Abstract
Free full text

Exogenous Pathogen and Plant 15-Lipoxygenase Initiate Endogenous Lipoxin A4 Biosynthesis
Abstract
Lipoxin A4 (LXA4) is a potent endogenous lipoxygenase-derived eicosanoid with antiinflammatory and proresolving properties. Supraphysiological levels of LXA4 are generated during infection by Toxoplasma gondii, which in turn reduces interleukin (IL) 12 production by dendritic cells, thus dampening Th1-type cell-mediated immune responses and host immunopathology. In the present work, we sought evidence for the structural basis of T. gondii's ability to activate LXA4 biosynthesis. Proteomic analysis of T. gondii extract (soluble tachyzoite antigen [STAg]), which preserves the immunosuppressive and antiinflammatory activity of the parasite, yielded several peptide matches to known plant lipoxygenases. Hence, we incubated STAg itself with arachidonic acid and found using LC-UV-MS-MS–based lipidomics that STAg produced both 15-HETE and 5,15-diHETE, indicating that T. gondii carries 15-lipoxygenase activity. In addition, T. gondii tachyzoites (the rapidly multiplying and invasive stage of the parasite) generated LXA4 when provided with arachidonic acid. Local administration of a plant (soybean) lipoxygenase itself reduced neutrophilic infiltration in murine peritonitis, demonstrating that 15-lipoxygenase possesses antiinflammatory properties. Administration of plant 15-lipoxygenase generated endogenous LXA4 and mimicked the suppression of IL-12 production by splenic dendritic cells observed after T. gondii infection or STAg administration. Together, these results indicate that 15-lipoxygenase expressed by a pathogen as well as exogenously administered 15-lipoxygenase can interact with host biosynthetic circuits for endogenous “stop signals” that divert the host immune response and limit acute inflammation.
Introduction
Arachidonic acid is the substrate for the biosynthesis of a range of bioactive eicosanoids that include the prostaglandins, thromboxane, leukotrienes, and lipoxins (1). Lipoxins are trihydroxy eicosanoids that contain a conjugated tetraene structure that arises from two sequential lipoxygenase-catalyzed oxygenations (2). Lipoxins are potent antiinflammatory lipid mediators that are formed locally in settings of heterotypic cell–cell interactions, such as occurring during inflammation, and are considered endogenous “stop signals” that limit the accumulation and action of neutrophils, and are “proresolution” (2) (for review see reference 3). Lipoxin A4 (LXA4; 5S,6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid) inhibits PMN adherence, migration, degranulation, and superoxide anion generation, as well as eosinophil migration and lymphocyte activation (4–6), and has potent actions on cytokine formation and release (7). Although endogenous lipoxygenases are recognized for their importance in lipoxin biosynthesis, the interaction of exogenous lipoxygenases that may be present in microbes and/or pathogens or other exogenous biological sources with endogenous lipoxin biosynthesis has not been investigated.
Along these lines, we recently found that the apicomplexan protozoan Toxoplasma gondii upon infection in mice markedly activates LXA4 biosynthesis. Inoculation with T. gondii tachyzoites (the rapidly multiplying and invasive stage of the parasite) or administration of parasite extracts (denoted STAg) induces an early splenic dendritic cell activation, which is characterized by a T cell–independent induction of IL-12 production that involves stimulation of the chemokine receptor CCR-5 on dendritic cells (8). This early innate immune response wanes within 1 d and is followed by a state of dendritic cell “paralysis,” in which dendritic cells lose their ability to generate IL-12 for several days, and restimulation with T. gondii during this period cannot trigger IL-12 generation (9).
T. gondii–induced suppression of dendritic cell function is mediated, in part, via down-regulation of dendritic cell CCR5 by the T. gondii–triggered formation of remarkably high levels of LXA4 production in vivo (10, 11). An extract of T. gondii, STAg, possesses all the biological activity necessary for inducing in vivo dendritic cell paralysis (12). Apparently, specific T. gondii components are present in STAg that are able to dampen dendritic cell IL-12 generation via dramatic stimulation of LXA4 that, in turn, mediates CCR5 down-regulation. To identify the principles enabling T. gondii to specifically activate formation of endogenous LXA4, we performed a proteomic analysis of STAg with the objective of characterizing putative polypeptide ligands and/or recognition motifs expressed by T. gondii that might be responsible for the activation of lipoxin biosynthesis during infection. Instead, the present experiments reveal that T. gondii itself possesses 15-lipoxygenase activity that can contribute to the formation of endogenous LXA4 derived from host precursors. Moreover, the recognition that an exogenous 15-lipoxygenase can activate endogenous lipoxin biosynthesis was further supported by the finding that administration of plant 15-lipoxygenase is bioactive in an acute inflammatory locus, stimulating LXA4 formation and limiting leukocyte recruitment.
Materials and Methods
Parasites.
T. gondii tachyzoites (RH88 strain) were cultured in human fibroblasts using Mycoplasma-free conditions. The parasites were isolated by passage over a glass wool column and washed twice in cold PBS before being resuspended at 108 organisms/ml in PBS. A protein-rich aqueous extract, denoted soluble tachyzoite antigen (STAg), was prepared from sonicated tachyzoites as described previously (12). In brief, purified tachyzoites were pelleted and sonicated (Misonix) with five pulses of 30 s each. The pellets were resuspended in Dulbecco's phosphate buffered saline (DPBS) without divalent cations, pH 7.4, and centrifuged at 35,000 rpm (100,000 g) for 45 min, and the supernatant was collected and assayed for its protein content.
Proteomic LC-MS-MS Analysis.
A tryptic digest of STAg was prepared by incubating 50 μg STAg protein with 2.5 μg of modified sequencing grade trypsin (Promega) in 50 mM ammonium bicarbonate for 12 h at 27°C. The digest was taken to dryness under reduced pressure by vacuum centrifugation (SpeedVac; Savant), resuspended in ultrapure water, and stored at −80°C until analysis. For LC-MS analysis, the peptides were suspended in mobile phase A and injected using a Rheodyne 7725i injector port. The tryptic peptides were separated by liquid chromatography on a Jupiter 50 × 2.0 mm reversed phase column at a flow rate of 200 μl/min delivered by an HPLC pump (SpectraPhysics P4000; Finnigan MAT). The LC gradient used was: 0–1 min 100% A, 1–35 min 0–60% B, 35–40 min 60–100% B, and 40–45 min 100% B, and the mobile phases were as follows: A, H2O/acetonitrile/formic acid/TFA 95:5:0.1:0.02 (vol/vol/vol); and B, H2O/acetonitrile/formic acid/TFA 10:90:0.1:0.02 (vol/vol/vol).
