Abstract
Free full text

On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators”
Abstract
When one nerve cell acts on another, its postsynaptic effect can vary greatly. In sensory systems, inputs from “drivers” can be differentiated from those of “modulators.” The driver can be identified as the transmitter of receptive field properties; the modulator can be identified as altering the probability of certain aspects of that transmission. Where receptive fields are not available, the distinction is more difficult and currently is undefined. We use the visual pathways, particularly the thalamic geniculate relay for which much relevant evidence is available, to explore ways in which drivers can be distinguished from modulators. The extent to which the distinction may apply first to other parts of the thalamus and then, possibly, to other parts of the brain is considered. We suggest the following distinctions: Cross-correlograms from driver inputs have sharper peaks than those from modulators; there are likely to be few drivers but many modulators for any one cell; and drivers are likely to act only through ionotropic receptors having a fast postsynaptic effect whereas modulators also are likely to activate metabotropic receptors having a slow and prolonged postsynaptic effect.
When one nerve cell signals to another, the effect can vary greatly. One distinction recognized currently is that between “drivers” and “modulators” (1–4). The former carry the message, defining the essential patterns of activity, whereas the latter can alter the effectiveness of the drive without contributing significantly to the general pattern of the message. However, it is often difficult to define whether afferents are drivers or modulators; it also is difficult to define precisely what is meant by either term. We here explore the distinction between drivers and modulators and add a third category: “disrupters.” We base our analysis on the thalamus, where these categories are likely to prove useful, and we explore the extent to which they also may apply to other parts of the brain.
In many sensory relays, drivers can be recognized because they transmit information from peripheral receptors to the brain. For the major sensory relays of the thalamus, we proposed (5) that afferents can be assigned, on the basis of their fine structure and function, to one of two classes (Fig. (Fig.1).1). They are either “primary afferents” (i.e., drivers), which define the receptive field properties of the postsynaptic cells, or else modulators, which change the pattern of transmission but do not alter the receptive field properties significantly. The clearest examples of drivers are visual, auditory, or somatosensory axons that innervate the lateral geniculate, the medial geniculate, or ventrobasal nucleus, respectively. These all have similar fine structural characteristics and synaptic relationships, and we argued (5) that other comparable axons innervating ventral lateral, ventral anterior, or the anterior thalamic nuclei could be inferred to have a similar “driving” function for their relay nuclei (Fig. (Fig.1).1).
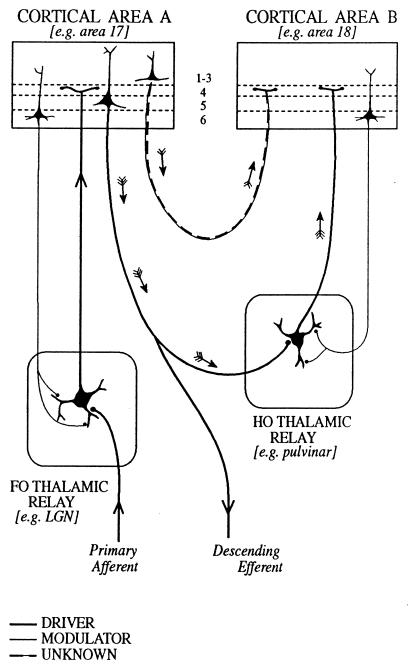
Schema to illustrate cortical and thalamic pathways. Two thalamic nuclei are shown: a first order (FO) relay on the left and a higher order (HO) relay on the right. A first order relay receives its driver inputs on proximal dendrites from subcortical sources via ascending pathways whereas a higher order relay receives its driver inputs from cells in cortical layer 5 (see ref. 40). The first order relay sends a driver input to layer 4 of cortical area A (thick line), and that same cortical area sends a modulator input (thin line with small terminals onto distal dendrites of the thalamic relay cell) from layer 6 back to the same first order thalamic nucleus. Cortical area A in turn sends a driver input from layer 5 to the higher order thalamic relay. This higher order relay sends its thalamocortical axons (shown as drivers, on the assumption that all thalamocortical inputs to layer 4 are drivers, although empirical data are lacking) to cortical area B and receives a modulator input back from layer 6 of cortical area B. Note that there are two paths by which cortical area A can influence area B. One is the transthalamic path, shown by small arrows and drawn as thick lines indicative of a driver pathway. The other is the direct corticocortical pathway (a “feed-forward” pathway, as defined by ref. 39), and this is shown by small arrows and as alternating thin and thick lines to indicate that we do not know whether this (or any other corticocortical pathway) represents a driver or a modulator input. As one specific example of such circuitry, we indicate the lateral geniculate nucleus (LGN) as a first order relay innervating area 17 and the pulvinar as a higher order relay receiving driver input from layer 5 of area 17 (41) and, in turn, innervating area 18.
