3 σ | 10 | 75 ± 21 | 60 ± 28 | 0.275 |
| NKD2 | 47 | 2 ± 4 (2) | 4 ± 7 (8.5) | 0.308 |
| PGRB | 48 | 3 ± 2 (0) | 2 ± 3 (0) | 0.594 |
| ERα | 40 | 7 ± 4 (3) | 5 ± 3 (5) | < 0.001 |
*Positive rate using the sample mean plus two times the SD of the pooled normal samples (and minimum 10% methylation) as a cut-off point.
**p value computed using the Wilcoxon signed rank test for paired data. SD, standard deviation.
Correlation of methylation between different genes and to age
On the basis of data from continuous marker methylation analyses in normal tissues, the methylation levels of RASSF1A and HIN-1 exhibited a strong correlation to each other (R = 0.66, p < 0.001). Some moderate correlations were found between RARβ2 and CDH13 (R = 0.40, p < 0.001) and between RIL and CDH13 (R = 0.30, p = 0.004). Similarly, in tumor tissues, a moderate correlation existed between RASSF1A and HIN-1 (R = 0.51, p < 0.001) and a weak correlation existed between RIL and CDH13 (R = 0.19, p = 0.068). In addition, the global methylation marker LINE1 was negatively correlated to RIL (R = -0.25, p = 0.019) and RASSF1A (R = -0.26, p = 0.014) in breast cancer samples.
In normal tissues, RIL methylation levels increased with age (R = 0.336, p = 0.001), whereas other markers had little correlation to age. In the cancer tissues, HIN-1 levels exhibited a weak correlation to age (R = 0.229, p = 0.03), whereas other markers had little correlation to age.
Correlation between methylation in malignant and adjacent normal tissues
To study whether gene methylation in cancer would affect adjacent normal-appearing mammary gland tissues, the pair-wise correlation between paired tumor and adjacent normal breast tissues was estimated for each marker. Methylation of RIL, HIN-1, RASSF1A, and CDH13 exhibited a positive correlation between breast cancer and normal tissues. The Pearson correlation values ranged from 0.25 to 0.38 (p values ranged from 0.02 to 0.0002).
Relationship between methylation of individual genes and clinical characteristics
Using the ANOVA model, no statistically significant associations were found between clinical stage, tumor size, or node status. Tumors that were poorly differentiated (grade 3) according to Black's nuclear grade (BNG) had lower methylation levels of HIN-1 than well- and moderately differentiated tumors (grade 1 + 2; p = 0.01). Similarly, RASSF1A methylation levels were lower in poorly differentiated cancers than well- and moderately differentiated tumors (p = 0.053). In addition, CDH13 and RIL methylation levels cancers were marginally higher in poorly differentiated than well- and moderately differentiated cancers (p = 0.059 and 0.096, respectively).
Relationship between methylation of individual genes and ER, PR, HR, and HER-2/neu status
In univariate analyses, the ER status was positively associated with high HIN-1 and RASSF1A methylation levels but negatively correlated to high RIL methylation levels (p < 0.001, 0.002, and 0.01, respectively). Specifically, patients with a positive ER status had higher methylation levels of HIN-1 (mean of 29% in ER-positive tumors versus 9% in ER-negative tumors), whereas patients with a negative ER status had higher methylation levels of RIL (mean of 36% in ER-negative tumors versus 22% in ER-positive tumors). In addition, the PR status was positively associated with high HIN-1 methylation levels (p < 0.001) and negatively associated with high CDH13 methylation levels (p = 0.03). By defining the HR status as positive if the status of ERs and/or PRs is positive, the associations between the methylation markers and HRs are similar to those between the methylation markers and ERs. In multivariate logistic models, high levels of both HIN-1 and RIL methylation are independent predictors of the status of ERs and HRs, and high levels of HIN-1 and CDH13 methylation are independent predictors of PR status (Table (Table3).3). Higher methylation levels of HIN-1 were more likely to be associated with ER-positive than ER-negative tumors, whereas higher methylation levels of RIL were more likely to be associated with ER-negative than ER-positive tumors. Using multivariable logistic models, we have assessed the effect of each gene (LINE1, RIL, HIN-1, RASFF1A, CDH13, and RARβ2) on HR (adjusted for age), ER (adjusted for age and PR status), or PR (adjusted for age and ER status) status (Additional file 3). The results shown in Additional file 3 are similar to those in Table Table3.3. Among the markers studied, the higher methylation levels of HIN-1 were significantly correlated to a lack of HER-2/neu amplification (p = 0.014).