A capillary LC system was used as well for some analyses. A tryptic digest corresponding to 2 μg STAg protein was suspended in acetonitrile/water/formic acid/TFA 2:98:0.1:0.005 (vol/vol/vol/vol), loaded onto a Peptide MacroTrap (Michrom Bioresources), and placed in the injection loop of a Rheodyne 7725i injector port just before the column. The adsorbed peptides were washed with 30 μl of mobile phase A and eluted off the trap onto the separating column during the LC run. Tryptic peptides were separated using a reversed phase column (PepMap; ID 300 μm; length: 15 cm, 3 μm particle size; LC Packings Dionex, equipped with a thermostat set at 30°C) at a flow rate of 4 μl/min, which was obtained with a precolumn flow splitter (Accurate; LC Packings Dionex). The LC gradient used was: 0–70 min 2–60% B, 70–75 min 60–100% B, and 75–80 min 100% B, and the mobile phases were as follows: A, H2O/acetonitrile/formic acid 95:5:0.1 (vol/vol/vol); and B, H2O/acetonitrile/formic acid 10:90:0.1 (vol/vol/vol).
The mass, charge, and tandem mass spectra of singly and multiply positively charged peptide ions with a minimum ion intensity of 104 were determined using dynamic exclusion with a LCQ Classic electrospray ionization ion trap mass spectrometer, or an LCQ Advantage electrospray ionization ion trap mass spectrometer (ThermoFinnigan). The mass spectrometers were tuned with synthetic angiotensin II (Sigma-Aldrich) before running a set of samples. The column eluate was sprayed from a fused silica capillary (ID 100 μm; OD 190 μm) using sheath and auxiliary nitrogen gas. The temperature of the heated capillary was 180°C. Peptide fingerprinting was performed with Sequest (BioWorks version 2.1) or TurboSequest (BioWorks version 3.1; ThermoFinnigan), using the National Center for Biotechnology Information nr.fasta protein database. Tandem MS matching was performed, taking into account the presence of tryptic cleavage sites, and possible methionine residue oxidation. Cross-species peptide mapping was also performed with the consideration that a tryptic cleavage site would not necessarily have to be present. The quality of the measured and theoretical tandem mass spectra matches was inspected.
Eicosanoid Lipidomics.
To assess lipoxygenase activity in STAg, 15 μg STAg total protein extract was added to 0.5 ml DPBS containing 0.88 mM CaCl2 and 0.49 mM MgCl2, pH 7.4, with or without 30 μg arachidonic acid (Cayman Chemical). After incubating for 30 min at room temperature, two volumes of ice-cold methanol were added, and samples were stored at −80°C. The samples were extracted using solid phase extraction (reversed phase Extract Clean/RC; Alltech), as described previously (10, 13). In brief, the eicosanoids generated by STAg were identified using liquid chromatography–photodiode array detector–tandem mass spectrometry (ThermoFinnigan). Reverse-phase LC was conducted on a Discovery C18 (100 × 2 mm, particle size 5 μm; Supelco) column at a flow rate of 0.2 ml/min. The mobile phase consisted of methanol/water/acetic acid (65:35:0.01 vol/vol/vol) with an isocratic elution for 8 min, followed by a linear gradient to 100% methanol from 8 to 30 min, and isocratic elution at 100% methanol from 30 to 38 min. Tandem mass spectra were recorded with an electrospray ionization mass spectrometer (LCQ; ThermoFinnigan). The characteristic parent ions for the arachidonic acid–derived lipoxygenase products monoHETE (m/z = 319) and diHETEs (m/z = 335) were monitored, and tandem mass spectra of these ions were recorded. Ions of diagnostic value or signature ions for 5-HETE, 12-HETE, 15-HETE, and 5,15-diHETE were as follows: 5-HETE, m/z = 319 [M-H], 301, 275, 257, 203, and 119; 12-HETE, m/z = 319 [M-H], 301, 275, 257, 207, 179, and 163; 15-HETE, m/z = 319 [M-H], 301, 275, 257, 219, and 204; and 5,15-diHETE, m/z = 335, 317, 299, 291, 273, 253, 235, and 217 (Fig. 2; compare with reference 10).