Further, two distinct fiber types going from the cerebral cortex to the thalamus can be recognized. All thalamic nuclei appear to receive afferents from cells in cortical layer 6, and we shall argue that these are modulators. For nuclei like the lateral or medial geniculate or the ventrobasal nucleus, which were described as “first order” relays (FO in Fig. Fig.1;1; see also ref. 5), this is their only cortical innervation. Other thalamic nuclei, like the pulvinar, the mediodorsal nucleus, or the posterior thalamic group (labeled HO for “higher order” relays in Fig. Fig.1)1) also receive afferents from cortical layer 5. These axons have terminals whose fine structural and synaptic relationships resemble the primary afferents from the retina or the medial lemniscus, and some of these afferents from layer 5 also appear to be the drivers. That is, they define the receptive field properties of their postsynaptic cells (6, 7). In nuclei on sensory pathways, then, drivers carry the receptive field properties and modulators do not. In nuclei like the mediodorsal nucleus, and in many other thalamic nuclei, receptive fields are not defined, and there is as yet no criterion for distinguishing a driver from a modulator.
Beyond the thalamus, the problem is more difficult and of broader significance. For instance, Crick and Koch (1) recently used a classification of “drivers” and “modulators” in a discussion of corticocortical pathways, and part of their argument concerned the distinction between drivers and modulators. They showed that the functional significance of a set of connections depends on whether a component is a driver or a modulator. They propose connectional ground rules for identifying a pathway as a driver or modulator, but at present, these are ad hoc rules. We know of no critical evidence to identify a corticocortical pathway as one or the other in the way that receptive field properties can be used to identify afferents in some thalamic relays.
The distinction between drivers and modulators may prove useful for some pathways other than thalamic and cortical circuits. A first step has to be a rigorous definition. We start with the sensory relay nuclei of the thalamus, particularly the lateral geniculate. Here, we have information about afferents, receptive fields, and relay cells. The thalamus may be unique from the point of view of the classification that we are exploring, and, further, circuits concerned with sensory relays may have special properties not shared by other thalamic nuclei. However, our analysis of the geniculate aims not so much to show well defined rules for the proposed classification but, rather, to explore observations that may be relevant in other thalamic nuclei and other brain centers. There is currently a lack of critical factual observations for establishing criteria to distinguish between drivers and modulators, either just for the thalamus or for a broad range of brain centers.
Afferents to the Lateral Geniculate Nucleus
It is almost a tautology to define retinal afferents to the lateral geniculate nucleus as drivers. We start with this assertion and treat all of the other afferents as modulators so that we can explore what distinguishes the drivers (5). The retinal afferents pass their receptive field properties to geniculate cells with only minor changes. These afferents have relatively large axon terminals that make synaptic contacts on dendrites close to the cell body of relay cells. They generally form multiple synaptic zones, often in glomeruli, which are complex synaptic zones related to the innervation of some relay cells. The retinal afferents use glutamate as a neurotransmitter and activate only ionotropic receptors. One can argue that some or all of these features will characterize other drivers in the thalamus, and possibly also in other parts of the brain, but they are not a reliable identifier of drivers, in the thalamus or elsewhere.