Table 3
Fitted multivariate logistic models for ER, PR, and HR expression
| Single gene model | Two-panels model | ||||
| Odds ratio (95% CI) | pvalue | Odds ratio (95% CI) | pvalue | ||
| ER | ER | ||||
| HIN-1 (× 10-1) | 0.399 (0.25 to 0.63) | 0.0001 | HIN-1/RASSF1A | 0.06 (0.01 to 0.25) | < 0.0001 |
| RIL (× 10-1) | 1.525 (1.11 to 2.10) | 0.0094 | CDH13/RIL | 10.14 (2.65 to 38.76) | 0.001 |
| PR | PR | ||||
| HIN-1 (× 10-1) | 0.551 (0.41 to 0.74) | 0.0001 | HIN-1/RASSF1A | 0.17 (0.06 to 0.46) | < 0.0001 |
| CDH13 (× 10-1) | 1.674 (1.05 to 2.67) | 0.0305 | CDH13/RIL | 3.51 (1.17 to 10.51) | 0.025 |
| HR | HR | ||||
| HIN-1 (× 10-1) | 0.319 (0.18 to 0.57) | 0.0001 | HIN-1/RASSF1A | 0.05 (0.01 to 0.23) | < 0.0001 |
| RIL (× 10-1) | 1.481 (1.08 to 2.03) | 0.0153 | CDH13/RIL | 10.18 (2.67 to 38.81) | 0.001 |
CI, confidence interval; ER, estrogen receptor; HR, hormone receptor; PR, progesterone receptor.
Correlation of two panels of methylation profiles to opposite hormone status
Because of a correlation between the methylation levels of several of the genes studied, we assembled four potential markers into two panels: HIN-1/RASSFIA and RIL/CDH13. A univariate analysis was performed and a multivariate logistic model was fitted to the two panels. The results of the univariate analysis for each panel indicated that the panels had a greater power to predict ER, PR, and HR status (Table (Table44 and Figure Figure3)3) than the methylation of individual genes (Table (Table3).3). Methylation of the HIN-1/RASSFIA panel was strongly correlated to positive ER, PR, and HR expression; 88% of HIN-1/RASSFIA-positive breast cancers were ER-positive and only 12% of breast cancers were ER-negative (p < 0.001). Conversely, methylation of the RIL/CDH13 panel was strongly correlated to negative ER, PR, and HR expression, because 69% of methylation-positive tumors were ER-negative and only 31% were ER-positive (p = 0.001).
Table 4
Univariate analysis of clinical variables by HIN-1/RASSF1A and RIL/CDH13panels, according to Fisher's exact test
| HIN-1/RASSF1A panel | RIL/CDH13 panel | |||||
| Number negative (percentage) | Number positive (percentage) | p value | Number negative (percentage) | Number positive (percentage) | p value | |
| Tumor size | 0.502 | 0.999 | ||||
| 1 | 11 (20) | 8 (24) | 14 (22) | 5 (19) | ||
| 2 | 20 (36) | 16 (47) | 25 (39) | 11 (42) | ||
| 3 | 12 (21) | 6 (18) | 13 (20) | 5 (19) | ||
| 4 | 13 (23) | 4 (12) | 12 (19) | 5 (19) | ||
| Tumor grade (BNG) | 0.015 | 0.162 | ||||
| 1 + 2 | 25 (45) | 25 (74) | 39 (61) | 11 (44) | ||
| 3 | 30 (55) | 9 (26) | 25 (39) | 14 (56) | ||
| Tumor stage | 0.16 | 0.745 | ||||
| 1 | 8 (14) | 7 (21) | 12 (19) | 3 (12) | ||
| 2 | 20 (36) | 17 (50) | 25 (39) | 12 (46) | ||
| 3 | 28 (50) | 10 (29) | 27 (42) | 11 (42) | ||
| Lymph-node metastasis | 0.558 | 0.78 | ||||
| PN0 | 24 (43) | 15 (44) | 26 (41) | 13 (50) | ||
| PN1 | 16 (29) | 12 (35) | 22 (34) | 6 (23) | ||