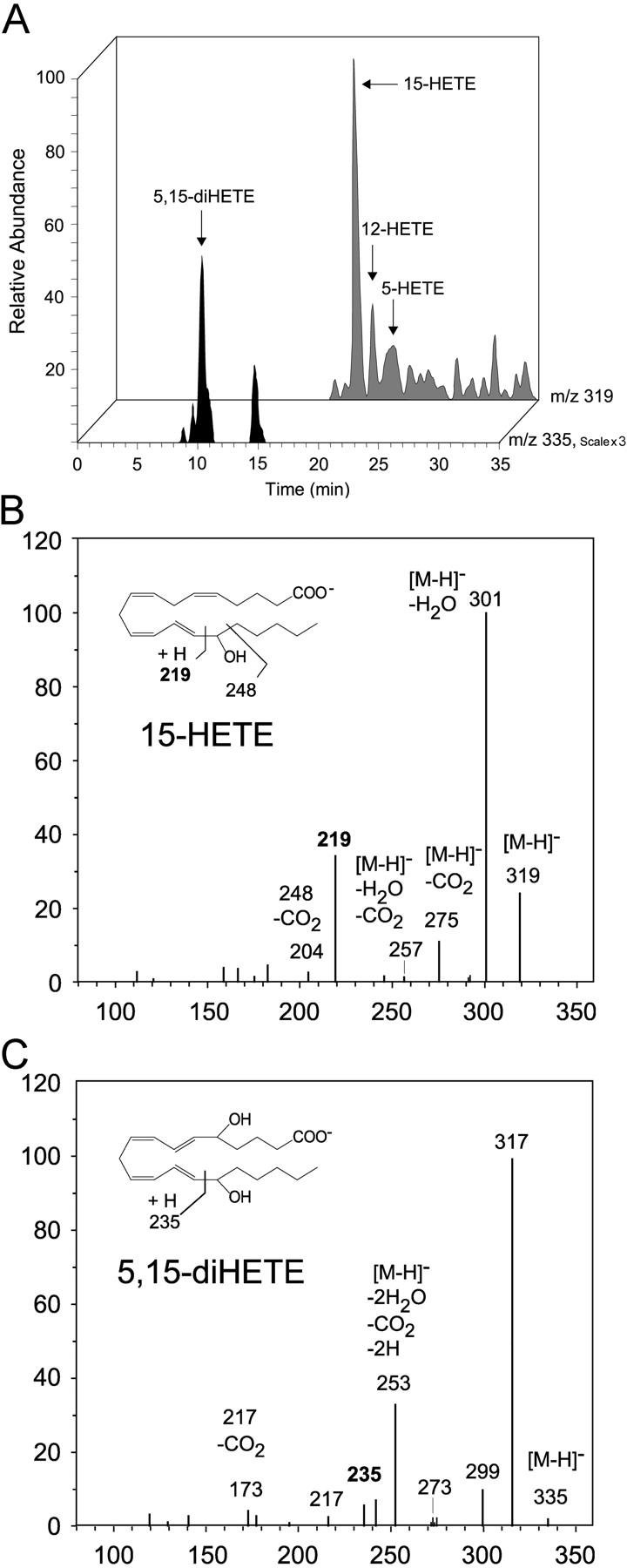
Lipidomic analysis. Profile of lipoxygenase products formed by STAg extracts. (A) Selected ion chromatogram for 15-HETE, 12-HETE, 5-HETE, and 5,15-diHETE formed by 15 μg STAg incubated with 30 μg arachidonic acid in 0.5 ml DPBS, pH 7.4, for 30 min (see Materials and Methods). Tandem mass spectra for the lipoxygenase products 15-HETE (B) and 5,15-diHETE (C). Results are representative of three independent incubations.
Analysis of 15-HETE by Enzyme-linked Immunosorbent Assay.
For incubations with STAg and inhibitors, 15 μg STAg total protein extract was added to 0.5 ml DPBS containing 0.88 mM CaCl2 and 0.49 mM MgCl2, pH 7.4, at 37°C, with a lipoxygenase inhibitor (50 μM ketoconazole; Sigma-Aldrich), 10 μM esculetin (Sigma-Aldrich), 10 μM baicalein (Sigma-Aldrich), or 10 μM nordihydroguaiaretic acid (BIOMOL Research Laboratories, Inc.), a cytochrome P450-inhibitor (SKF 525A; 100 μM; BIOMOL Research Laboratories, Inc.) and 100 μM 8-methoxypsoralen (BIOMOL Research Laboratories, Inc.), or vehicle (0.1% ethanol; references 14, 15). After 5 min, arachidonic acid was added to a final concentration of 20 μM. The incubations were stopped after 30 min by rapid freezing in a dry ice/isopropanol bath, and samples were stored at −80°C until analysis. 15-HETE was measured with a 15S-HETE ELISA (AssayDesigns). Stereospecificity of the 15-HETE 15-carbon alcohol was determined by coupling 15S-ELISA and LC-MS quantitative analysis; 15-HETE that was formed in identical and simultaneous incubations of STAg with arachidonic acid was quantitated by LC-MS analysis, and compared with the amount of 15-HETE measured by ELISA using standard curves for either 15S-HETE and 15R-HETE. An additional standard curve for synthetic 15R-HETE (Cayman Chemical) was made, which displayed ~10% cross-reactivity compared with 15S-HETE.
Tachyzoite Incubations.
Isolated culture-derived tachyzoites (107 organisms/well in RPMI 1640 containing 1% BSA) were incubated for 30 min at 37°C in the presence or absence of 20 μM arachidonic acid (Sigma-Aldrich) and/or 5 μM calcium ionophore A23187 (Sigma-Aldrich). The incubations were stopped by adding 2 ml/well of ice-cold methanol and stored at −80°C until assayed. LXA4 was measured by ELISA (Neogen), after evaporation of the methanol and dilution of samples with ELISA assay buffer.
Native Isoelectric Focusing and In-gel Lipoxygenase Staining.
Soybean lipoxygenase (Glycine max; Type IV, 360.000 U/mg; 1 U defined as the amount of enzyme that oxygenates 0.11 nmol linoleic acid/min at 25°C at pH 9.0; Sigma-Aldrich) was suspended in native rehydration buffer composed of 0.1% Triton X-100 and 0.2% ampholytes (BioLyte pH range 3–10; Bio-Rad Laboratories). Immobilized pH gradient strips (pH range 3–10, 11 cm; Bio-Rad Laboratories) were rehydrated with the lipoxygenase-containing native rehydration buffer for 12 h at 10°C. The protein was focused in a Protean isoelectric focusing unit (Bio-Rad Laboratories) at 10°C by applying a linear voltage gradient that increased during 1 h to 250 V, for another 2.5 h to 8,000 V, followed by an 112-min focusing period at 8,000 V. The gel was thereafter placed in a solution containing o-dianisidine and 90 μg/ml arachidonic acid in 50 mM potassium phosphate buffer, pH 7.4. Lipoxygenase activity was visualized with the development of an orange–brown band within the gel.
Zymosan A–stimulated Peritonitis.
To determine whether 15-lipoxygenase itself carries antiinflammatory activity, its action was determined in a murine model of acute neutrophilic inflammation, zymosan A-stimulated peritonitis (13). All animal studies were approved by and performed in accordance with guidelines provided by the Institutional Review Board. Peritonitis was induced in mice (male, FVB, 20–25 g) by intraperitoneal administration of 1 mg zymosan A (Sigma-Aldrich) in 1 ml of sterile saline. Soybean lipoxygenase was administered intraperitoneally 5 min before zymosan A. 2 h after zymosan administration, the mice were killed using isoflurane inhalation, and peritoneal exudate cells were harvested by lavaging the peritoneum with 5 ml DPBS without calcium and magnesium. Exudate cells were enumerated by bright-field microscopy using a Neubauer chamber. For LXA4 determinations, 6 ml of ice-cold methanol was added to 3 ml of peritoneal exudate lavage, mixed, and stored at −80°C until analysis. The samples were extracted using solid phase extraction (as aforementioned) and quantitated using ELISA for LXA4 (Neogen).