When we look at modulator inputs to geniculate relay cells, the situation is no clearer. They include glutamatergic afferents from cortex, cholinergic, serotonergic, and noradrenergic cells from the brainstem, and histaminergic cells from the hypothalamus. Other modulatory afferents come from local GABAergic cells (GABA = γ-aminobutyric acid), including interneurons and cells in the adjacent thalamic reticular nucleus. Synaptic terminals from modulator inputs are relatively small and generally have single active zones. Modulator inputs from the cortex make synapses primarily onto distal dendrites, those from the brainstem are found primarily proximally, adjacent to retinal inputs, and those from GABAergic sources make contacts with all parts of the dendritic arbor (8–11). Modulators outnumber drivers significantly. Earlier estimates of ≈15–20% for the proportion of synaptic junctions in the lateral geniculate nucleus of the cat contributed by retinal afferents (9, 11) have been revised recently to only ≈7% (12). Further, whereas possibly all of the modulators entering the thalamus contact the inhibitory thalamic reticular cells, the retinal afferents do not send any branches into the reticular nucleus.
A first step stab at distinguishing drivers from modulators in the thalamus, then, suggests that drivers have relatively few, large axon terminals making contacts close to the cell body whereas modulators have many more, smaller terminals dominating on peripheral dendrites. Further, drivers may activate only ionotropic receptors whereas modulators also activate metabotropic receptors. However, these distinctions are not diagnostic, and if the classification is to be useful in other brain regions, further criteria are needed.
Receptive Fields and Cross-Correlograms
In the awake or the lightly anesthetized animal, retinal inputs dominate geniculate relay cell responses in terms of their receptive field properties. The center/surround receptive field of a geniculate relay cell is more like that of its retinal afferent(s) than that of afferents from visual cortex or thalamic reticular nucleus. It is probably unlike those from the brainstem or the hypothalamus, which almost certainly lack classical visual center/surround receptive fields. Thus, in terms of receptive field properties, retinal afferents dominate the output of a geniculate relay cell when it is transmitting visual information, and all of the other afferents modulate the input/output relationships to control quantitative features of the relay (4, 5).
Defining drivers as transmitters of receptive field properties takes us a step further, but it will not serve to distinguish drivers from modulators in nuclei other than the first order sensory or some of the higher order thalamic nuclei. First and higher order nuclei are defined in Fig. Fig.1;1; the former receive primary (driving) afferents from ascending pathways whereas the latter receive primary, driving afferents from pyramidal cells in layer 5 of the cortex. Thus, in the first order relays for visual or somatosensory pathways, receptive fields of relay cells come from the ascending primary afferents (retinal or lemniscal fibers). These receptive field properties are resistant to cortical lesions or cooling (reviewed in refs. 4 and 5). However, in the pulvinar and posterior thalamic group, which are higher order relays, the relay cell receptive fields are lost when the afferents from the cortex are inactivated (6, 7). Although this receptive field criterion may work for sensory relays, as noted above, receptive field properties cannot be used in nuclei like the mediodorsal nucleus or in other regions where receptive fields have not been shown. It is necessary to look at other features in the geniculate relay to arrive at more useful generalizations.
The linkage between retinal afferents and geniculate relay cells involves more than the structure of their receptive fields. When individual action potentials are recorded simultaneously in relay cells and their retinal afferents, most retinal action potentials are followed by a single action potential in the relay cell with a fixed latency (13–15). A cross-correlogram between the spikes in the retinal axon and the geniculate relay cell shows the close link of the relay cell and its retinal afferents. Such a cross-correlogram has a relatively narrow peak with a latency of several milliseconds and a relatively low, flat baseline (Fig. (Fig.22 A and C; refs. 16 and 17 provide a further discussion of cross-correlograms).