| PN2 | 5 (9) | 4 (12) | 6 (9) | 3 (12) | ||
| PN3 | 11 (20) | 3 (9) | 10 (16) | 4 (15) | ||
| Lymphatic invasion | 0.244 | 0.805 | ||||
| 1 | 25 (52) | 20 (67) | 32 (59) | 13 (54) | ||
| 2 | 23 (48) | 10 (33) | 22 (41) | 11 (46) | ||
| Vascular invasion | 0.346 | 0.623 | ||||
| 1 | 26 (54) | 20 (67) | 33 (61) | 13 (54) | ||
| 2 | 22 (46) | 10 (33) | 21 (39) | 11 (46) | ||
| ER status | < 0.001 | 0.001 | ||||
| Positive | 22 (42) | 30 (88) | 44 (72) | 8 (31) | ||
| Negative | 31 (58) | 4 (12) | 17 (28) | 18 (69) | ||
| PR status | < 0.001 | 0.033 | ||||
| Positive | 16 (30) | 24 (71) | 33 (54) | 7 (27) | ||
| Negative | 37 (70) | 10 (29) | 28 (46) | 19 (73) | ||
| HR status | < 0.001 | 0.001 | ||||
| Positive | 24 (45) | 31 (91) | 46 (75) | 9 (35) | ||
| Negative | 29 (55) | 3 (9) | 15 (25) | 17 (65) | ||
| HER-2/neu status | 0.041 | 0.999 | ||||
| Positive | 7 (14) | 0 (0) | 5 (9) | 2 (8) | ||
| Negative | 43 (86) | 30 (100) | 50 (91) | 23 (92) | ||
| Triple-negative | 0.001 | < 0.001 | ||||
| No | 33 (59) | 31 (91) | 53 (83) | 11 (42) | ||
| Yes | 23 (41) | 3 (9) | 11 (17) | 15 (58) | ||
BNG, Black's nuclear grade; ER, estrogen receptor; HR, hormone receptor; PR, progesterone receptor.
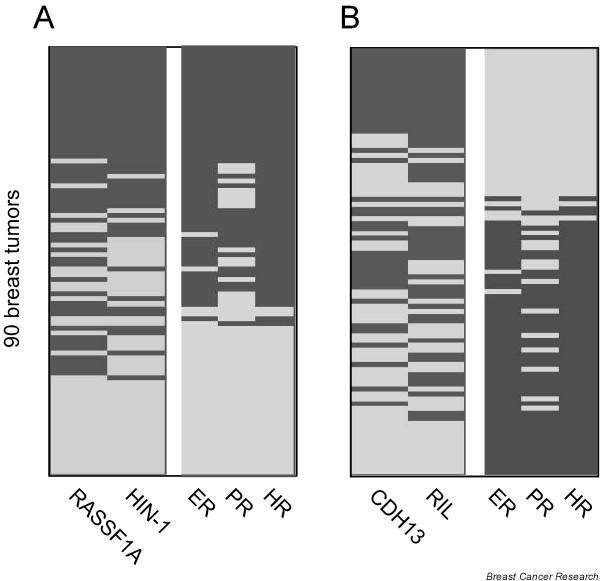
Comparison of two methylation panels with hormone receptor status. (a) Dichotomous heat map representing DNA methylation in the HIN-1/RASSF1A panel (left) and hormone status of each tumor (right). (b) Dichotomous heat map representing DNA methylation data in the RIL/CDH13 panel (left) and hormone receptor status of each tumor (right). Dark gray, positive; light gray, negative.
We summarize the fitted multivariate logistic models of the HIN-1/RASSF1A and RIL/CDH13 panels by categorical variables (Table (Table3).3). Specifically, a patient with a positive value in the RIL/CDH13 panel is ten times more likely to have an ER-negative tumor than a patient with a negative value. By contrast, a patient with a positive value in the HIN-1/RASSF1A panel is 17 times more likely to have an ER-positive tumor than a patient with a negative value.
Interestingly, we found that triple-negative breast cancers (in other words, those with negative ER, PR, and HER-2/neu status) were positively correlated to methylation of the RIL/CDH13 panel and negatively correlated to methylation of the HIN-1/RASSFIA panel (Table (Table4).4). In particular, 58% (15 out of 26) of triple-negative cancers exhibited methylation of the RIL/CDH13 panel, whereas 17% (11 out of 64) of triple-negative cancers failed to methylate in this panel (p < 0.001). Only 9% (3 out of 34) of triple-negative cancers exhibited HIN-1/RASSFIA panel methylation, whereas 41% (23 out of 56) of triple-negative cancers failed to methylate in this panel (p = 0.001). In addition, triple-negative cancers were significantly associated with advanced tumor grade (24% in grade 1 and 2 versus 76% in grade 3; p = 0.001).