Dendritic Cell Function In Vivo.
For in vivo analysis of dendritic cell function, mice (C57Bl/6J) were injected i.p. with sterile DPBS (control), STAg (1 μg/mouse), or with several concentrations of soybean 15-lipoxygenase (102, 103, 104, or 105 U/mouse) in a total volume of 0.5 ml sterile DPBS. 18 h later, the animals received a second injection with DPBS, STAg, or soybean 15-lipoxygenase. 6 h later, spleens were removed and digested with Liberase CI (Roche Biochemicals). Dendritic cells were semi-purified by dense BSA gradient as described previously (10, 16). After 12 h incubation in media alone, culture supernatants were harvested and assayed for IL-12 p40 levels by ELISA, as described previously (8, 10).
Results
Proteomic Analysis of T. gondii Protein Extract (STAg).
STAg injection and live T. gondii infection induce LXA4, 5,15-diHETE, and 15-HETE from endogenous sources at very high levels that appear to be two orders of magnitude greater than those produced during resolution of inflammation (10, 17). To address the possibility that specific T. gondii products evoked LXA4 biosynthesis to suppress host defense, we performed a proteomic analysis of a protein extract of T. gondii (STAg). STAg was digested with trypsin, tryptic peptides were separated by liquid chromatography (Fig. 1 A), and peptide masses and charges were determined by mass spectrometry. Using peptide fingerprinting, 11 known T. gondii proteins were identified (Fig. 1 B). Protein coverage ranged from 4.5 (putative translation initiation factor 5A2) to 44% (lactate dehydrogenase).

LC-MS-MS analysis of tryptic peptides from T. gondii protein extract (STAg). (A) Arrows indicate the position of the following protein peptide fragments: A, lactate dehydrogenase; B, enolase; C, dense granule protein 1; D, heat shock protein 70; E, dense granule protein 2; L1, peptide match to Lycopersicon esculentum lipoxygenase A; and L2, peptide match to Persea americana lipoxygenase. (inset) Base peak ion trace for peptide L1. (B) T. gondii proteins identified in STAg.
Of particular interest, three peptides were found that matched plant lipoxygenases. These were to tomato lipoxygenase A and potato lipoxygenase (peptide sequence QLISSVQSDPANGLQK; L1), avocado lipoxygenase (LVQSTKFSAPA), and soybean lipoxygenase-3 (MLGGLLHRGHKIK) (Fig. 1 B). The quality of the matches ranged from relatively strong (cross correlation score = 2.00 and 12 out of 32 predicted ions matched) to weak (cross correlation score = 1.07 and 10 out of 20 predicted ions matched). A base peak ion trace for peptide L1 is shown in Fig. 1 (inset). Tomato lipoxygenase A and soybean lipoxygenase-3 are known to oxygenate polyunsaturated fatty acids with a positional specificity for a 15- or 12-lipoxygenase with arachidonic acid as a substrate. These findings suggest that among several identified T. gondii proteins, a plantlike lipoxygenase may be present within STAg and carried by the parasite. T. gondii is known to have incorporated plantlike genomic components of a possible green algal plastid origin (18, 19). Our proteomic analyses did not indicate the presence of any of the known human lipoxygenases (or other human proteins), and, thus, it was unlikely that a lipoxygenase derived from the host human fibroblast cell line in which T. gondii is propagated contaminates the STAg preparation. Along these lines, it is noteworthy that immunoblot analysis of STAg lysates did not reveal the presence of a protein that reacted with polyclonal antibodies directed against murine leukocyte-type 12-lipoxygenase or rabbit reticulocyte 15-lipoxygenase (unpublished data).
Eicosanoid Lipidomics: STAg Converts Arachidonic Acid.
Because the proteomic analysis of STAg suggested that it contained a lipoxygenase, we tested this directly by assessing enzymatic activity. To determine whether STAg itself contains lipoxygenase-like enzymatic activity, we incubated STAg with arachidonic acid and assessed the formation of eicosanoids. After solid-phase extraction, methyl formate fractions obtained from incubations of STAg with arachidonic acid showed a pronounced UV absorbance at 234 nm that was indicative of the presence of products containing conjugated diene chromatophores characteristic of monohydroxy eicosatetraenoic acids (HETEs; unpublished data). Using LC-UV-MS-MS–based lipidomic analyses, the presence and formation of the main monoHETE were established, namely 15-HETE, 12-HETE, as well as the double dioxygenation product 5,15-diHETE (Fig. 2 A). Tandem mass spectra obtained from these isolated STAg incubations established the identification of 15-HETE and 5,15-diHETE (Fig. 2 B). The ratio of 15-HETE/12-HETE formation was ~4:1, 1.7:1, and 0.6:1 in three independent series of analyses. It is noteworthy that although 5,15-diHETE, the double dioxygenation product of 15-lipoxygenase (20), was indeed generated, and lipoxins did not appear to be generated by STAg alone (Fig. 2 A); each of these products were identified in vivo at high levels after injection of STAg (compare with reference 10). The formation of 15-HETE from arachidonic acid by STAg was inhibited by lipoxygenase inhibitors (ketoconazole, esculetin, baicalein, and nordihydroguaiaretic acid; 59, 52, 57, and 69% inhibition, respectively), but not by high concentrations of the cytochrome P450 inhibitors SKF 525A and 8-methoxypsoralen (21 and 7% inhibition, respectively; references 14, 15). A comparison of the amount of 15-HETE measured by coupling LC-MS analysis to antibody-dependent analysis using standard curves for 15S-HETE and 15R-HETE (Materials and Methods), indicated that STAg generated 15-HETE carrying the alcohol group at carbon-15 in >80% S-configuration.
LXA4 Formation by T. gondii Tachyzoites.
Having established that STAg produces 15-HETE, a potential substrate for lipoxin biosynthesis, and 5,15-diHETE, which is associated with the activation of lipoxin pathways, we next addressed whether intact T. gondii tachyzoites were capable of forming LXA4. Tachyzoites were incubated with or without the substrate arachidonic acid and an agonist, calcium ionophore A23187. T. gondii tachyzoites formed LXA4 in the presence of added arachidonic acid (Fig. 3). The formation of LXA4 was further enhanced by simultaneous activation of the tachyzoites with calcium ionophore. Tachyzoites alone did not form appreciable amounts of LXA4 in the absence of added arachidonic acid, or with calcium ionophore alone. These results indicate that intact T. gondii tachyzoites can generate LXA4, but only when supplied with exogenous sources of arachidonic acid.