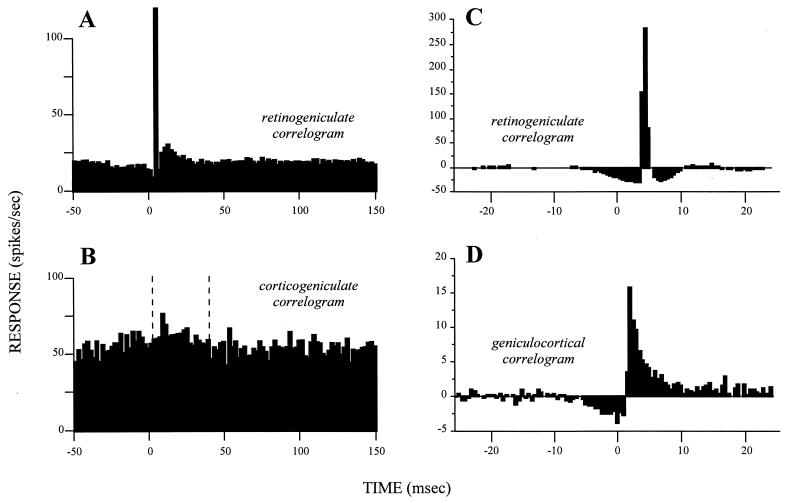
Cross-correlograms displaying the difference between drivers and modulators. Each is based on simultaneous recordings in cats from two neurons, one presynaptic to the other. The cross-correlograms represent the firing of the postsynaptic cells relative to a spike at time zero for the presynaptic cell. (A) Retinogeniculate cross-correlogram based on spontaneous activity in both the retinal and geniculate neurons. Note the narrow peak rising out of a flat, low baseline that marks this as a driver connection. Redrawn from Fig. 3A of ref. 14, with permission of the publisher. (B) Corticogeniculate cross-correlogram based on spontaneous activity in both the layer 6 cell in area 17 and geniculate neuron. Glutamate was applied to the cortex to enhance the spontaneous firing of the afferent cell. Between the vertical, dashed lines, it is possible to discern a very gradual, prolonged, and small peak arising from a noisy, high baseline that marks this as a modulator connection. Redrawn from Fig. Fig.22A of ref. 42, with permission of the publisher. (C and D) Cross-correlograms taken from the same laboratory by using identical techniques for easier comparison. Both are based on visually driven activity and involve a “shuffle correction” (43), and they are normalized against the firing level of the afferent, which is why some bins fall below zero. Both represent driver inputs and include another retinogeniculate pair (C) plus a geniculocortical pair (D). Note the difference in vertical scale, indicating that the retinal input accounts for more postsynaptic spikes in the geniculate cell (C) than does the geniculate input to the layer 4 cell of the striate cortex (D). Note also that the time represented by these cross-correlograms is much briefer than that for A and B. Nonetheless, both cross-correlograms have narrow peaks rising from a flat, low baseline, marking them as driver inputs. Data kindly provided by the authors for replotting. C is redrawn from data of Usrey et al.(15), and D is redrawn from Fig. Fig.22 of Reid and Alonso (44).
Here, then, is a relationship based on individual action potentials that can be applied to relays where receptive fields cannot be defined. Where, as in the transmission of receptive fields, critical temporal relationships are a key function of the driver, any transmission not producing a sharp cross-correlogram (i.e., a narrow peak arising from a flat baseline) would lose information along the time axis. An additional feature for the identification of drivers is the shape of the cross-correlogram This should be sharp for a driver, providing a crucial test for cerebral regions where we know nothing about receptive fields.
One problem about the cross-correlogram arises when a cell receives input from more than one major source: for example, axons with adjacent but nonoverlapping receptive fields converging to innervate a single postsynaptic cell as in the geniculocortical path (18, 19). If the synaptic influences sum linearly, the postsynaptic receptive field will reflect all of the receptive fields of the inputs. Action potentials can occur in the postsynaptic cell in relation to action potentials from any of its inputs or only if several of its inputs are firing concurrently. If the afferents fired independently of each other, the cross-correlogram based on one of these afferents could still be fairly sharp, but the peak would be smaller and the baseline would be higher and noisier because of the firing of the other afferents. A cross-correlogram can identify the driver as long as the number of convergent, independently firing afferents does not prevent the baseline from obscuring the peak that each alone would produce. Clearly, convergence among independently firing drivers must be limited if a diagnostic, sharp cross-correlogram is to be produced. Large numbers of convergent inputs could produce a sharp cross-correlogram only if their firing were highly correlated. This idea is well shown in geniculocortical connections: Cross-correlograms for geniculate and cortical cells indicate a driver input (Fig. (Fig.22D) but are sharper when the relevant geniculate cells fire in synchrony (20).