Breast tumors with p53 mutations lacked methylation
We studied the mutation status of p53 in the 84 tumors in which DNA sequences were available for analysis. Overall, p53 mutations were found in 9 out of 84 (11%) cases. Seven cases with p53 mutations belonged to the group that lacked methylation in both panels; one case was positive in the HIN-1/RASSF1A panel and one case was positive in the RIL/CDH13 panel. In the methylation-negative group (both panels were negative), 18% (7 out of 38) of the specimens had p53 mutations; in the methylation-positive group (either panel or both panels were positive), only 4% (2 out of 46) of specimens had p53 mutation (p = 0.072 in Fisher's exact test). Interestingly, five of the cases with p53 mutations occurred in triple-negative (ER-, PR-, and HER2-negative) cancers.
Discussion
Although a large body of evidence has demonstrated that aberrant DNA methylation has an important role in breast carcinogenesis, variation in the data is still a problem. In this study, we used bisulfite pyrosequencing to quantitate the methylation of 12 known tumor-suppressor genes in breast cancers. To avoid contamination with normal tissues, we isolated breast cancer cells by microdissection and compared levels of methylation with those in normal breast epithelial cells from the same patients. Bisulfite pyrosequencing provided sensitive and reproducible measurements. The variation between the different assays was < 6%. Our data confirmed that RIL, HIN-1, RASSF1A, CDH13, and RARβ2 were frequently methylated in breast cancers but not in normal breast tissues. The other six genes were not highly methylated in breast cancers or methylated in either malignant and normal breast tissues; ARHI, as an imprinted gene, has a different methylation status. All the inconsistencies indicate that these seven genes are unsuitable for methylation profile studies.
In the past, methylation data have been correlated to clinical pathologic parameters, to clarify the role of methylation in breast carcinogenesis. Our most interesting finding was a correlation between gene methylation and the HR status. Methylation in breast cancer has already been connected to hormone regulation, but the correlation is not clear yet. Campan and colleagues [26] reviewed the DNA methylation profiles of breast, endometrial, ovarian, and proximal colon cancers but did not find evidence for global hormone-specific DNA methylation alterations. Widschwendter and colleagues [16] reported significant differences in the HR status between clusters of DNA methylation profiles. Their results suggested the existence of an interaction between DNA methylation and HR biology in breast cancer cells. In our own study, we found that the ER status was positively associated with the methylation of HIN-1 and RASSF1A but negatively correlated to the methylation of RIL. In addition, the PR status was positively associated with the methylation of HIN-1 and negatively associated with the methylation of CDH13. Moreover, if data from the methylation of individual genes were combined into two panels, methylation of the HIN-1/RASSF1A panel strongly predicted the expression of ERs, PRs, and HRs and methylation of the RIL/CDH13 panel strongly predicted the negative expression of ERs, PRs, and HRs.
The status of ERs, PRs, and HRs has been recognized as an important prognostic factor in patients with breast cancer, in addition to a predictive marker for the response to treatment with endocrine therapy. The presence of ERs and/or PRs is predictive of the response to treatment with the antiestrogen tamoxifen [27]. ER and PR expression patterns are heavily influenced by changes in the chromatin structure during transcription. Indeed, both the predominant mammalian DNA methyltransferase and histone deacetylases have crucial roles in maintaining transcriptionally repressive chromatin by forming suppressive complexes at replication foci [28]. Our current studies provide evidence that epigenetic changes are tightly connected with HR regulation in breast cancer.
Interestingly, ERs and PRs were not frequently methylated in breast cancers at levels comparable with those observed in the five tumor-suppressor genes. Consequently, alterations in methylation do not seem to have silenced these receptors directly. Increased methylation of certain genes was associated with the expression of ERs and PRs, suggesting that silencing of, at least some, tumor suppressors might affect the transcriptional regulation of HRs, possibly by upregulating HR co-stimulators. Conversely, the downregulation of ERs or PRs might relate to the increased expression of HR co-repressors. These hypotheses will be tested in future studies.