LXA4 formation by T. gondii tachyzoites. T. gondii tachyzoites (107 organisms/well) were incubated for 30 min in the presence or absence of 5 μM A23187 and 20 μM arachidonic acid. LXA4 was monitored (see Materials and Methods) and expressed as mean values ± SEM (n = 3). Student's t test; *, P < 0.05; **, P < 0.005.
Local Administration of Plant 15-Lipoxygenase Diminishes Murine Peritonitis.
To determine whether exogenous 15-lipoxygenase itself carries antiinflammatory properties, we administered neat soybean lipoxygenase into murine peritoneum before inducing leukocytic inflammation with zymosan A. By native isoelectric focusing and in-gel lipoxygenase staining, the soybean lipoxygenase preparation used in this analysis appeared to consist of a single active isoenzyme with an apparent pI of ~6.4 (Fig. 4 A, inset). By SDS-PAGE, followed by LC-MS-MS and peptide mapping, this lipoxygenase preparation was shown to be mainly composed of soybean lipoxygenase type-1 (unpublished data). Unexpectedly, as shown in Fig. 4 A, local administration of soybean 15-lipoxygenase type-1 gave a marked reduction in inflammation. Peritoneal exudate cell viability was >95% by trypan blue staining. Administration of soybean lipoxygenase gave a dose-dependent increase in exudate LXA4 levels, indicating that the enzyme remained active in vivo (Fig. 4 B). Together, these results indicate that administration of plant 15-lipoxygenase promotes local endogenous LXA4 biosynthesis, and attenuates zymosan A–stimulated murine peritonitis.
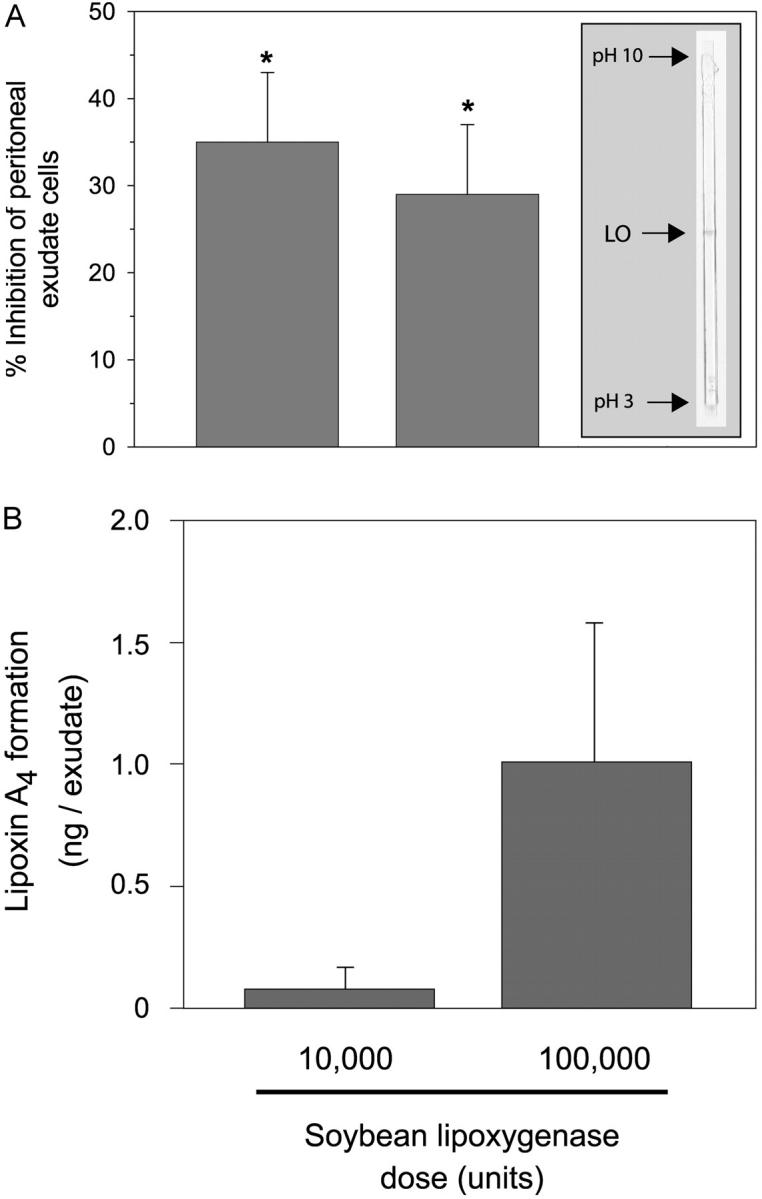
Local administration of plant 15-lipoxygenase diminishes zymosan A–stimulated leukocyte infiltration, a hallmark of acute inflammation. Peritonitis was induced by intraperitoneal injection of 1 mg zymosan A. Soybean lipoxygenase (10 or 100 × 103 U) or vehicle alone was administered 5 min before zymosan A (1 mg/ml saline i.p.), and after 2 h, peritoneal exudates were collected. (A) Inhibition of peritoneal exudate cell numbers (mean ± SEM; n = 5–7; *, significantly different from vehicle; P < 0.05). (inset) In-gel lipoxygenase activity stain with substrate arachidonic acid after native isoelectric focusing of soybean 15-lipoxygenase (LO; 5 × 103 U). (B) Peritoneal exudate LXA4 levels expressed as mean values ± SEM (n = 7–8).
Intraperitoneal Administration of Plant 15-Lipoxygenase Mimics STAg-induced Paralysis of Splenic Dendritic Cells.