Geniculate cells and other thalamic sensory relay cells have an unusual property that makes them into poor exemplars for a general definition of drivers versus modulators. The lateral geniculate relay appears to be the only relay, from retinal receptor to higher cortical visual areas, that produces no significant spatial change in receptive field properties. Geniculate receptive fields are essentially like those of retinal ganglion cells. Where new receptive field structures are synthesized, as at the geniculocortical synapse, we must expect a more complex grouping of afferents, and a cross-correlogram with a sharp peak rising from a flat baseline, like that in Fig. Fig.22 A, C, and D, may not always be apparent. Nonetheless, the relatively sharp cross-correlogram seen for the geniculocortical synapse (Fig. (Fig.22D) identifies this as a possible driver identifiable at a synapse beyond the confines of the thalamus.
A cross-correlogram obtained from a concurrent recording of a layer 6 cortical cell and its target geniculate cell (Fig. (Fig.22B) shows a small peak with a broad foundation on a high baseline. The difference between this and Fig. Fig.22 A, C, and D is critical for the distinction between modulators and drivers. It eventually may be necessary to quantify the difference, but at present, there is too little relevant evidence for this.
The difference between driver and modulator cross-correlograms relates to the fact that there are likely to be many modulators but few drivers. The modulators may show significant convergence, and the effect of any one modulator, possibly one of thousands, may be miniscule. A driver, if it is to drive, must produce a distinct, measurable effect. The quantitative relationships of putative drivers to their postsynaptic neurons almost certainly will prove important, and the extent to which any one driver in any other relay can actually produce a cross-correlogram as sharp as that in Fig. Fig.22 A and C is untested and is likely to be to be a useful feature to explore. Because modulator inputs may often far outnumber driver inputs, the numerical strength of an afferent component, which is sometimes used to gauge its importance in signal transfer, may instead often indicate modulatory influences. That is, one cannot simply ascribe the dominant (i.e., driver) input on the basis of large numbers. These inputs do not act like a democratic assembly, and understanding the functioning of circuits requires identifying drivers and modulators by criteria other than density of inputs.
If other afferents to geniculate cells (e.g., from brainstem or local GABAergic cells) are all to be regarded as modulators, their relevant properties have yet to be defined. Corticogeniculate axons outnumber geniculate relay cells by a factor of 10–100 (21). Because corticogeniculate axons branch richly and are likely to be contacting many geniculate cells, the amount of convergence is likely to be much greater. Except for the special and physiologically implausible case of correlated firing in all corticogeniculate axons (e.g., by electrical stimulation), this convergence would lead to cross-correlograms with small peaks embedded in a noisy baseline (cf. Fig. Fig.22B).
Tonic and Burst Modes in Thalamic Relay Cells
Although a very sharp cross-correlogram can identify a thalamic driver, this cannot be the complete story. Thalamic relay cells exhibit two different response modes, “tonic” and “burst,” that necessarily affect the appearance of these cross-correlograms. Which is present depends on the activation state of a voltage-dependent Ca2+ conductance: When the conductance is inactive, the cell fires in tonic mode, and when it is active, the cell fires in burst mode. Details of this Ca2+ conductance can be found elsewhere (5, 22, 23). Switching between states requires maintaining the appropriate membrane potential for roughly ≥100 msec, faster voltage fluctuations being ineffective. Tonic firing results in a steady stream of unitary action potentials. However, the Ca2+ conductance leads to a large, spike-like depolarization that produces a burst of conventional action potentials, and these bursts are separated by silent periods. This firing pattern characterizes burst firing.
Action potentials in the tonic firing mode result directly from excitatory postsynaptic potentials, but during bursting, they result from the Ca2+ spike and thus are linked indirectly to the excitatory postsynaptic potential, which would affect the cross-correlograms. During tonic firing, an action potential in the retinal afferent may evoke an action potential in the relay cell with a tight one-to-one coupling, resulting in a cross-correlogram with a peak only a few milliseconds across (Fig. (Fig.22 A and C). During burst firing, an action potential in the retinal afferent may activate a Ca2+ spike, and the resultant burst of several action potentials lasts for ≈20 msec. Thus, the coupling between input and output action potentials is no longer one-to-one. There are as yet no published retinogeniculate cross-correlograms for burst firing of the relay cell. However, we can expect that the peak in the cross-correlogram would be broader during burst than tonic firing, but it would still be quite sharp compared with that produced by modulators.
