
| PMC full text: |
|
Figure 4
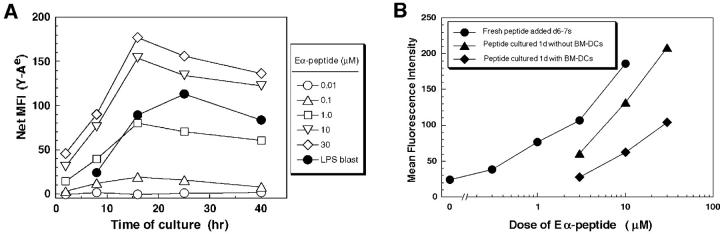

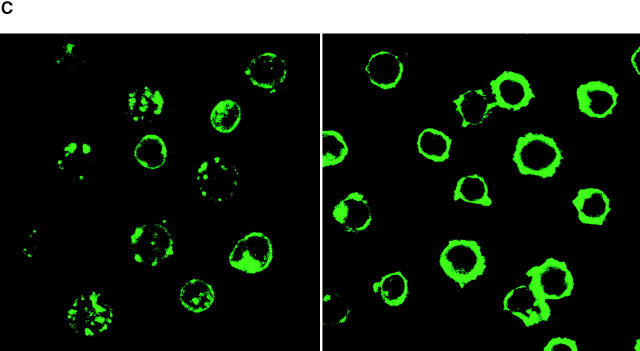
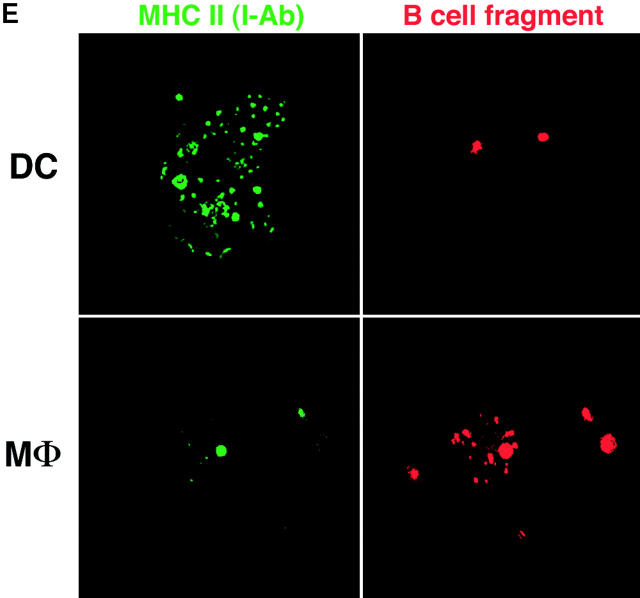
Efficiency of antigen transfer after phagocytosis. (A) As in Fig. Fig.11 A, C57BL/6 DCs were cultured for the indicated times with doses of I-Eα peptide, or 106 B blasts from BALB/c mice, and double labeled for Y-Ae and CD86. Y-Ae signals (Mean Fluorescence Index) are shown for CD86+ mature DCs. (B) Assays for the stability of the I-E peptide during culture. Graded doses of I-E peptide were added to day 6 marrow DCs, and the levels of Y-Ae (Mean Fluorescence Intensity) were measured 20 h later (circles). At this time, supernatants were harvested from the cultures (diamonds) in parallel with peptide that was cultured in the absence of cells for 20 h (triangles). Both sources of peptide were then added to fresh cultures of immature marrow DCs, and Y-Ae epitope formation was compared 20 h later. (C) Distribution of MHC II in fresh B cells (left) and 4-d splenic B blasts (right). The latter are processed best by DCs and express ~1.5 × 105 I-E molecules/cell, mostly mature heterodimers (see text). MHC II was visualized with a rabbit polyclonal antibody to I-Eα. The majority is on the cell surface of blasts, but in intracellular vacuoles in fresh B cells. (D) Most of the I-E in B blasts consists of mature I-E heterodimers. 4-d LPS-induced B blasts from I-E+ BALB/c and I-E− C57BL/6 mice were lysed and reacted with mAb 14-4-4S to mature I-E molecules. The immune complexes were bound to beads and then a rabbit anti–I-E antibody, which recognizes all forms of I-E, was used to blot bound and nonbound fractions. Most I-E molecules from B blasts were bound by 14-4-4S. (E) Macrophages versus immature DCs are the main site for phagocytosis of B cell fragments in 6-d GM-CSF–stimulated mouse marrow cultures. Immature MHC II–rich (green) DCs phagocytose B cell fragments (B220 stain, red), but firmly adherent MHC II–low macrophages contained more numerous intracellular B cell fragments.