To determine whether the 15-lipoxygenase activity could account for the in vivo paralysis of splenic dendritic cells that is known to occur after T. gondii or STAg administration (compare with reference 10), we determined IL-12 generation by splenic dendritic cells from STAg- and/or 15-lipoxygenase–treated mice. Administration of 1,000 U of soybean 15-lipoxygenase (i.p.) markedly reduced splenic dendritic cell IL-12 generation induced by a second challenge with STAg, namely the dendritic cell paralysis procedure (Fig. 5 A; reference 10). Intraperitoneal administration of the plant arachidonate 15-lipoxygenase induced a dose-dependent reduction in splenic dendritic cell IL-12 production with a nadir at 10.000 U (Fig. 5 B). The shape of the dose–response curve indicates that a complex relationship exists between activity and the temporal and spatial distribution of the immunosuppressive action of exogenously administered 15-lipoxygenase. Heat inactivation of the soybean 15-lipoxygenase (100°C for 60 s) before in vivo administration abolished its impact on IL-12 formation, strongly suggesting that the catalytic activity of 15-lipoxygenase is required for the inhibition in vivo of dendritic cell IL-12 generation. We also observed that local administration of high doses of the lipoxygenase (5 × 105 and 106 U) led to local bleeding and death of mice. This indicates that adverse actions by lipoxygenase, namely overdosing, can occur, that may also be responsible for the observed lack of inhibitory action on dendritic cell IL-12 formation observed after local administration of 105 U, giving rise to the V-shaped dose–response (Fig. 5 B). Together, these results indicate that at the lower doses used, 15-lipoxygenase administered in vivo mimics the suppression of dendritic cell IL-12 generation that is evoked with T. gondii infection and STAg administration.
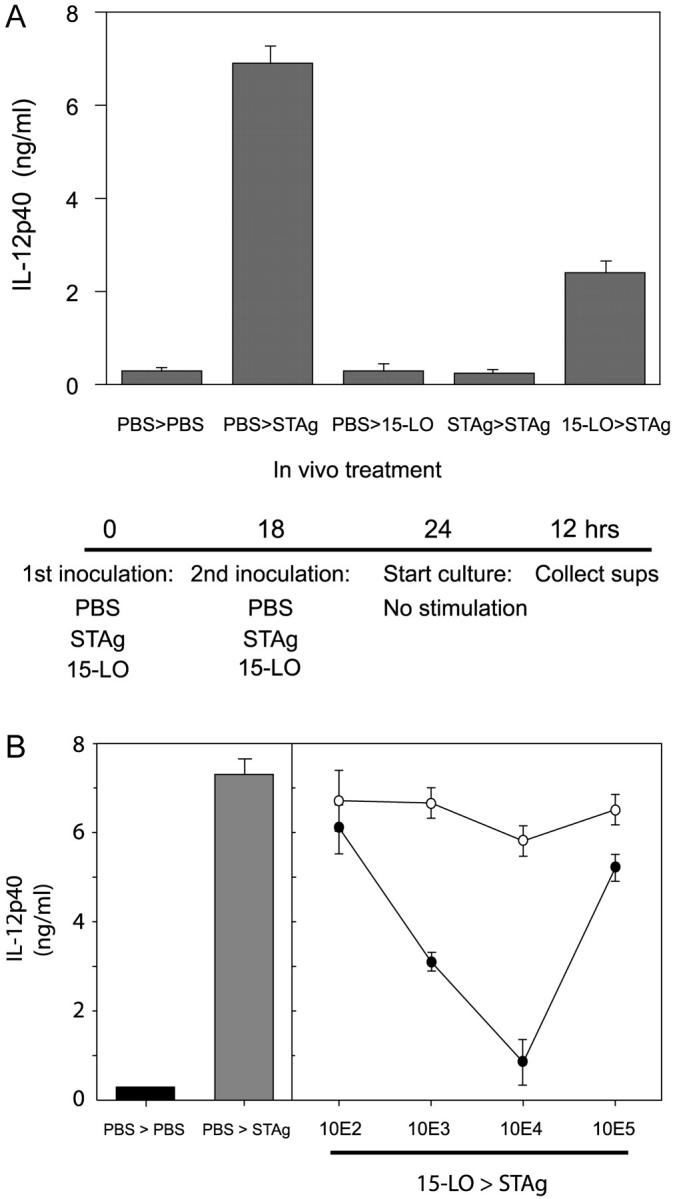
Plant 15-lipoxygenase reduces dendritic cell IL-12 production in vivo. (A) Intraperitoneal administration of plant 15-lipoxygenase mimics STAg-induced paralysis of splenic dendritic cells. Mice were injected i.p. with 1 μg STAg, 103 U soybean lipoxygenase, or vehicle alone 18 h before a second challenge with 1 μg STAg, 103 U soybean lipoxygenase, or vehicle. 6 h later, splenic dendritic cells were harvested and cultured for another 12 h. Dendritic cell formation of IL-12 p40 was measured (see Materials and Methods) and expressed as mean values ± SEM (n = 3). (B) Dose-dependent induction of dendritic cell paralysis (see Introduction and references 8–10 and 16 for details) by i.p. administration of plant 15-lipoxygenase requires catalytic activity. Mice were injected intraperitoneally with native (closed circles), boiled (open circles) soybean lipoxygenase, or vehicle alone 18 h before a second challenge with 1 μg STAg (i.p.) or vehicle. 6 h later, splenic dendritic cells were harvested and cultured for another 12 h. IL-12 p40 was measured (see Materials and Methods) and expressed as mean values ± SEM (n = 3).
Discussion
We report here using proteomic and lipidomic LC-MS-MS–based analysis that both microbial lipoxygenase–like activity and plant 15-lipoxygenase can, upon infection or administration in mice, participate in and enhance endogenous LXA4 biosynthetic circuits. T. gondii tachyzoites carry a 15-lipoxygenase–like activity (Figs. 1–3) that can initiate and contribute to parasite-activated biosynthesis of high levels of LXA4 demonstrated earlier during infection that down-regulates the biosynthesis of splenic dendritic cell IL-12 formation, that in turn suppresses host immunopathology (10). Large scale expressed sequence tag analysis indicates that T. gondii has commandeered several plantlike genes, probably via secondary endosymbiosis of a green algal plastid (18, 19). Surprisingly, these key microbial lipoxygenase-mediated actions were also mimicked by local administration of a plant 15-lipoxygenase type-1. Together, these unexpected results illustrate that exogenous lipoxygenases themselves can activate endogenous lipoxin biosynthetic pathways and impact the inflammatory status of the host.