Recently, on the basis of the microarray profiling of invasive breast carcinomas, five distinct subtypes of tumors (luminal A, luminal B, normal breast-like, HER-2/neu overexpressing, and basal) associated with different clinical outcomes have been identified [29,30]. The basal subtype is associated with poor clinical outcomes and the subtype observed in BRCA1-related breast cancers. All basal-like tumors tested in the current study were triple-negative (that is to say, negative for ER, PR, and HER-2/neu expression), poorly differentiated, and high-grade [29,30]. An early study of HIN-1 methylation revealed lower frequencies of HIN-1 promoter methylation in sporadic breast tumors with a 'BRCA1-like' histopathologic phenotype [31]. In the 90 cases we tested, 76% of the 26 triple-negative tumors were high-grade. Interestingly, we found these tumors to be positively associated with methylation of the RIL/CDH13 panel but negatively correlated to methylation of the HIN-1/RASSFIA panel. Our results suggested that methylation reflected by the RIL/CDH13 panel might have a role in the phenotype of basal-like breast tumors.
Recent advances in molecular biology have revealed numerous genetic alterations involved in breast tumorigenesis; p53 mutation is among the most important of those alterations, and studies have reported that p53 mutations are strongly associated with poor prognoses in breast cancer [32]. In colorectal cancer, methylation phenotypes define two groups with significantly different genetic lesions (K-RAS and p53 mutations) [33]. In this study, we have identified 11% of breast tumors that had p53 mutations. Equivalent to the observation in colorectal cancers, most cases with p53 mutations belong to the group that lack methylation, suggesting that p53 mutation and methylation can be two distinct mechanisms that deactivate tumor-suppressor genes in breast cancer. The correlation of p53 mutation to hypomethylation is also likely to be owing to the fact that p53 mutations and hypomethylation both occur in basal-like triple-negative breast cancers.
The methylation of multiple tumor-suppressor genes has been correlated to poor prognoses in cancers [34]. The use of epigenetic information has shown promise in the identification of patients with gastrointestinal cancers who have poor prognoses [35]. In esophageal carcinoma, the cancers with frequent methylation had significantly poorer survival, and methylation was a better predictor of outcome than the disease stage or patient age [36]. Methylation, as a prognosis factor, has also been described in bladder cancer [37], head and neck cancer [38], ovarian cancer [39], and acute lymphocytic leukemia [40]. In our study, we did not have sufficient survival data to correlate methylation to prognosis (most patients were treated in 2004 and 2005), but we did confirm that the methylation of multiple tumor-suppressor genes was an early event in the subgroups of patients with breast cancer. To understand whether methylation is a prognostic factor in breast cancer, we must conduct studies with longer follow-up times to obtain adequate survival data.
Finally, epigenetic therapy, including the use of demethylating agents (for example, 5-aza-cytidine and 5-aza-2'-deoxycytidine) and histone deacetylase inhibitors (for example, suberoylanilide hydroxamic acid and valproic acid), is currently in clinical trials for myelodysplastic syndrome, leukemia, ovarian cancer, and lung cancer. Previous reports indicate that the 'cross-talk' between inhibitors of DNA methylation and histone deacetylase can result in synergistic activation of silent tumor-suppressor genes in breast cancer. These observations suggest that combination of these inhibitors might be an effective form of epigenetic therapy for breast cancer. It is possible that epigenetic therapy will have a role in the management of breast cancer. The information from this study of methylation profiles will be useful for the study of the biology of breast cancer and refining epigenetic therapy.
Conclusion
By pyrosequencing methylation analysis, we have examined the methylation profile of 90 normal/breast cancer paired samples. Our data indicated that 5 out of 12 tumor-suppressor genes were frequently methylated in breast cancers but not the normal breast. We have defined two panels (HIN-1/RASSFIA and RIL/CDH13) of methylation profiles, which correlated, either positively or negatively, to HR status.
Abbreviations
BNG = Black's nuclear grade; CI, confidence interval; COBRA = combined bisulfite restriction analysis; ER = estrogen receptor; FISH = fluorescence in situ hybridization; H & E = hematoxylin and eosin; HR = hormone receptor; MCA = methylated CpG island amplification; MSP = methylation-specific PCR; PCR = polymerase chain reaction; PR = progesterone receptor; UTR = untranslated region.
Authors' contributions
WF, LS, XH, and JJ carried out the methylation assays and helped prepare the manuscript. SH and MH carried out the p53 mutation assays. SW and YS performed the statistical analysis. AAS and KKH provided tissues and clinical data. DGR and JL provided pathologic supports for tissue microdisection. YY, JJI, and RCB conceived the study and prepared the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Primers and PCR conditions for pyrosequencing assays.
Methylation levels of 12 tumor-suppressor genes, ERα, PGRB, and LINE1 in six breast cancer cell lines and two normal breast epithelial cell cultures.