We thus further define a driver as an input that produces a narrow peak in the cross-correlogram against a low, flat baseline. The width of the peak at half-height would be less than a few milliseconds during tonic firing and perhaps 25–50 msec during burst firing. This may be regarded as the signature of a driver, and, as we shall see, inputs to relay cells from modulators would be expected to produce broader and less clear peaks in their cross-correlograms. For instance, the modulator (corticogeniculate) cross-correlogram of Fig. Fig.22B has a sharp peak (though less sharp than for the drivers of Fig. Fig.22 A, C, and D), but it rises out of a broad, noisy bulge and not a flat baseline. It is plausible that this broad peak in Fig. Fig.22B represents activation from the cortex of metabotropic receptors and the narrower peak rising from it reflects activation of ionotropic receptors.
Latencies and Duration of Postsynaptic Responses
There is another factor besides the morphology and cross-correlogram that is probably important for distinguishing drivers from modulators. Postsynaptic potentials from drivers must be fast to permit a sharp cross-correlogram because every postsynaptic action potential must be tightly coupled in time to each presynaptic one. A prolonged, gradual excitatory postsynaptic potential would produce too much latency variability for an evoked action potential and thus would create too much temporal summation for closely spaced presynaptic action potentials to have individual postsynaptic signatures. The main temporal determinant of postsynaptic potentials for relay cells is their postsynaptic receptors. These come in two general forms: ionotropic and metabotropic (24–28). Ionotropic receptors are linked directly to ion channels, and their activation produces a fast postsynaptic conductance change resulting in very fast (typically 5–10 msec) postsynaptic potentials. This fast response meets the requirements of a driver and supports a sharp cross-correlogram. In contrast, metabotropic receptors are linked indirectly to postsynaptic ion channels via complex second messenger pathways, and these pathways eventually produce a postsynaptic potential with a gradual rise and a prolonged response (hundreds of milliseconds to seconds)§. This adds considerable temporal scatter to the latency distribution of the postsynaptic action potentials so that cross-correlograms, even without any convergence of inputs, would have peaks much broader than defined above for drivers. The slow, prolonged changes in membrane potential produced by activation of metabotropic receptors are ideal for modulation because these sustained changes affect excitability of the relay cell and serve to control activation of the many voltage-dependent conductances. Also, activation of second messenger pathways via metabotropic receptors can produce other long-term changes in the relay cell.
Retinal inputs activate only ionotropic receptors in relay cells (31). All of the other inputs, from cortex, brainstem, and local GABAergic cells, activate both ionotropic and metabotropic receptors, indicating that the receptor type may be an important property distinguishing drivers from modulators. Also, although retinal inputs to relay cells activate only ionotropic receptors, these same axons often innervate presynaptic dendritic terminals of interneurons, where they activate metabotropic receptors (32). It thus is likely that individual axons or pathways cannot be classified as drivers or modulators. Rather, the classification must refer to the synaptic contacts established by particular axons at particular sites. For instance, a single retinal axon is a driver for a relay cell and may be a modulator for an interneuron. Further, interneurons in the thalamus have two distinguishable parts—the postsynaptic somadendritic surface and the dendritic appendages that themselves can be presynaptic also—and these parts may relate to differing functions of the interneuron (33). Therefore, the action of a retinal afferent on an interneuron may depend on where on the interneuron the synapse is made.
The importance of synaptic latency and duration in the distinction between drivers and modulators may not apply to systems that react slowly (e.g., those concerned with autonomic functions or peripheral portions of olfactory, gustatory, or nociceptive pathways). Only if the precise temporal patterning of action potentials proves important to the neural code (e.g., ref. 34) would one expect to be able to recognize driver inputs on the criteria we have suggested. The issue is tantalizingly unresolved.
Key Differences Between Drivers and Modulators
We can now summarize our definition for drivers versus modulators innervating relay cells of the lateral geniculate nucleus. The key feature is the nature of cross-correlograms (see Fig. Fig.2).2). For driver inputs, they have a sharp peak rising out of a low, flat baseline. For modulators, they would have a broad, gradual peak, if any, set against a noisy baseline. The sharp cross-correlogram conforms to the need of the relay cell to convey faithfully the signal of its driver input to the cortex, and a lack of such cross-correlograms for the modulator inputs means that the signals they carry are not relayed faithfully to cortex. These critical features of cross-correlograms are merely products of a variety of circuit and cellular properties that characterize the contacts made by these inputs. Thus, retinogeniculate inputs show little convergence and make relatively few contacts, which are near cell bodies, and they activate only ionotropic receptors. Modulating inputs make more contacts, which are generally further from the cell body and can show considerable convergence; they all activate metabotropic receptors in addition to ionotropic receptors.