In healthy humans, vascular and mucosal LXA4 biosynthesis typically occurs in scenarios of heterotypic cellular interactions, where a lipoxygenase in one cell type catalyzes the first hydrogen abstraction and oxygenation of arachidonic acid to form a monoH(p)ETE, followed by the action of a second lipoxygenase and subsequent reactions to form LXA4 in an interacting or second cell type (for review see reference 11). For example, PMN 5-lipoxygenase can convert 15-H(p)ETE generated by a 15-lipoxygenase in cells present in mucosal surfaces to form LXA4 (11). Within the vasculature, activated neutrophils generate leukotriene A4 (LTA4) by 5-lipoxygenase and as much as 50–80% is released from the cell and rapidly converted by interacting platelets, for example, to lipoxins by platelet 12-lipoxygenase, or to cysteinyl-leukotrienes by leukotriene C4-synthase (21–23). Multiple routes for transcellular lipoxin biosynthesis are now known, and 15-, 12-, and 5-lipoxygenase can each contribute to lipoxin biosynthesis (2, 11). Transcellular lipoxin biosynthesis occurs and has been studied mainly in inflammation, during which the formed LXA4 signals the resolution of the inflammatory state (17, 24). LXA4 can also be formed in a single cell type, e.g., when PGE2 present in the inflammatory exudate activates the rapid translation of 15-lipoxygenase message that, together with already present 5-lipoxygenase, establishes a complete lipoxin biosynthetic circuit within the human neutrophil (17).
In the current studies, we sought to identify the bioactive principle present in the parasite because T. gondii gives high levels of LXA4 and 5,15-diHETE in vivo (10). Interestingly, we found that T. gondii itself appears to catalyze the first step in lipoxin biosynthesis.
Support for an active initiation of lipoxin biosynthesis from within the parasite itself comes from several independent sets of findings. First, by proteomic analysis, we identified several known T. gondii proteins. The analysis was limited to the number of T. gondii proteins that are described and deposited in protein databases (estimated at ~600 proteins at the time of preparation of this paper). Hence, we sought to identify additional proteins that have structurally similar homologues in T. gondii by cross-species peptide matching. We also took into consideration that measured tryptic peptide masses could match a peptide from a similar protein present within different species that did not contain a preceding tryptic cleavage site. Although tryptic peptides are less well conserved across species, identification of orthologous proteins by peptide mass via LC-MS-MS can be performed as the level of peptide conservation is above that observed by chance (25, 26). Among several known T. gondii tachyzoite proteins, we were intrigued by the finding that several peptides were present that matched peptide sequences present in plant lipoxygenases. The hypothesis emerged that T. gondii might contain lipoxygenases that could contribute to LXA4 biosynthesis observed in vivo (10).
Second, parasite extracts (STAg) tested for eicosanoid biosynthesis gave results indicating rapid oxygenation of arachidonic acid with the formation of the reduced alcohol-containing products 15-HETE, 12-HETE, and 5,15-diHETE that were identified by lipidomic analysis. This product profile points to the presence of an arachidonic acid 15-lipoxygenase (27, 28). Moreover, the identification of the double dioxygenation product 5,15-diHETE strongly supports the presence of an arachidonic acid 15-lipoxygenase, which is able to both generate 15-H(p)ETE and use this product again as a substrate that positions in an inverse orientation in the substrate binding center of the enzyme to form 5,15-diHETE (20). A marked inhibition of 15-HETE formation by a series of lipoxygenase inhibitors, but not by cytochrome P450 inhibitors, further supports the presence of a 15-lipoxygenase enzyme in T. gondii. The 15-HETE generated by STAg was predominantly in an S-configuration, consistent with reported properties of lipoxygenase-based catalysis. Blast searches using the T. gondii genomic sequence that is currently available have not revealed the genomic origin of this 15-lipoxygenase activity, and the complete identification of the T. gondii gene awaits future studies. The peptide matches obtained with STAg for lipoxygenase point to the existence of a plantlike lipoxygenase in T. gondii. T. gondii is an apicomplexan protozoan that contains several genes with a probable algal plastid origin (18, 19, 29). Thus, it is possible that a lipoxygenase with a plant plastid origin could be functional in T. gondii.
Third, we found that intact T. gondii tachyzoites can form LXA4 in the presence of arachidonic acid. The parasite 15-lipoxygenase could contribute to part of LXA4 biosynthesis in vivo as a result of infection by catalyzing the first step of lipoxin biosynthesis, a scenario that would require a host 5-lipoxygenase for conversion of 15-HETE to LXA4 (Fig. 6). Indeed, 5-lipoxygenase–deficient mice are incapable of producing LXA4 during T. gondii infection, and dendritic cell paralysis does not occur (10). The requirement for exogenously supplied arachidonic acid suggests that tachyzoites do depend on host cell arachidonic acid for the parasite-stimulated LXA4 biosynthesis (Fig. 6). T. gondii infection of macrophages stimulates 15- and 12-HETE, the formation of which is dependent on the incorporation of live tachyzoites into the host cells (30). T. gondii tachyzoites may also trigger the release of arachidonic acid from host phospholipid stores, which is subsequently used for lipoxin biosynthesis. STAg can induce dendritic cell paralysis in vivo, but not in isolated dendritic cells in vitro, indicating that host 5-lipoxygenase activity is likely to be contained in another cell type in the spleen. Treatment of mice with STAg markedly increases the number of 5-lipoxygenase–containing resident macrophages in the spleen (10), suggesting that resident macrophages or newly recruited monocytes can use T. gondii–derived 15-HETE as a substrate to form LXA4. In addition, a T. gondii lipoxygenase could use host 5-lipoxygenase–derived leukotriene A4 as a substrate to generate LXA4 (22).
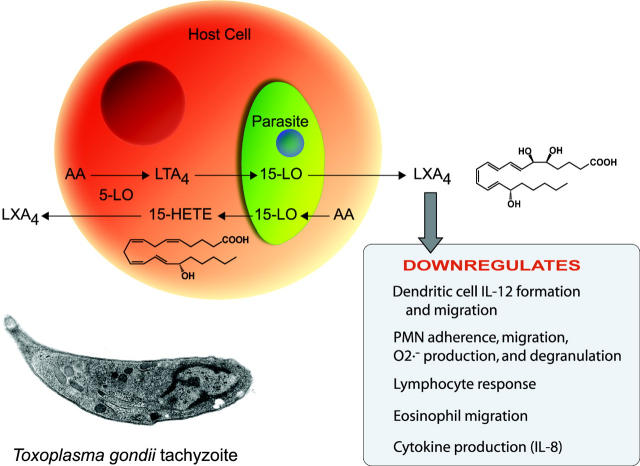
Hypothetical scheme for bidirectional microbial and plant 15-lipoxygenase–initiated biosynthetic pathways that enhance endogenous LXA4 formation.