Multivariable logistic models for assessing effect of each gene (LINE1, RIL, HIN-1, RASFF1A, CDH13, and RARβ2) on HR (adjusted for age), ER (adjusted for age and PR status), or PR (adjusted for age and ER status) status.
Acknowledgements
This work was supported by National Institutes of Health grants CA 80957 and CA 64602 and National Foundation for Cancer Research grant LF 2004-00009224HM.
References
- Yang X, Yan L, Davidson NE. DNA methylation in breast cancer. Endocr Relat Cancer. 2001;8:115–127. 10.1677/erc.0.0080115. [Abstract] [CrossRef] [Google Scholar]
- Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002;21:5462–5482. 10.1038/sj.onc.1205606. [Abstract] [CrossRef] [Google Scholar]
- Yuan J, Luo RZ, Fujii S, Wang L, Hu W, Andreeff M, Pan Y, Kadota M, Oshimura M, Issa J-P, et al. Aberrant methylation and silencing of ARHI, an imprinted tumor suppressor gene whose function is lost in breast cancer. Cancer Res. 2003;63:4174–4180. [Abstract] [Google Scholar]
- Fujii S, Luo RZ, Yuan J, Kadota M, Oshimura M, Dent SR, Kondo Y, Issa JP, Bast RC, Jr, Yu Y. Reactivation of the silenced and imprinted alleles of ARHI is associated with increased histone H3 acetylation and decreased histone H3 lysine 9 methylation. Hum Mol Genet. 2003;12:1791–1800. 10.1093/hmg/ddg204. [Abstract] [CrossRef] [Google Scholar]
- Dammann R, Yang G, Pfeifer GP. Hypermethylation of the CpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001;61:3105–3109. [Abstract] [Google Scholar]
- Krop IE, Sgroi D, Porter DA, Lunetta KL, LeVangie R, Seth P, Kaelin CM, Rhei E, Bosenberg M, Schnitt S, et al. HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc Natl Acad Sci USA. 2001;98:9796–9801. 10.1073/pnas.171138398. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fackler MJ, McVeigh M, Evron E, Garrett E, Mehrotra J, Polyak K, Sukumar S, Argani P. DNA methylation of RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107:970–975. 10.1002/ijc.11508. [Abstract] [CrossRef] [Google Scholar]
- Murata H, Khattar NH, Kang Y, Gu L, Li GM. Genetic and epigenetic modification of mismatch repair genes hMSH2 and hMLH1 in sporadic breast cancer with microsatellite instability. Oncogene. 2002;21:5696–5703. 10.1038/sj.onc.1205683. [Abstract] [CrossRef] [Google Scholar]
- Ferguson AT, Evron E, Umbricht CB, Pandita TK, Chan TA, Hermeking H, Marks JR, Lambers AR, Futreal PA, Stampfer MR, Sukumar S. High frequency of hypermethylation at the 14-3-3 sigma locus leads to gene silencing in breast cancer. Proc Natl Acad Sci USA. 2000;97:6049–6054. 10.1073/pnas.100566997. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Du Y, Carling T, Fang W, Piao Z, Sheu JC, Huang S. Hypermethylation in human cancers of the RIZ1 tumor suppressor gene, a member of a histone/protein methyltransferase superfamily. Cancer Res. 2001;61:8094–8099. [Abstract] [Google Scholar]
- Di Vinci A, Perdelli L, Banelli B, Salvi S, Casciano I, Gelvi I, Allemanni G, Margallo E, Gatteschi B, Romani M. p16(INK4a) promoter methylation and protein expression in breast fibroadenoma and carcinoma. Int J Cancer. 2005;114:414–421. 10.1002/ijc.20771. [Abstract] [CrossRef] [Google Scholar]
- Hu XC, Loo WT, Chow LW. E-cadherin promoter methylation can regulate its expression in invasive ductal breast cancer tissue in Chinese woman. Life Sci. 2002;71:1397–1404. 10.1016/S0024-3205(02)01843-X. [Abstract] [CrossRef] [Google Scholar]
- Khan S, Kumagai T, Vora J, Bose N, Sehgal I, Koeffler PH, Bose S. PTEN promoter is methylated in a proportion of invasive breast cancers. Int J Cancer. 2004;112:407–410. 10.1002/ijc.20447. [Abstract] [CrossRef] [Google Scholar]
- Wei M, Grushko TA, Dignam J, Hagos F, Nanda R, Sveen L, Xu J, Fackenthal J, Tretiakova M, Das S, Olopade OI. BRCA1 promoter methylation in sporadic breast cancer is associated with reduced BRCA1 copy number and chromosome 17 aneusomy. Cancer Res. 2005;65:10692–10699. 10.1158/0008-5472.CAN-05-1277. [Abstract] [CrossRef] [Google Scholar]