There are several characteristic features of drivers in the lateral geniculate nucleus that distinguish them from modulators. These characteristics include their fine structural appearance, their synaptic relationships, their degree of convergence on relay cells, their relatively small number relative to the modulators, their absence of connections to the thalamic reticular nucleus, their transmitter and receptor characteristics, and the nature of the cross-correlogram they produce when stimulated. Many of these properties are seen in all first order thalamic nuclei where the drivers can be identified. Other properties remain to be studied in these other nuclei, and some, such as the cross-correlograms, have not been defined empirically for modulators in any other thalamic nucleus. This summary for the lateral geniculate nucleus leads to a number of clear questions about other thalamic nuclei, where current knowledge of their functional organization does not make clear which is the driver, although in some, the morphological evidence has provided a useful clue. In extending the definition of drivers and modulators to other cerebral centers, such as the cortex, the characteristics we have identified may prove to be relevant.
The Sleeping Thalamus
There are two different functional states for thalamic nuclei. One involves the active, dynamic relay of driver activity to the cortex and characterizes the waking state. The other involves rhythmic, synchronized bursting of relay cells in which the relay cells no longer respond to driver inputs and are seen often during slow wave sleep. The switch from the waking to the sleeping thalamus effectively disengages the drivers.
Cells of the thalamic reticular nucleus operate this switch, a switch that seems to depend on the same sort of voltage-dependent Ca2+ conductance and bursting described above for relay cells, although the bursts in the reticular cells last longer. This leads to rhythmic bursting both in reticular cells and relay cells, and modeling studies suggest that the interconnections between reticular and relay cells and among reticular cells serve to create and maintain synchrony (for details of this behavior, see refs. 23, 35–37).
During this synchronized bursting, input from the thalamic reticular nucleus dominates relay cells, and excitatory postsynaptic potentials generated by driver inputs are insufficient to break the stranglehold of reticular inputs on thalamic relay cell responses, which is why effective thalamic relay functions are blocked during slow wave sleep. Note that the relay is disengaged not by silencing relay cells but, rather, by forcing these cells to burst rhythmically and independently of driver input. Thus, instead of silence, the cortex receives a positive signal that the relay is disrupted. Silence alone would be ambiguous; the absence of a visual stimulus could not be distinguished from the absence of an effective relay of the stimulus. The rhythmic bursting, by signaling the “no-relay” alternative, avoids this ambiguity.
If we apply our cross-correlogram criterion, reticular input to relay cells looks like a driver input during slow wave sleep because there would be a fairly sharp peak with little baseline in the cross-correlogram during the synchronized, rhythmic bursting. One could argue that the thalamic relay cells are responding to a “message” sent by the reticular cells. However, this form of driving occurs because of the special relationships that produce the highly correlated firing of the synchronized reticular cells. Without such a correlation, there would be no driving. It seems that the action of these afferents has to be distinguished from drivers considered earlier but that it cannot be regarded as modulation. We suggest that this action be treated as a “disrupting” action that is distinct from driving and modulation and that may be special to the thalamic reticular nucleus and its thalamic connections.
Conclusions
The distinction between drivers and modulators (and disrupters) is important for understanding thalamic relays, and it may prove particularly critical for defining the functional organization of thalamic nuclei where this cannot be studied in terms of readily defined receptive field properties. Possibly, the distinction can be applied much more broadly to the cerebral cortex, as suggested by Crick and Koch (1), and possibly to other cerebral centers as well. One would expect thalamocortical axons, especially those going to cortical layer 4 and possibly all of them, to be drivers, whether from a sensory relay nucleus or a higher order nucleus like the pulvinar. The classification of corticocortical pathways largely is untested in terms of the criteria proposed here.