A scheme is proposed (Fig. 6) that takes into account these present observations; namely, hypothetical bidirectional contributions to lipoxin formation by the intracellular parasite using substrates and intermediates generated by both the host and parasite lipoxygenases. A similar scenario may apply to other microbial infections because other parasites such as Brugia malayi, Trypanosoma brucei, and Schistosoma mansoni have also been shown to generate cyclooxygenase- and lipoxygenase-derived eicosanoids that are generated from host polyunsaturated fatty acids, and that are essential for parasite development, possibly via modulating the host immune responses and physiology (31–34). Our findings suggest that T. gondii itself initiates LXA4 formation not by producing an epitope or ligand that activates lipoxin biosynthesis, but rather by wielding its own 15-lipoxygenase. Future goals will include nucleotide sequencing of T. gondii 15-lipoxygenase and related genes.
Administration of lipoxygenase in a model of neutrophilic infiltration indicated that plant 15-lipoxygenase itself imparts antiinflammatory activity, and down-regulates PMN infiltration. Soybean 15-lipoxygenase stimulated the formation of LXA4, indicating that the enzyme remains active in vivo (Fig. 5). Administration of soybean lipoxygenase mimicked the impact of T. gondii infection or STAg administration on host dendritic cell formation of IL-12. These findings indicate that cell-free lipoxygenase can form an essential part of lipoxin biosynthetic pathways, and function in vivo to produce LXA4 and initiate endogenous antiinflammation. A lack of leukocyte recruitment to tissues harboring T. gondii is a typical feature of T. gondii infections (35). Our results are consistent with this observation because LXA4 is a potent inhibitor of leukocyte trafficking to sites of infection (11). T. gondii infection in 5-lipoxygenase–deficient mice leads to an uncontrolled Th1-type immune response and lethality that can be partly controlled by IL-10, and fully corrected by administration of a LXA4-stable analogue (36). Our present results indicate that activation of endogenous lipoxin biosynthetic pathways by either T. gondii–initiated biosynthesis or via administration of exogenous 15-lipoxygenase constitutes a novel means to stimulate antiinflammation or modify Th1-type host-damaging immune responses that could be used by microbes to enhance invasion and/or protect the host against excess parasite-induced IL-12 production.
Acknowledgments
The authors thank S. Hieny, R.-L. Moussignac, and E. Tjonahen for skillful technical assistance, and M.H. Small for assistance in preparing the manuscript.
The present work was supported in part by National Institutes of Health grants GM-38765, P01-DK50305, and P01-DE13499 (to C.N. Serhan). G.L. Bannenberg is a recipient of an Arthritis Foundation Postdoctoral Fellowship.
Note added in proof. The authors would like to note that Vance et al. using a different approach, also uncovered recently that the pathogenic bacterium Pseudomonas aeruginosa also possesses a novel secreted 15-lipoxygenase (Vance, R.E., S. Hong, K. Gronert, C.N. Serhan, and J.J. Mekalanos. 2004. Proc. Natl. Acad. Sci. USA. In press.).
Notes
Abbreviations used in this paper: DPBS, Dulbecco's phosphate buffered saline; LXA4, lipoxin A4; STAg, soluble tachyzoite antigen.
References
Articles from The Journal of Experimental Medicine are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1084/jem.20031325
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jem/article-pdf/199/4/515/1148850/jem1994515.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102684410
Article citations
Lipoxygenases at the Intersection of Infection and Carcinogenesis.
Int J Mol Sci, 25(7):3961, 02 Apr 2024
Cited by: 0 articles | PMID: 38612771 | PMCID: PMC11011848
Review Free full text in Europe PMC
Pseudomonas aeruginosa cytochrome P450 CYP168A1 is a fatty acid hydroxylase that metabolizes arachidonic acid to the vasodilator 19-HETE.
J Biol Chem, 298(3):101629, 24 Jan 2022
Cited by: 6 articles | PMID: 35085556 | PMCID: PMC8913318
Tityus serrulatus (Scorpion): From the Crude Venom to the Construction of Synthetic Peptides and Their Possible Therapeutic Application Against Toxoplasma gondii Infection.
Front Cell Infect Microbiol, 11:706618, 20 Jul 2021
Cited by: 6 articles | PMID: 34354963 | PMCID: PMC8329421
Pro-Resolving Ligands Orchestrate Phagocytosis.
Front Immunol, 12:660865, 10 Jun 2021
Cited by: 11 articles | PMID: 34177900 | PMCID: PMC8222715
Review Free full text in Europe PMC
Narrative Review of n-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation.
Nutrients, 13(2):662, 18 Feb 2021
Cited by: 34 articles | PMID: 33670710 | PMCID: PMC7922327
Review Free full text in Europe PMC
Go to all (57) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Lipoxin A4 and lipoxin B4 stimulate the release but not the oxygenation of arachidonic acid in human neutrophils: dissociation between lipid remodeling and adhesion.
J Cell Physiol, 143(3):512-523, 01 Jun 1990
Cited by: 27 articles | PMID: 2162850
Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection.
J Exp Med, 196(9):1253-1262, 01 Nov 2002
Cited by: 142 articles | PMID: 12417634 | PMCID: PMC2194099
Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins.
J Clin Invest, 92(3):1572-1579, 01 Sep 1993
Cited by: 128 articles | PMID: 8376607 | PMCID: PMC288306
Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution.
Prostaglandins Leukot Essent Fatty Acids, 73(3-4):141-162, 01 Sep 2005
Cited by: 242 articles | PMID: 16005201
Review
Funding
Funders who supported this work.
NIDCR NIH HHS (2)
Grant ID: P01-DE13499
Grant ID: P01 DE013499
NIDDK NIH HHS (2)
Grant ID: P01 DK050305
Grant ID: P01-DK50305
NIGMS NIH HHS (3)
Grant ID: R01 GM038765
Grant ID: GM-38765
Grant ID: R37 GM038765