- Mehrotra J, Vali M, McVeigh M, Kominsky SL, Fackler MJ, Lahti-Domenici J, Polyak K, Sacchi N, Garrett-Mayer E, Argani P, Sukumar S. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin Cancer Res. 2004;10:3104–3109. 10.1158/1078-0432.CCR-03-0118. [Abstract] [CrossRef] [Google Scholar]
- Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. 10.1158/0008-5472.CAN-03-3852. [Abstract] [CrossRef] [Google Scholar]
- Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, Widschwendter M. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. 10.1158/0008-5472.CAN-05-2508. [Abstract] [CrossRef] [Google Scholar]
- Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. [Abstract] [Google Scholar]
- Tost J, Dunker J, Gut IG. Analysis and quantification of multiple methylation variable positions in CpG islands by Pyrosequencing. Biotechniques. 2003;35:152–156. [Abstract] [Google Scholar]
- Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, Talpaz M, Garcia-Manero G, Kantarjian HM. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. 10.1200/JCO.2005.11.981. [Abstract] [CrossRef] [Google Scholar]
- Yu YH, Xu FJ, Peng HQ, Fang XJ, Zhao SL, Li Y, Cuevas B, Kuo W-L, Gray JW, Siciliano M, et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci USA. 1999;96:214–219. 10.1073/pnas.96.1.214. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Protocol used at National Cancer Institute (NCI) and related information http://cgap-mf.nih.gov/Protocols/Microdissection/Overview.html
- Boumber YA, Kondo Y, Chen X, Shen L, Gharibyan V, Konishi K, Estey E, Kantarjian H, Garcia-Manero G, Issa JP. RIL, a LIM gene on 5q31, is silenced by methylation in cancer and sensitizes cancer cells to apoptosis. Cancer Res. 2007;67:1997–2005. 10.1158/0008-5472.CAN-06-3093. [Abstract] [CrossRef] [Google Scholar]
- Estecio MR, Yan PS, Ibrahim AE, Tellez CS, Shen L, Huang TH, Issa JP. High-throughput methylation profiling by MCA coupled to CpG island microarray. Genome Res. 2007 Sep 4. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. 10.1093/nar/gnh032. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Campan M, Weisenberger DJ, Laird PW. DNA methylation profiles of female steroid hormone-driven human malignancies. Curr Top Microbiol Immunol. 2006;310:141–178. [Abstract] [Google Scholar]
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. 10.1200/JCO.2003.09.099. [Abstract] [CrossRef] [Google Scholar]
- Yan L, Yang X, Davidson NE. Role of DNA methylation and histone acetylation in steroid receptor expression in breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:183–192. 10.1023/A:1011308707512. [Abstract] [CrossRef] [Google Scholar]
- Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19:617–621. 10.1038/modpathol.3800570. [Abstract] [CrossRef] [Google Scholar]
- Tischkowitz MD, Foulkes WD. The basal phenotype of BRCA1-related breast cancer: past, present and future. Cell Cycle. 2006;5:963–967. [Abstract] [Google Scholar]
- Krop I, Maguire P, Lahti-Domenici J, Lodeiro G, Richardson A, Johannsdottir HK, Nevanlinna H, Borg A, Gelman R, Barkardottir RB, et al. Lack of HIN-1 methylation in BRCA1-linked and "BRCA1-like" breast tumors. Cancer Res. 2003;63:2024–2027. [Abstract] [Google Scholar]
- Feki A, Irminger-Finger I. Mutational spectrum of p53 mutations in primary breast and ovarian tumors. Crit Rev Oncol Hematol. 2004;52:103–116. 10.1016/j.critrevonc.2004.07.002. [Abstract] [CrossRef] [Google Scholar]
- Toyota M, Ohe-Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA. 2000;97:710–715. 10.1073/pnas.97.2.710. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Issa JP. Methylation and prognosis: of molecular clocks and hypermethylator phenotypes. Clin Cancer Res. 2003;9:2879–2881. [Abstract] [Google Scholar]
- Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004;127:1578–1588. 10.1053/j.gastro.2004.09.007. [Abstract] [CrossRef] [Google Scholar]