Current views of corticocortical communication (see refs. 38, 39) could be influenced significantly by experiments that demonstrated which connections are drivers and which are modulators. One distinct and intriguing possibility is that the major source of a functional drive for corticocortical communication actually goes through the thalamus and derives from cells in layer 5 of one cortical area, which then provide a driver input to relay cells in a higher order thalamic nucleus such as the pulvinar (see Fig. Fig.1).1). These thalamic cells then, in turn, send their axons as drivers to layer 4 of another cortical area. An extreme corollary might be that most direct corticocortical pathways serve as modulators, which would mean that information flowing from one cortical area to another, by passing through a thalamic relay, would be subject to the same control of information flow as exists for information coming into cortex from subcortical sources. The thalamus thus serves as a “gate” not only in the control of information to particular cortical areas about sensory events but also in the control of information passed to other cortical areas from the descending outputs emanating from layer 5.
If the categorization of inputs as driver or modulator (or possibly as disrupter) is to have an agreed significance, or if one is to determine whether disrupters are unique to the thalamus or also can be identified in other parts of the brain, then it becomes important that experimental criteria for identifying the class of an input be understood clearly. We have tried to provide an introduction to the problems that need to be addressed if a classification that has wide applicability is to be used. Possibly, it will prove that there are too many problem areas and intermediate positions for the distinction to be of any service outside the thalamus. The observations remain to be made.
Acknowledgments
We thank Paul Adams, Ed Bartlett, Sherry Feig, Lew Haberly, Clay Reid, Phil Smith, Dan Uhlrich, and Martin Usrey for many helpful comments on an earlier draft of this manuscript. Sherry Feig also produced Fig. Fig.1.1. R.W.G. has been supported by U.S. Public Health Service Grant EY11494, and S.M.S. has been supported by U.S. Public Health Service Grant EY03038.
Footnotes
§One can divide ionotropic glutamate receptors into N-methyl-d-aspartate and non-N-methyl-d-aspartate types. The former has a slower time course than the latter, but both respond much faster than do metabotropic glutamate receptors. Some metabotropic receptors [e.g., those involved in the synapse from rods to on-bipolar cells (29, 30)] respond rapidly, but this seems rare.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.95.12.7121
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc22761?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.95.12.7121
Article citations
Border-ownership tuning determines the connectivity between V4 and V1 in the macaque visual system.
Nat Commun, 15(1):9115, 23 Oct 2024
Cited by: 0 articles | PMID: 39438464 | PMCID: PMC11496508
Synaptic integration of somatosensory and motor cortical inputs onto spiny projection neurons of mice caudoputamen.
Eur J Neurosci, 60(8):6107-6122, 24 Sep 2024
Cited by: 0 articles | PMID: 39315531
Correlated variability and its attentional modulation depend on anatomical connectivity.
Proc Natl Acad Sci U S A, 121(35):e2318841121, 22 Aug 2024
Cited by: 0 articles | PMID: 39172780
Transthalamic Pathways for Cortical Function.
J Neurosci, 44(35):e0909242024, 28 Aug 2024
Cited by: 0 articles | PMID: 39197951
Review
Convergent direct and indirect cortical streams shape avoidance decisions in mice via the midline thalamus.
Nat Commun, 15(1):6598, 04 Aug 2024
Cited by: 0 articles | PMID: 39097600 | PMCID: PMC11297946
Go to all (427) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices.
J Neurophysiol, 105(1):279-292, 03 Nov 2010
Cited by: 74 articles | PMID: 21047937 | PMCID: PMC3023380
Drivers and modulators from push-pull and balanced synaptic input.
Prog Brain Res, 149:147-155, 01 Jan 2005
Cited by: 91 articles | PMID: 16226582
Review
Thalamic Circuit Diversity: Modulation of the Driver/Modulator Framework.
Front Neural Circuits, 9:86, 12 Jan 2016
Cited by: 45 articles | PMID: 26793068 | PMCID: PMC4709853
Review Free full text in Europe PMC
Drivers from the deep: the contribution of collicular input to thalamocortical processing.
Prog Brain Res, 149:207-225, 01 Jan 2005
Cited by: 19 articles | PMID: 16226586
Review
Funding
Funders who supported this work.
NEI NIH HHS (3)
Grant ID: R01 EY003038
Grant ID: EY11494
Grant ID: EY03038