- Brock MV, Gou MG, Akiyama Y, Muller A, Wu TT, Montgomery E, Deasel M, Germonpre P, Rubinson L, Heitmiller RF, et al. Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res. 2003;9:2912–2919. [Abstract] [Google Scholar]
- Maruyama R, Toyooka S, Toyooka KO, Harada K, Virmani AK, Zochbauer-Muller S, Farinas AJ, Vakar-Lopez F, Minna JD, Sagalowsky A, et al. Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features. Cancer Res. 2001;61:8659–8663. [Abstract] [Google Scholar]
- Ogi K, Toyota M, Ohe-Toyota M, Tanaka N, Noguchi M, Sonoda T, Kohama G, Tokino T. Aberrant methylation of multiple genes and clinicopathological features in oral squamous cell carcinoma. Clin Cancer Res. 2002;8:3164–3171. [Abstract] [Google Scholar]
- Wei SH, Chen CM, Strathdee G, Harnsomburana J, Shyu CR, Rahmatpanah F, Shi H, Ng SW, Yan PS, Nephew KP, et al. Methylation microarray analysis of late-stage ovarian carcinomas distinguishes progression-free survival in patients and identifies candidate epigenetic markers. Clin Cancer Res. 2002;8:2246–2252. [Abstract] [Google Scholar]
- Shen L, Toyota M, Kondo Y, Obata T, Daniel S, Pierce S, Imai K, Kantarjian HM, Issa JP, Garcia-Manero G. Aberrant DNA methylation of p57KIP2 identifies a cell cycle regulatory pathway with prognostic impact in adult acute lymphocytic leukemia. Blood. 2003;101:4131–4136. 10.1182/blood-2002-08-2466. [Abstract] [CrossRef] [Google Scholar]
Articles from Breast Cancer Research : BCR are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/bcr1762
Read article for free, from open access legal sources, via Unpaywall:
https://breast-cancer-research.biomedcentral.com/counter/pdf/10.1186/bcr1762
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Droplet digital PCR analysis of CDH13 methylation status in Slovak women with invasive ductal breast cancer.
Sci Rep, 14(1):14700, 26 Jun 2024
Cited by: 0 articles | PMID: 38926485 | PMCID: PMC11208553
Omics Technologies Improving Breast Cancer Research and Diagnostics.
Int J Mol Sci, 24(16):12690, 11 Aug 2023
Cited by: 4 articles | PMID: 37628869 | PMCID: PMC10454385
Review Free full text in Europe PMC
Aberrant DNA Methylation, Expression, and Occurrence of Transcript Variants of the ABC Transporter ABCA7 in Breast Cancer.
Cells, 12(11):1462, 24 May 2023
Cited by: 4 articles | PMID: 37296582 | PMCID: PMC10252461
DNA methylation in peripheral blood leukocytes for the association with glucose metabolism and invasive breast cancer.
Clin Epigenetics, 15(1):23, 13 Feb 2023
Cited by: 1 article | PMID: 36782224 | PMCID: PMC9926571
An Optimized CoBRA Method for the Microfluidic Electrophoresis Detection of Breast Cancer Associated RASSF1 Methylation.
BioTech (Basel), 12(1):7, 09 Jan 2023
Cited by: 4 articles | PMID: 36648833 | PMCID: PMC9844460
Go to all (99) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Methylation of HIN-1, RASSF1A, RIL and CDH13 in breast cancer is associated with clinical characteristics, but only RASSF1A methylation is associated with outcome.
BMC Cancer, 12:243, 13 Jun 2012
Cited by: 36 articles | PMID: 22695491 | PMCID: PMC3476972
Tumor suppressor genes are frequently methylated in lymph node metastases of breast cancers.
BMC Cancer, 10:378, 20 Jul 2010
Cited by: 35 articles | PMID: 20642860 | PMCID: PMC2914707
Aberrant methylation of RASSF1A is associated with poor survival in Tunisian breast cancer patients.
J Cancer Res Clin Oncol, 136(2):203-210, 06 Aug 2009
Cited by: 24 articles | PMID: 19657672
Clinicopathological significance and potential drug target of CDH1 in breast cancer: a meta-analysis and literature review.
Drug Des Devel Ther, 9:5277-5285, 18 Sep 2015
Cited by: 13 articles | PMID: 26425077 | PMCID: PMC4583122
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: P01 CA064602
Grant ID: R01 CA080957
Grant ID: CA 80957
Grant ID: CA 64602

 1
1